Significant outcomes
-
1. 60 g of casein glycomacropeptide (with 12.0 g Leu and 1.8 g Trp) produced the strongest depletion of plasma aromatic amino acids (Phe and Tyr) and seemed to be safe for consumption.
-
2. The effect appeared to be highest after 3–4 h.
-
3. 60 g of casein glycomacropeptide should be the dose considered for future studies involving CGMP as a potential modulator of manic symptoms.
Limitations
-
1. We measured aromatic amino acids (Phe and Tyr) in the blood but did not measure the level of dopamine in the brain.
-
2. We only included men and do not know if the same effect is seen in women.
-
3. A placebo arm was not included.
Introduction
There is a great need for more efficient treatments of the manic condition. Preclinical studies in rats from our research group showed that an intake of casein glycomacropeptide (CGMP) lowered the precursors of dopamine, tyrosine (Tyr) and phenylalanine (Phe) in the blood and in the brain and had an anti-manic effect (Liebenberg et al., Reference Liebenberg, Jensen, Larsen, Kousholt, Pereira, Fischer and Wegener2018). The aim of this study is to build on the promising results from the rat study by performing a proof-of-concept study in healthy adults.
Bipolar disorder
The population lifetime risk of bipolar disorder ranges from 2.8% to 6.5% depending on the level of defined severity (Bauer & Pfennig, Reference Bauer and Pfennig2005). The biological mechanism of action of anti-psychotics used to treat acute mania is a blockade of the dopamine D2 receptor and to some degree antagonism of the serotonin 5-HT2A receptors, and agonism of the 5-HT1A receptor.
Amino acids and the brain
The synthesis of monoamines, especially serotonin (5-hydroxytryptamine) in the brain, is influenced by the concentration of the ingested precursor amino acid, tryptophan (Trp). Trp and other aromatic and branched-chain amino acids (BCAA) are transported into the brain via the LAT1 transporter where they compete for entry (Scalise et al., Reference Scalise, Galluccio, Console, Pochini and Indiveri2018). Plasma Trp can be lowered in healthy individuals by administering a mixture of amino acids lacking in Trp. Such mixtures may cause a lowering of mood in patients who were previously in remission, but probably also in people at risk of depression (Smith et al., Reference Smith, Fairburn and Cowen1997).
Dopamine plays an important role in bipolar disorder. Post-mortem data support the notion that an abnormality within the dopaminergic pathways, in particular involving D2/3 receptors, might play a role in the pathogenesis of bipolar disorder (Ashok et al., Reference Ashok, Marques, Jauhar, Nour, Goodwin, Young and Howes2017). BCAA leucine (Leu), isoleucine (Ile) and valine (Val) compete with Tyr and Phe for entry into the brain, lowering the available amino acid pool for dopamine and noradrenaline synthesis. BCAA, in particular leucine, play a major role in stimulating peripheral protein synthesis (Badawy, Reference Badawy2013) thereby depleting Phe and Tyr.
Glutamatergic dysregulation is implicated in the neuropathology of bipolar disorder as well. BCAA are metabolised in the human brain by BCAA aminotransferase to branched-chain keto acids, converting 2-oxoglutarate to glutamate (Blacker et al., Reference Blacker, Lewis, Frye and Veldic2017). BCAAs are thus nitrogen donors for the synthesis of glutamate and GABA (γ-aminobutyric acid), with Leu playing a major role (Yudkoff, Reference Yudkoff2017).
Clinical studies
The administration of a mixture of BCAAs compared to tap water has been shown to lower plasma Tyr with 50% and Phe with 40% in healthy adults in hours (Sheehan et al., Reference Sheehan, Tharyan, Mctavish, Campling and Cowen1996). In the tap water condition, the plasma Tyr was reduced with 10% and Phe with 3%. In the study from our research group, Tyr was reduced with 50% compared to tap water (Liebenberg et al., Reference Liebenberg, Jensen, Larsen, Kousholt, Pereira, Fischer and Wegener2018).
A review article from 2003 states that there is no evidence that lowering Tyr and Phe in healthy adults induces depressive symptoms (Booij et al., Reference Booij, van der Does and Riedel2003).
In a study from 2001, 20 inpatients meeting DSM-IV criteria for mania (McTavish et al., Reference McTavish, Mcpherson, Harmer, Clark, Sharp, Goodwin and Cowen2001) were blindly randomised to receive a single dose of either a Tyr and Phe free amino acid mixture (17.5 g Val, 15 g Ile, 22.5 g Leu, 17.5 g lysine, 5 g methionine, 10 g threonine and 2.5 g Trp) or a control mixture of free amino acids containing Tyr and Phe and were observed for 6 h. Subjects given the Tyr and Phe free mixture exhibited a reduction of 35% in manic symptomatology over the 6-h observation period. Subjects in the control group showed only minor changes in symptomatology. However, the amino acid mixture was foul tasting and unsuitable for repeated administration, as mentioned by the study group (Scarna et al., Reference Scarna, Gijsman, Mctavish, Harmer, Cowen and Goodwin2003). In a blinded randomised study (Scarna et al., Reference Scarna, Gijsman, Mctavish, Harmer, Cowen and Goodwin2003), patients fulfilling the DSM-IV criteria for bipolar I disorder with a mean baseline severity of manic symptoms on the YMRS of 29.3 were given a simpler amino acid mixture with daily intake of 60 g of BCAA (Val, Ile and Leu in the ratio 3:3:4) for 7 days. In the study, 13 manic patients were compared to 12 persons receiving a placebo. The BCAA significantly (p < 0.05) reduced manic symptoms among completers over the first 6 h, lasting for 7 days. Five withdrew from the BCAA group, one complained of drowsiness and another of nausea, both possibly related to the drink.
Casein glycomacropeptide
CGMP is a 64-amino acid bioactive protein isolated from whey, which is a by-product of cheese-making. It is a light, mild tasting soluble powder. CGMP is more soluble than amino acid salts and has a neutral taste, which is easily optimised with standard flavourings. As an intact protein, CGMP’s structure also lends itself to more optimal digestion and absorption compared to amino acids (Badawy, Reference Badawy2013). The protein structure lacks Trp, Tyr and Phe and is rich in threonine, Leu, Ile and Val, which in total, comprise 44.7% of CGMP’s amino acid profile. The product is safe to consume and is currently sold for use as a protein substitute for patients with phenylketonuria (PKU) and tyrosinemia, which are genetic disorders where patients cannot metabolise Phe and Tyr, respectively. CGMP’s low levels of the aromatic amino acids make it an ideal candidate for a natural, well-tasting alternative to free amino acids to investigate the effects of Tyr and Phe depletion in manic patients. Due to the naturally low levels of Trp in CGMP, any test product must be supplemented with Trp to avoid the depletion of serotonin in the brain which could increase the risk of depression (Booij et al., Reference Booij, van der Does and Riedel2003). An additional potential of CGMP is its ability to deliver BCAA into the brain and thereby influence glutamatergic and GABAergic functions (Yudkoff, Reference Yudkoff2017).
Aims of the study
The aim of the study was to investigate whether CGMP can provide at least the same change in the amino acid ratio obtained by BCAA, but without considerable side effects or a mood deteriorating effect in healthy men.
The primary endpoint of the study was the degree of reduction in Tyr and Phe in the blood among participants receiving three different doses of CGMP (20, 40 or 60 g) with at least 14 days washout between sessions.
Secondary endpoints of the study were to ensure that there are no considerable side effects measured with different psychiatric and neurological scales.
Method
Study design and participants
The design was a double-blind randomised dose–response study of CGMP in healthy men.
The 15 participants were divided into 3 blocks of 5 persons each and the blocks received 20, 40 and 60 g in a random order. Women were excluded to avoid the influence of hormonal fluctuations during the menstrual cycle as this influences the measurements of amino acids in plasma. The randomisation was done on a computer using a randomisation programme. The randomisation code was stored at the Translational Neuropsychiatry Unit in Aarhus, Denmark, and not accessible for the investigators involved in the study. The participants and investigators were not informed of the amount of CGMP given in the drink.
Ethics
The project was approved by the Central Denmark Region Committee of Health Research Ethics (No.1-10-72-318-16), the Danish Data Protection Agency (No. 1-16-02-651-16) and the Danish Health Authority. The Danish Health Authority does not consider CGMP as a medical product.
Inclusion criteria
Males aged 25–40 years with a BMI of 18.5–25.9 kg/m2.
Exclusion criteria
Candidate participants were excluded if they fulfilled one or more of the following criteria: having the first-degree relative with a history of a psychiatric disorder; self-reporting of the prior or current harmful use or dependence on psychoactive drugs; prior or current severe head trauma; use of prescribed medication during the last 3 months; and being a smoker.
Recruitment
Participants were recruited via the Aarhus University web page with a link to the Translational Neuropsychiatry site. There, candidate participants submitted their demographic data and were subsequently contacted by the investigator where they were able to obtain information about the study. Participants fulfilling the criteria were invited for an informative meeting at the hospital. After providing written consent for participation, a time for physical examination and psychiatric evaluations was scheduled. Participants received a minor fee covering their loss of earnings for 3 days.
Participant screening and baseline procedures
Selected participants were invited to attend the study site for the screening and collection of baseline data where the following tests were performed.
-
1. Blood screening, including red blood cell count, haemoglobin, haematocrit, white blood cell count with differential, platelet count, red blood cell indices (MCV, MCH and MCHC), alanine aminotransferase, albumin, alkaline phosphatase, aspartate aminotransferase, total bilirubin, creatinine, gamma-glutamyl transferase, potassium, sodium, lactate dehydrogenase, glucose and calcium.
-
2. Electrocardiogram with 12-lead ECG.
-
3. Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al., Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller, Hergueta, Baker and Dunbar1998).
-
4. Major Depression Inventory (MDI) (Bech et al., Reference Bech, Rasmussen, Olsen, Noerholm and Abildgaard2001).
-
5. Becks Anxiety Index (BAI) (Beck et al., Reference Beck, Epstein, Brown and Steer1988).
-
6. Visual Analogue Mood Scales (VAMS) (Arruda et al., Reference Arruda, Stern and Legendre1996).
-
7. UKU Side Effect Scale (Lingjaerde et al., Reference Lingjaerde, Ahlfors, Bech, Dencker and Elgen1987).
The participants were informed of their somatic or psychiatric investigations. If the results were not normal, they were advised to get an appointment with their GP. They also received a copy of the findings. They also received a copy of the findings.
Description of psychological measures
The rating scales were used in a semi-structured way to ensure that participants understood the content of the self-rating scales.
The MINI is a short-structured diagnostic interview, developed jointly by psychiatrists and clinicians in the United States and Europe, for DSM-IV and ICD-10 psychiatric disorders (Sheehan et al., Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller, Hergueta, Baker and Dunbar1998). The MINI comprises of modules for 17 psychiatric diagnoses.
The MDI was developed in 1998 according to the diagnostic criteria for moderate to severe depression in the ICD-10 and major depression in the DSM-IV. The MDI is used to screen for the level of depressive symptoms by using a total scale score and to discriminate between clinical levels of depression by the use of cut-off points (Bech, Reference Bech2012). The scale score ranges from 0 to 50, and a score of 26 or higher has been suggested as the most appropriate cut-off point for moderate depression. The MDI differs from the more commonly known Hamilton Depression Rating Scale or Beck Depression Inventory in that it asks about the frequency of depressive symptoms within the last 2 weeks, rather than their intensity.
The BAI (Beck et al., Reference Beck, Epstein, Brown and Steer1988), created by Beck et al,, is a 21-question multiple-choice self-report inventory that is used for measuring the severity of anxiety in children and adults. The BAI contains 21 questions, each answer being scored on a scale value of 0 (not at all) to 3 (severely). The total score is calculated by finding the sum of the 21 items. Higher total scores indicate more severe anxiety symptoms. The standardised cut-offs are: 0–7: minimal anxiety; 8–15: mild anxiety; 16–25: moderate anxiety; and 26–63: severe anxiety.
The VAMS developed by Stern (Reference Stern1996) is a reliable and valid picture-based screening measure with eight specific mood states and place minimal cognitive or linguistic demands on the respondent. Therefore, it has been used in ECT patients and in neurologically impaired patients. Other scales typically involve 3 or 7 days as in the Hamilton score or 14 days as in the MDI score. However, in this trial, we only had 6 h for testing during the intake of CGMP. The VAMS scale includes the following depressive mood states: afraid, confused, sad, angry, tired, tense and the manic mood states: energetic and happy. For clinical purposes, each item is considered on its own, but for research purposes, a total score is calculated with the items happy and energetic scored separately (Kontou et al., Reference Kontou, Thomas and Lincoln2012).
The UKU Side Effect Scale (Lingjaerde et al., Reference Lingjaerde, Ahlfors, Bech, Dencker and Elgen1987) is primarily used for evaluation of psychotropic drugs. It is a semi-structured scale that consists of 48 items. Each item is defined by a scale from 0 to 3 as follows: absence of side effect: 0; minor side effects: 1; moderate side effects: 2; severe side effects: 3.
Statistical Analyses
Our a priori method used variance analyses to evaluate the change in Tyr and Phe in the blood as well as the evaluation of side effects. In the primary analyses, the percentage change in Tyr and Phe in the blood among participants receiving CGMP in three different doses were compared by area under the curve estimations by use of the average of the Y values for points with shared X values.
A one-way ANOVA followed by Dunnett’s multiple comparisons test was performed using GraphPad Prism version 8.3.1 for Windows (GraphPad Software, San Diego, CA, USA, www.graphpad.com.) Trp availability to the brain was calculated by the ratio of plasma free Trp to the sum of the other five competitors (Leu, Ile, Val, Tyr and Phe). Similarly, Tyr and Phe availability to the brain was calculated by the ratio of the sum of Phe+Tyr to that of BCAA+Trp.
Power analyses
In preclinical studies, we found a Tyr and Phe reduction in the blood of 39–56% (Liebenberg et al., Reference Liebenberg, Jensen, Larsen, Kousholt, Pereira, Fischer and Wegener2018). In the study by Sheehan, the reduction was 40–50% as well (Sheehan et al., Reference Sheehan, Tharyan, Mctavish, Campling and Cowen1996). If we include a 40% reduction, an alpha of 0.05, a power of 0.80 and a SD of 5.0, the average sample size of a paired and a non-paired test is estimated to be 2 in each dosing group. We choose to include 15 participants each of whom received 3 different doses.
Access to data
Data were entered in RedCap (Research Electronic Data Capture), a secure web platform at the university for building and managing online databases and surveys. Arla Foods Ingredients were given access to the results of the analyses. Processing of personal data will take place in accordance with the provisions of Chapters 1 and 2 of the Ministry of Justice’s Order nos. 528 of 15 June 2000 on safeguards for the protection of personal data, which are dealt with by the public administration. The EU General Data Protection Regulation (GDPR) has also been complied with.
Intervention
All subjects each randomly received one of three mixtures of either 20, 40 or 60 g of CGMP (Lacprodan® CGMP-20, Arla Foods Ingredients Group P/S) added Trp (0.6, 1.2 or 1.8 g, respectively) and Leu (4.0, 8.0 and 12 g, respectively). The doses administered were calculated based on previous work described by Badawy (Reference Badawy2013). The study was carried out in a windowless room.
The coding and production of the CGMP test powders was done by employees at Arla Foods Ingredients P/S and unknown for participants and investigators. Subjects came to the hospital at 7:30 a.m. on the morning of each visit, having consumed a low-protein pizza (protein less than 20 g) provided for them on the evening before each visit and fasted from 10 p.m. Ensuring low intakes of protein on the evening before each visit maximised the effect of the CGMP mixtures. Following their arrival at 7:30 a.m., subjects were cannulated with an indwelling venous antecubital cannula and baseline blood samples were taken for amino acids estimation. Subjects were then rated with MDI and BAI. In addition, they completed the UKU Side Effect Rating Scale under supervision and semi-structured as well as the VAMS.
At 8:00 a.m., the subjects received a mixture of either the 20, 40 or 60 g of CGMP with added Trp and Leu. Cooled mineral water was added to give a volume of 200 ml and was chocolate flavoured with Nesquik. All drinks were prepared and administered by the medical laboratory assistant involved in collecting blood samples during the study. Subjects received a low-protein meal twice during each visit, at 8:00 a.m. and after 4 h with 80 g of white bread, 20 g of marmalade, and 10 g of butter (570 calories) + 250 ml of water). The VAMS ratings were repeated at 1-h intervals for the following 6 h. Finally, the UKU Side Effect Scale was repeated at the end of each visit.
Blood analyses
For plasma amino acid analysis, approximately 1 ml of EDTA-plasma is isolated immediately after blood sampling and cryo-preserved at minus 80° Celsius. After all subjects were included, the analysis was performed at the laboratory at the Translational Neuropsychiatry Unit as earlier described (Liebenberg et al., Reference Liebenberg, Jensen, Larsen, Kousholt, Pereira, Fischer and Wegener2018). Briefly, plasma samples were prepared by adding perchloric acid to yield a final concentration of 0.2M and then centrifuged at 4°C for 30 min at 21 000×g and the supernatant transferred to Costar cellulose acetate filter tubes (0.22 µm; Corning Inc., Corning, NY, USA) and centrifuged at 4°C for 10 min at 21 000×g. For the measurement of Tyr and Trp, 0.2M perchloric acid was used to dilute the samples a further 80×. For the measurement of AAs, the samples were diluted a further 80× for Glu, Asp, Gln, Ser, Ala, Arg, Gly, His and Met and 8× for the remaining AAs. Plasma samples were diluted 100× for the measurement of Tyr and Trp. Chromatographic conditions consisted of Thermo Scientific Ultimate 3000 model isocratic pump and autosampler (Waltham, MA, USA) equipped with a Hypersil™ BDS C18 3 μm, 3 × 150 mm particle column kept at 28°C. Detection was carried out using a Thermo Scientific™ Dionex™ model 6011RS ultra Coulometric Analytical cell (E1: +250 mV: E2: +550 mV vs. Pd reference). The column was maintained at 27°C while eluting the analytes with a MDTM mobile phase (Thermo Scientific™ Dionex™ Test Phase, 70-3829) at a flow rate of 0.5 ml/min (Dionex, 2015).
For measurement of AA profile, the equipment consisted of a Thermo Scientific Ultimate 3000 model 4-line gradient pump, autosampler and fluorometric detector (Waltham, MA, USA) equipped with a Kinetex EVO C18 5 µm 4.6 × 150 mm particle column kept at 40°C. Mobile phase A consisted of 10 mm Na2HPO4 adjusted to pH 7.8 with phosphoric acid and filtered through a 0.2-µm membrane under vacuum. Mobile phase B consisted of 1:1 methanol to acetonitrile. The gradient was as follows: 5 min equilibration at a 3% mobile phase B, and gradually increased to a 60% mobile phase B over 20 min followed by 100% mobile phase B for 3 min. The flow rate was 1 ml/min and detection was carried out at 337 nm (excitation) and 442 nm (emission). The samples and standards underwent an in-needle pre-column o-phthaldialdehyde derivatisation reaction as previously described (Dionex, 2015). All mobile phase components, standards and reagents were purchased from Sigma Aldrich (St. Louis, MO, USA).
Results
Fifteen healthy men were included with a mean age (years) of 27 (range 25–39), a mean height (in metres) of 1.84 (range 1.63–2.02); a mean weight (kg) of 75.9 (range 62–98), a mean BMI (weight/height2) of 22.4 (range 19.1–26.3). All blood screenings and ECGs were normal. The MINI did not reveal any psychiatric disease in any participants. The baseline MDI was low at 3.07 (SD 2.34) and did not increase during the intervention (Table 1). The baseline BAI was low as well 1.9 (SD:1.9) and did not increase.
Table 1. Major Depression Inventory (MDI) and Beck Anxiety Inventory (BAI) scores throughout the intervention period *
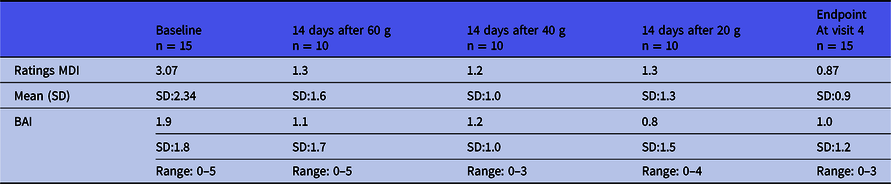
* Participants were not followed after the last CGMP intake (visit 4).
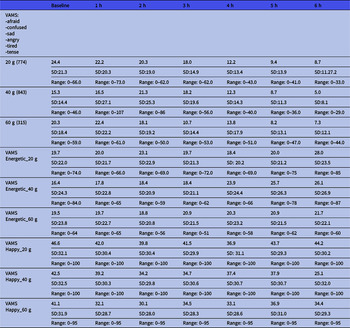
The VAMS results are shown in Table 2. This shows the results divided into the depressive mood states and the energetic/happy states according to the dose. Raw scores below 50 are considered normal. There seems to be a trend of the lowering of scores from the baseline to 6 h. This means that we cannot find any trend towards a depressive position (higher scores).
Table 2. Mean visual Analogue Mood Scale (VAMS) ratings of the total score of depressive states and the manic (energetic and happy) states over the course of the intervention period according to dose *
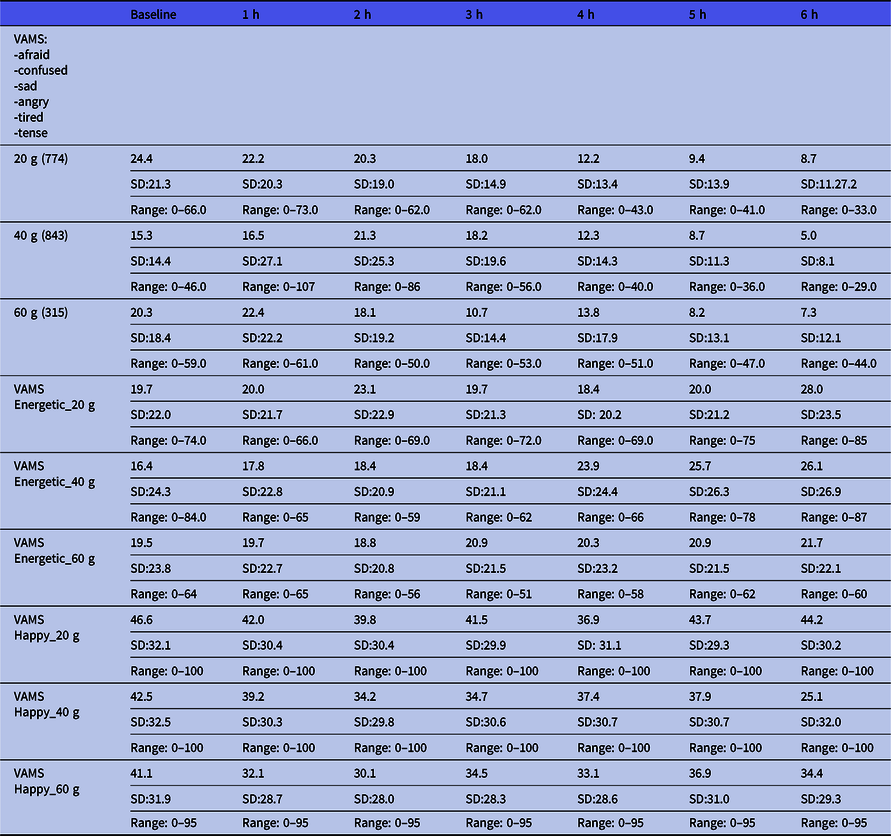
* Mean. Lower values indicate closer to neutral state.
Results from the UKU Side Effect Scale in Table 3 showed that only few side effects were found. All symptoms were mild and included drowsiness (increased by 80%), tiredness (increased by 54%) and headache (increased by 20%). Incidences of nausea were low (7%). On the UKU score from 0 to 3, no one scored more than 1 with regards to drowsiness, tiredness or headache.
Table 3. UKU Side Effect Scale before and after intake of the CGMP in different doses *
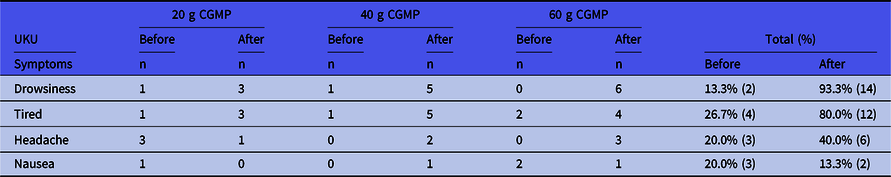
No other symptoms were found. The level of side effect was not higher than level 1 (mild symptoms).
* On the UKU score from 0 to 3, no one scored more than 1.
The blood plasma Tyr changes (Fig. 1) showed a rapid decrease from baseline to 4 h after CGMP ingestion (added Trp and Leu), but no increase thereafter. The total area under the curve is 323.6 [SE:13.36] (20 g), 277.2 [SE:13.53] (40 g) and 257.3 [SE:12.81] (60 g). Compared to one-way ANOVA with Dunnett’s multiple comparisons test, F = 0.01535, p = 0.9848, CGMP 20 g versus CGMP 40 g, p = 0.9898, CGMP 20 g vs. CGMP 60 g, p = 0.9789.
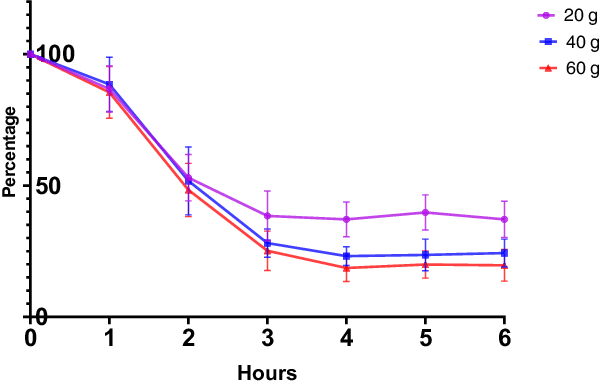
Fig. 1. Change in tyrosine levels after intake of CGMP in different doses compared to baseline level.
The blood plasma Phe changes (Fig. 2) showed a rapid decrease from baseline to 3 h after CGMP ingestion (added Trp and Leu) and thereafter a minor increase. To evaluate the effect size of the different doses, the total area under the curve was calculated. This showed that 60 g resulted in the biggest decrease. The total area under the curve is 359.2 [SE:20.37] (20 g), 273.6 [SE:22.16] (40 g), and 263.7 [SE:19.36] (60 g). Compared to one-way ANOVA with Dunnett’s multiple comparisons test, F = 6.511, p = 0.0055, CGMP 20 g versus CGMP 40 g, p = 0.0132, CGMP 20 g versus CGMP 60 g, p = 0.0061.

Fig. 2. Change in phenylalanine levels after intake of CGMP in different doses compared to baseline level.
The blood plasma Trp changes showed a rapid increase in the Trp level 1 h after CGMP ingestion (added Trp and Leu). The total area under the curve is 746 [SE:49.61] (20 g), 1022 [SE:76.98] (40 g) and 1225 [76.03] (60 g). Compared to one-way ANOVA with Dunnett’s multiple comparisons test, F = 1.079, p = 0.3609, CGMP 20 g versus CGMP 40 g, p = 0.6231, CGMP 20 g versus CGMP 60 g, p = 0.2692.
As an approximation of the reduction of Phe and Tyr uptake into the brain (Fig. 3), the plasma ratio of Phe and Tyr is compared with the level of BCAA+Trp as mentioned by Badawy (Reference Badawy2013). The total area under the curve was CGMP (20 g): 3.648 [SE:0.3281]; CGMP (40 g): 2.368 [SE:0.1858]; and CGMP: (60 g): 1.887 [SE:0.2591]. A comparison of the groups showed a dose-dependent statistical difference, one-way ANOVA F = 11.87, p = 0.0003, CGMP 20 g versus CGMP 40 g, p = 0.0042, CGMP 20 g versus CGMP 60 g, p = 0.0002.
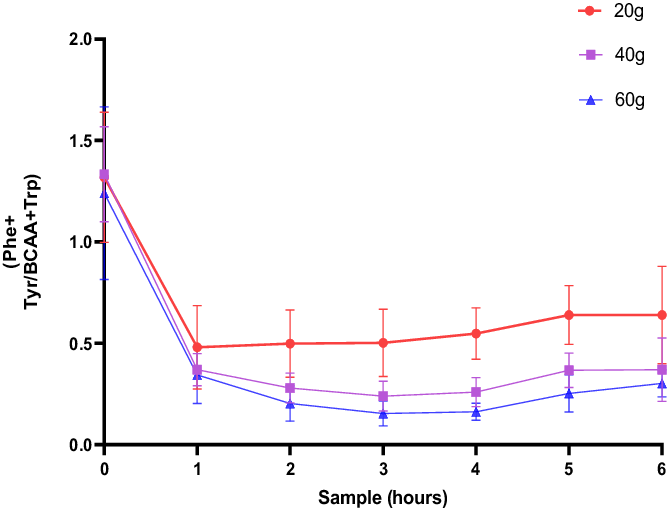
Fig. 3. The blood plasma [Phe+Tyr]/[BCAA+Trp] ratio of fasted male subjects prior to, and hourly after consumption of a drink containing 20, 40 and 60 g of CGMP, respectively.
Discussion
In this double-blind, randomised dose–response study, we investigated the plasma amino acid, and psychological responses in healthy human adults following the consumption of three doses of a mixture of CGMP with added Leu and Trp. We found that CGMP produced the greatest dose-dependent depletion of plasma Phe and Tyr, with 60 g of CGMP (with added 12 g Leu and 1.8 g Trp).
The mechanism by which CGMP with Leu and Trp reduces the peripheral appearance of Phe and Tyr is theoretically related to at least two possible mechanisms through which CGMP mixtures may induce the depletion. Firstly, the ingested AAs (especially Leu) would stimulate peripheral protein synthesis, leading to the residual AAs that are absent in the mixture (i.e. Phe, Tyr and/or Trp) to become depleted in plasma (Badawy, Reference Badawy2013). Secondly, Phe, Tyr and Trp use the same transporter and therefore, compete for entry into the brain with other LNAAs (His, Ile, Leu, Met, Thr and Val). Their relatively lower plasma levels cause them to be outcompeted and further reduced in the brain. The rate-limiting enzymes that synthesise DA and 5-HT, that is, Tyr hydroxylase and Trp hydroxylase, respectively, are unsaturated under normal physiological conditions, and the rate of monoamine synthesis is therefore vulnerable to changes in Tyr and Phe availability. As shown previously, this can reduce dopamine in the brain and exert anti-manic effects in rodent models and manic patients (Boado et al., Reference Boado, Li, Nagaya, Zhang and Pardridge1999; Scarna et al., Reference Scarna, Gijsman, Mctavish, Harmer, Cowen and Goodwin2003; Liebenberg et al., Reference Liebenberg, Jensen, Larsen, Kousholt, Pereira, Fischer and Wegener2018).
No signs of depression or anxiety were revealed using the MDI scale, the VAMS scale and the BAI scale. When the UKU Side Effect Scale is used, only few side effects are found. Therefore, CGMP is a safe and palatable alternative to free amino acids. The reason why the test persons experienced more drowsiness and tiredness may have the following reasons. One is that the subjects received a small carbohydrate breakfast, including white bread. This usually increases insulin production after ingestion and results in a subsequently lower glucose level after some time. CGMP may also contribute to these side effects caused by lowering the dopamine level in the brain as Phe and Tyr uptake in the brain was decreased (Pagano et al., Reference Pagano, Molloy, Bain, Rabiner, Chaudhuri, Brooks and Pavese2016). Another explanation is the increase in Trp uptake, and a subsequent higher synthesis of serotonin in the brain (Blomstrand, Reference Blomstrand2001).
When used in manic patients, it is preferable for the patients to become a little sleepy and tired. A headache might be caused by Trp (Greenwood et al., Reference Greenwood, Lader, Kantameneni and Curzon1975) or it may be caused by fasting the evening before and having a small carbohydrate breakfast the morning after. Only two participants reported symptoms of nausea when consuming 60 g of CGMP.
Phe drops considerably during the first 3 h following ingestion of 60 g CGMP which may be an indicator of a rapid anti-manic effect as seen in earlier studies using BCAA in mania. Tyr shows a similar fast decrease during the first 4 h. Trp increases as we would expect, as Trp was added to the CGMP mixture to avoid lowering serotonin in the brain.
The ratio of (Phe+Tyr)/(BCAA+Trp) indicates the degree of depletion of Phe and Tyr and the degree of BCAA increases in the brain and thus the availability of Phe and Tyr to enter the brain. We found that 60 g of CGMP plus 12.0 g Leu and 1.8 g Trp gave the highest degree of depletion shown as the smallest area under the curve. Treatment of manic symptoms seems to be a possibility with CGMP, but it needs to be investigated in a randomised clinical study with bipolar patients. We assume that CGMP increases BCAA uptake in the brain as the competing aromatic amino acids are lowered with CGMP. This might be important to re-establish the balance between glutamate and GABA and to decrease glutamate excess in the brain in bipolar patients. The rapid increase in Trp may have contributed to the effect. In our next study in patients with a manic episode, we intend to include both men and women.
We chose not to measure s-prolactin, as it was not the aim of the present study. For treatment with antipsychotics that block the dopamine receptors, a 1–5-fold increase in s-prolactin can be seen. Depletion of Tyr and Phe has been shown to increase the levels of plasma prolactin in euthymic women with a past history of major depression. However, the ratings of depression were unaffected (McTavish et al., Reference McTavish, Mannie, Harmer and Cowen2005). S-prolactin must be measured at the earliest 3 h after awakening (Rojas Vega et al., Reference Rojas Vega, Hollmann and Strüder2012).
Conclusions
This study demonstrate CGMP is a well-tolerated and effective mixture, and that 60 g of CGMP produced the strongest depletion of plasma aromatic amino acids (phenylalanine and tyrosine). The effect seems to be highest after 3–4 h. We therefore conclude that this dose should be the one considered for future studies involving CGMP in humans.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/neu.2021.34
Acknowledgements
The authors would like to thank Per Fuglsang Mikkelsen and Marit Nyholm Nielsen at the Translational Neuropsychiatry Unit, Department of Clinical Medicine, Noerrebrogade 44, 8000 Aarhus C., Denmark.
Author contributions
ERL had full access to all study data and took responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: ERL, GW and EJ. Critical revision of the manuscript: all authors. Recruitment of subjects/intervention: AJ and ERL.
Financial support
Arla Foods Ingredients P/S financed the project.
Conflicts of interest
Besides this project, the principal investigators Erik Roj Larsen and Gregers Wegener have no affiliation to Arla Foods Ingredients P/S. Erik Jensen and Tristan R. Hollyer were both full-time employees of Arla Foods Ingredients P/S. G.W. is Editor-in-Chief of Acta Neuropsychiatrica but was not involved and actively withdrew during the review and decision process of this manuscript.
Disclosures
Erik Roj Larsen has received lecture fees from H. Lundbeck A/S. Gregers Wegener has received lecture/consultancy fees/research support from H. Lundbeck A/S, Servier SA, Astra Zeneca AB, Eli Lilly A/S, Sun Pharma Pty Ltd, Pfizer Inc., Shire A/S, HB Pharma A/S, Alkermes Inc., Mundipharma International Ltd., J&J Inc. and Jannsen Pharma A/S. No other conflicts of interests are reported.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975 as revised in 2008.