Introduction
Human population growth has resulted in the largest impacts on biodiversity ever recorded, mostly as a result of conversion of natural forest to agricultural land (Corlett Reference Corlett2014). Over the past four decades, global biodiversity has decreased at an alarming rate, with the main declines occurring primarily in tropical areas, where most threatened vertebrates are found (Butchart et al. Reference Butchart, Walpole, Collen, Van Strien, Scharlemann, Almond and Bruno2010, Hoffmann et al. Reference Hoffmann, Hilton-Taylor, Angulo, Böhm, Brooks, Butchart and Cox2010). In South-East Asia, extinction risk has increased markedly due to anthropogenic activities (Hoffmann et al. Reference Hoffmann, Hilton-Taylor, Angulo, Böhm, Brooks, Butchart and Cox2010, Duckworth et al. Reference Duckworth, Batters, Belant, Bennett, Brunner, Burton and Harris2012), such as overexploitation and deforestation, the rate of which are among the highest in the tropics (Heino et al. Reference Heino, Kummu, Makkonen, Mulligan, Verburg, Jalava and Räsänen2015), and are still increasing (Miettinen et al. Reference Miettinen, Shi and Liew2011). It is estimated that nearly 50% of the region’s mammal populations and 32% of bird populations will be extinct by the end of this century (Brook et al. Reference Brook, Sodhi and Ng2003). At least half of these could represent global extinctions, and the number could be even higher due to other threats such as climate change and invasive species (Brook et al. Reference Brook, Sodhi and Ng2003). Within the region, Cambodia had the highest deforestation rate for 2013 (Hansen et al. Reference Hansen, Potapov, Moore, Hancher, Turubanova, Tyukavina and Loveland2013, Corlett Reference Corlett2014) as a result of its Economic Land Concession (ELC) development and road system expansion with consequent increases in hunting and logging (Clements et al. Reference Clements, Lynam, Gaveau, Yap, Lhota, Goosem and Laurance2014).
Mainland South-East Asia mostly lies within the Indo-Burma Biodiversity Hotspot (Myers et al. Reference Myers, Mittermeier, Mittermeier, Da Fonseca and Kent2000) and supports 72 galliform species (World Pheasant Association 2017) mostly comprising three genera: Lophura, Arborophila and Polyplectron. Galliforms show a globally high extinction risk with 25% of the 308 species in the IUCN Red List listed as threatened, compared to 13% for all bird species (BirdLife International 2016), while for South-East Asia this rises to 27% of galliform species threatened with extinction. As for most biodiversity in the region, the major threats are habitat loss and fragmentation, and hunting. Unfortunately, the ecology and conservation status of most galliform species within the region is poorly known (Grainger et al. Reference Grainger, Garson, Browne, McGowan and Savini2018) and for some genera, such as Arborophila, almost no quantitative data are available, with the exclusion of a few case studies (Vy et al. Reference Vy, Ngoprasert, Browne and Savini2017).
The Chestnut-headed Partridge, Arborophila cambodiana, is restricted to the Cardamom Mountains in south-west Cambodia with a small population also found in south-east Thailand (Eames et al. Reference Eames, Steinheimer and Bansok2002). Initially described from what is now Bokor National Park in 1928 (Delacour Reference Delacour1929) and thought to be a common resident of the mid-elevation (400–1,400 m) semi-evergreen and evergreen hill forests (Goes and Furey Reference Goes and Furey2013), the species is little known and limited information has been collected and reported over the last 60 years, in part due to civil war in the area from 1967 to 1998 (Poole Reference Poole1999). This paucity of information prompted A. cambodiana to be classified as ‘Endangered’ in 2002 (BirdLife International 2016). However as more information slowly trickled in, primarily consisting of anecdotal observations by birdwatchers, the species was downgraded to ‘Vulnerable’ in 2004 and to ‘Least Concern’ in 2009, mainly based on the estimated available habitat (BirdLife International 2016) and survey reports (Samnang et al. Reference Samnang, Sary and Browne2009). However, these assessments may not now reflect the true situation. Large areas of the Cardamom Mountains have been zoned as economic land concessions, which either have been or are likely to be cleared for agro-industrial plantations. Areas of both Bokor National Park and Kirirom National Park are threatened with poorly controlled tourism development, whilst agricultural development (pepper farming) is increasing in Phnom Samkos Wildlife Sanctuary and Botum Sakor National Park. This resulted in 2,146 km2 (10% of the total area) of the Cardamom Mountain range being converted to agriculture through ELCs (Open Development Cambodia 2014). In addition, in 2007 hunting was believed to be the major threat, followed by land conversion (Samnang et al. Reference Samnang, Sary and Browne2009).
In order to assess the conservation status of A. cambodiana within its restricted and diminishing range, and to address the lack of detailed information on the species, we aimed to: 1) estimate the current distribution and population density of A. cambodiana in the Cardamom Mountain range; 2) assess habitat change over the past 20 years; and 3) provide an updated recommendation for the specie’s conservation status based on revised habitat availability and density information.
Methods
Study sites
We surveyed A. cambodiana at four sites in Cambodia’s Cardamom Mountains range: Bokor National Park (BKNP), Central Cardamom National Park (CCNP), Phnom Samkos Wildlife Sanctuary (PSWS) and Southern Cardamom National Park (SCNP) (Figure. 1). The Cardamom Mountains cover approximately 23,000 km2 and range in elevation from 0 to 1,800 m (Stuart and Emmett Reference Stuart and Emmett2006). The Cardamoms are covered with tropical evergreen and semi-evergreen forests (Eames et al. Reference Eames, Steinheimer and Bansok2002) and are subject to a tropical monsoonal climate with a wet season from May to October (2,000–5,000 mm total rainfall) and a dry season from November to March (2,000–3,000 mm). Average temperatures range from 25–30o C, but can drop below 15°C at higher elevations (Daltry and Momberg Reference Daltry and Momberg2000).
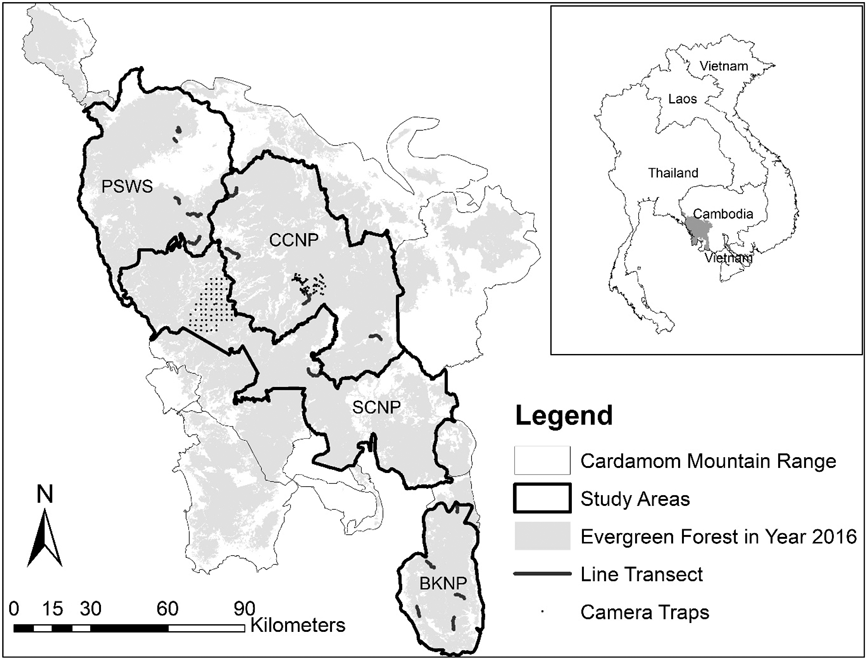
Figure 1. Arborophila cambodiana study sites at Bokor National Park (BKNP), Central Cardamom National Park (CCNP), Phnom Samkos Wildlife Sanctuary (PSWS) and Southern Cardamom National Park (SCNP) with the location of camera traps and line transects.
Bokor National Park (10047’N, 104001’E) is situated in the Elephant Mountains, a southern offshoot of the Cardamom Mountains, covering an area of 1,418 km2 with an elevation range from 30 to 1,079 m. The park is dominated by a large massif with an extensive plateau at around 1,000 m. It supports large and intact areas of evergreen forest, with wet evergreen forests found mostly in the south, and deciduous and semi-evergreen forests in the north.
The Central Cardamom National Park (11o59’N, 103o29’E) covers an area of 4,015 km2 and is characterised by large rivers and expanses of lowland evergreen forests on the rolling foothills with an elevation ranging from 300 to 1,300 m. Unlike the other sites in the Cardamom Mountains range, this area is derived from Mesozoic sandstones.
Phnom Samkos Wildlife Sanctuary (10o29’N, 102o57’E) covers 3,302 km2 and is named after Samkos Mountain, which is Cambodia’s second highest peak (1,717 m). The vegetation consists of lowland evergreen forest, medium altitude evergreen forest, semi-deciduous forest, dry deciduous forest, lowland and medium altitude forests on limestone, pine forests and montane grasslands. PKWS ranges in elevation from 300 to 1,700 m.
Southern Cardamom National Park (11o48’N, 103o06’E) covers 4,114 km2 with an elevation range from 10 to 980 m. The vegetation is like that present in CCNP and constitutes one of the region’s largest continuous areas of rainforest. It is ecologically important as it provides the main corridor for Cambodia’s largest remaining population of Asian elephant Elephas maximus, allowing them to move through the landscape, including into Thailand.
Density estimates
Bird densities were estimated using line transects in three different protected areas (BKNP, CCNP and PKWS). A total of 30 transects were established, 10 in each protected area, along existing human and animal trails when approximately straight. If trails were unsuitable, not straight, or not available, straight lines were cut through the forest, avoiding areas with land mines. In each protected area we established five study locations, each comprising two line transects spaced 300 m apart (Figure 1). Transect length varied from < 1 km, where they were cut through the forest, to > 4 km when existing human and animal trails were found. Line transects were surveyed from 28 January 2015 to 25 December 2015 and 9 January 2016 to 28 March 2016 which corresponded with the A. cambodiana breeding season (both nesting and mating) from April to June when detections are higher (Goes and Furey Reference Goes and Furey2013).
Each transect was walked by two different observers simultaneously four times a day (morning: 06h00–09h00 and 09h00–11h30 and afternoon 14h00–16h30 and 16h30–18h00) for three consecutive days, except during periods of heavy rain. The point at which a calling A. cambodiana was heard along a transect was recorded by GPS (Garmin 62SC), as was time, the estimated distance from the observer, and using a compass, the direction from the observer. These data were used to define the perpendicular distance of birds to the transect line using ArcGIS. To avoid double counting we assumed that if multiple birds were heard calling within three minutes of one another, within a bearing range of 10 degrees and within a radial distance of < 100 m they were a single calling group. We excluded from analysis eight line transects along which we did not detect any birds. Six of these were at an elevation below 400 m (four located in BKNP and two in CCNP), whilst two were in high elevation pine forests in CCNP.
We used distance sampling protocols to estimate A. cambodiana density (Buckland et al. Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001, Reference Buckland, Marsden and Green2008). Only calling male birds recorded from line transects were used to calculate density. Sighting-only detections were excluded from the analysis because only two groups of A. cambodiana were sighted during the survey period. Distance 7.1 (Thomas et al. Reference Thomas, Buckland, Rexstad, Laake, Strindberg, Hedley and Burnham2010) was used to estimate A. cambodiana detection probability and density. Key functions ‘uniform’, ‘half-normal’, and ‘hazard’ with cosine adjustments were used to run the analysis. Model fitness was selected using a combination of visual assessment of the distribution curve, goodness-of fit test, and the lowest Akaike’s Information Criterion (AIC) (Akaike Reference Akaike, Petrovand and Csàki1973). As the number of detections of A. cambodiana from each study site was small, we tested the difference among detection functions of each study site by comparing the value of AIC between global and stratified models. For the global model, we estimated density by pooling all detections from each study site. For the stratified models, because of the small sample sizes, we used the pooled global detection function (half-normal) to derive stratum specific density estimates. Finally, the best model selection was based on the AIC value and coefficient of variance (CV) from each model (Buckland et al. Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001).
Camera trap survey and habitat association
To increase the number of A. cambodiana detections used in the habitat selection analysis we used data from two camera trap surveys (Figure 1). The first dataset consisted of paired cameras installed at 74 locations (total 15,080 trap-nights) from December 2013 to March 2014 by the Wildlife Conservation Research Unit of Oxford University to target the common leopard Panthera pardus at CCNP between elevations of 565 and 1,169 m. The second survey consisted of a single camera at 66 locations (total 8,236 trap-nights) set from December 2015 to January 2016 by Wildlife Alliance - Cambodia to target Indochinese tiger Panthera tigris prey in SCNP between elevations of 105 and 620 m. In both cases camera traps were set in a systematic 2-km grid and placed 20–50 cm above the ground. Elevation, slope and distance to the nearest water source were considered as the main environmental variables likely to influence A. cambodiana. Elevation and slope were extracted from the ASTER GDEM at a scale of 30 x 30 m (Global Digital Elevation Model) downloaded from the Earth Remote Sensing Data Analysis Center (http://www.jspacesystems.or.jp/ersdac/GDEM/E/4-.html). Distance to the nearest water source (DS) was derived from the Cambodian Ministry of Environment topographic map. All data was re-projected to the WGS1984 datum before analysis.
We investigated A. cambodiana habitat use using camera trap and line transect data. Generalized linear mixed models with binomial distribution including the null model were developed to determine the association between ecological variables and the presence of A. cambodiana. The ‘glmmTMB’ (Template Model Builder) package (Bolker Reference Bolker2016) was used with R version 3.4 (R Development Core Team 2017) for fitting generalized linear mixed models and extensions when sampling methods (camera trap and line transect) were treated as random effects. Explanatory variables were elevation, slope, and distance to water sources. Habitat selection models were developed using 619 km surveys from 22 transects and 23,296 trap-nights of 140 camera trap locations. The detection of calling (from line transect) and captures (from camera trap) of birds from each survey were treated as the response variable (detection or non-detection). Forest type was excluded from analysis as only two birds were heard calling in semi-evergreen forest and none in the pine forest. Five binomial regression models, including the null model, were developed to determine the association between ecological variables and the presence of A. cambodiana.
Prior to running the models, the continuous variables including elevation, slope, and distance to water sources were checked and outliers were removed. These variables were then standardised by subtracting from the mean and dividing by its standard deviation (x variable – mean of x/sd of x) (Gelman Reference Gelman2008). We did not include highly correlated variables (r > 0.5) in the same model. The survey effort (number of visits multiplied by transect length and number of camera trap-nights) was treated as a fixed coefficient and set to 1 by using an “offset” (Gelman and Hill Reference Gelman and Hill2006). We selected models by comparing Akaike information criterion (AICc) values adjusted for small samples. Akaike model weights (AIC-w) were calculated as the weight of evidence in favour of a model among the models being compared. We assessed model accuracy using the area under the receiver operating characteristic curve (Hosmer and Lemeshow Reference Hosmer and Lemeshow2000, Franklin Reference Franklin2010) in the “Presence/Absence” package (Freeman and Moisen Reference Freeman and Moisen2008). We chose an optimal threshold cut-off value for classification using the minimised difference between the proportion of presences correctly predicted (sensitivity) and the proportion of absences correctly predicted (specificity) (Franklin Reference Franklin2010).
Current suitable habitat
A. cambodiana habitat loss was defined as the reduction of evergreen forest above 400 m and slope between 110 and 430 from 1996 to 2016. Loss was calculated using LANDSAT 5 (1996), LANDSAT 7 (2006) and LANDSAT 8 images from http://glovis.usgs.gov/ using supervised classification (ESRI 2011) in ArcGIS 10.1 (ESRI, Redlands, USA). Images were downloaded for the Cardamom Mountain range for February 1996, 2006 and 2016 when there was likely to be the lowest level of cloud scatter (< 10%).
The images were defined into different colour bands (different vegetation types) based on the Cambodian forest cover layer (Open Development Cambodia 2016), then the total area of evergreen forest above 400 m each year was calculated using summary statistics in ArcGIS 10.1. The evergreen forest above 400 m was calculated for two ten-year periods (1996–2006 and 2006–2016) and compared to the whole area of evergreen forest. Separate loss statistics were generated for the Cardamom Mountains as a whole, as well as BKNP, CCNP and PSWS.
Results
Density estimation
One hundred and forty-eight calling males were recorded from the three study areas and 619 km of surveyed line transects. The half-normal key function was the most supported model with detection probability P = 0.48. Calling birds were detected up to 97 m from the transect line (Figure 2) and the overall density estimate was 1.23 calling males/km2. Study area stratification was the most supported model with AIC = 189 compared to the global model (AIC = 1491). Estimated density was high in BKNP (2.65 calling males/km2), but lower in both PSWS (∼ 60% less) and CCNP (∼ 90% less) (Table 1). As there was minimal overlap between 95% confidence intervals between estimates for CCNP and BKNP the density within the latter was higher. There was little difference in density between PSWS and either of the other two sites (Table 1).
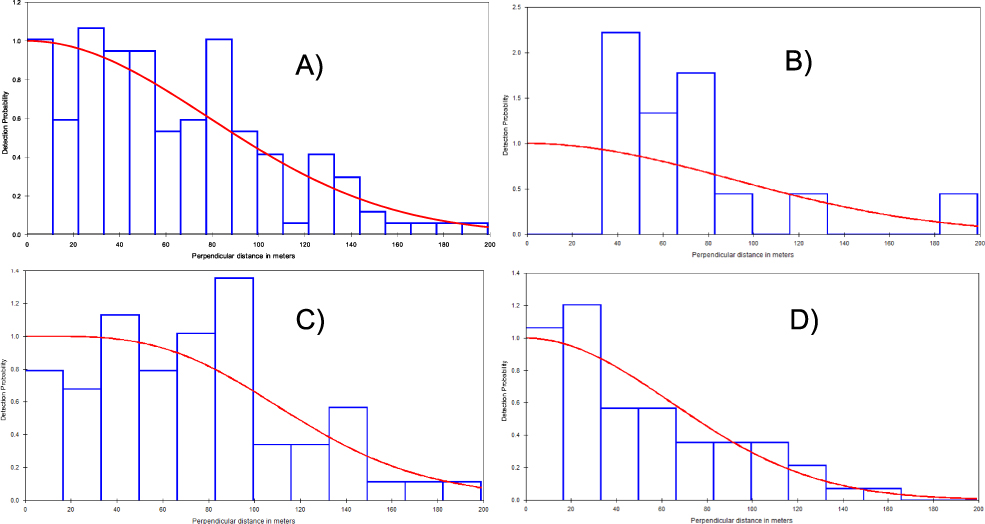
Figure 2. The detection function curve of A) the global model (all sites); B) Bokor National Park, C) Central Cardamom National Park and D) Phnom Samkos Wildlife Sanctuary.
Table 1. Distance sampling of detected male Arborophila cambodiana calls using line transects from Bokor National Park (BKNP), Central Cardamom National Park (CCNP), Phnom Samkos Wildlife Sanctuary (PSWS).

* Total length of line transect (line length in km multiplied by observation times)
** Global density estimation
Habitat association
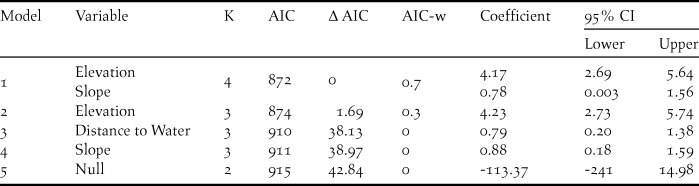
The presence of A. cambodiana was positively associated with elevation (> 400 m) and slope (11 and 43o), whereas distance to water had no effect (Table 2). The best fitted model provided reasonable discrimination between A. cambodiana presence and absence (AUC = 0.79). The AUC threshold cut-off value was 0.4 based on the minimised difference between sensitivity and specificity with the highest correct classification at 78%.
Table 2. Detail of parameters in accepted Arborophila cambodiana habitat use models with beta coefficient and 95% CI.
Using the best regression model, we estimated there to be 2,308 km2 of A. cambodiana habitat in Cambodia’s Cardamom Mountain range remaining in 2016. This comprises around 45% of the estimated total area of evergreen forest above 400 m and 96% (2,221 km2) of this habitat is located inside protected areas (Figure 3).

Figure 3. Change in suitable habitat for Arborophila cambodiana in the Cardamom Mountains over 20 years (1996–2016).
Habitat loss
In 2016 15,007 km2 of evergreen forest covered the Cardamom Mountains in Cambodia, which comprised (65%) of the total area. Of this, 2,308 km2 (15%) was suitable for A. cambodiana being located above 400 m and with a slope between 110 and 430. Over the past 20 years (1996–2016) the area of evergreen forest across the Cardamom Mountains has decreased by 20% (3,551 km2) including a 11% reduction in suitable habitat for A. cambodiana. This is equal on average to a rate of loss of around 100 km2 every 10 years. Previously the amount of available habitat was larger and had declined by 173 km2 (7%) between 1996 and 2006, a further 118 km2 (5%) between 2006 and 2016. Comparing the three study areas, in 20 years (1996–2006 and 2006–2016), total evergreen forest loss was highest in CCNP (127 km2, 11 km2) followed by PSWS (25 km2, 7 km2) and BKNP (10 km2, 6 km2) (Figure 3 and Table 3).
Table 3. Predicted A. cambodiana habitat (steeply sloping evergreen forest above 400 m from sea level) in 1996 to 2006 and 2016.

* Total predicted suitable habitat of A. cambodiana which including three study areas plus Phnom Aural Wildlife Sanctuary, Peam Krosob Wildlife Sanctuary, Southern Cardamom National Park, Kirirum National Park and new established wildlife corridors.
Discussion
Bird density
Our A. cambodiana density estimates were low when compared to similar partridge species. For example, the estimated density of A. davidi in southern Vietnam was 3.63/km2 (Vy et al. Reference Vy, Ngoprasert, Browne and Savini2017), A. chloropus (now Tropicoperdix chloropus Chen et al. Reference Chen, Liu, Davison, Dong, Chang, Gao and Zhang2015) in Khao Yai National Park, north-eastern Thailand was ∼ 18/km2 (Ong-in unpubl. data) and A. arde on Hainan Island, China was 6.54/km2 (Gao Reference Gao1999). However, our estimate was higher than the estimated 0.48/km2 density of A. rufipectus in Sichuan, China where much of the natural habitat had been replaced by non-native conifer plantations and what remained was highly fragmented (Dai et al. Reference Dai, Dowell, Rodney and Robert1998).
The higher density of A. cambodiana at BKNP was likely due to the low level of habitat loss at this site when compared to the others. The suitable habitat that remains at BKNP is isolated and largely inaccessible to humans, as much occurs on a plateau, surrounded by high, steep cliffs. As a result habitat fragmentation is low, which is favourable for many bird species (Ewers and Didham Reference Ewers and Didham2006, Chan Reference Chan2010). This is unlike the other study areas which are much more accessible and fragmented. In 2007, much of the southern part of BKNP was granted to a private company to develop ecotourism (Open Development Cambodia 2014). This area is now better protected, with the collection of NTFPs having been banned. Ecotourism can also benefit galliform conservation, for example birdwatching in Cat Tien National Park, Vietnam (Sukumal et al. Reference Sukumal, McGowan and Savini2015) has reduced the hunting pressure on Green Peafowl Pavo muticus as villagers now value this iconic species for its ability to attract tourists. The increase in tourism and consequent increase in financial revenue for the area, might have encouraged the adjacent rural communities to avoid disturbing the forest (i.e. hunting and grazing cattle in the park) as well as increased the park’s management effectiveness (Sukumal et al. Reference Sukumal, McGowan and Savini2015).
During the survey we also recorded male T. chloropus calling along line transects We estimated their density at approximately 15 calling males /km2 in BKNP (unpubl. data) which is like the 18 calling males/km2 recorded for the same species in the well protected Khao Yai National Park, Thailand (Ong-in unpubl. data). T. chloropus was also found at low density in the other two study areas (three calling males/km2 in CCNP and six calling males/km2 in PSWS).
The lower densities recorded for both partridge species in the CCNP are likely to be the result of habitat fragmentation. In the CCNP evergreen forest is interspersed with woodlands with a grassland understorey (Stuart and Emmitt 2006) which is unsuitable habitat for several Arborophila species (Dai et al. Reference Dai, Dowell, Rodney and Robert1998, Gao Reference Gao1999, Ong-in et al. Reference Ong-in, Pierce, Gale, Browne and Savini2016, Vy et al. Reference Vy, Ngoprasert, Browne and Savini2017). For instance, we did not detect A. cambodiana along the two transects located in open pine forest with grassy understorey. Similarly, Sichuan Hill-partridge (A. rufipect) in China were also absent from coniferous forest despite occurring in adjacent areas of plantation (Dai et al. Reference Dai, Dowell, Rodney and Robert1998).
Habitat use
In the Cambodian Cardamom Mountains A. cambodiana was most commonly recorded in evergreen forest at elevations above 400 m and on steep slopes. This shows the importance of the structure of the terrain for this species. Similar micro-habitat preferences were also found for other Arborophila species including the Common Hill-partridge A. torqueola (Liao et al. Reference Liao, Hu and Cao2007a) and Sichuan Hill-partridge A. rufipectus (Dai et al. Reference Dai, Dowell, Rodney and Robert1998, Liao et al. Reference Liao, Cao, Jin-chu and Xin2007b) in China and Orange-necked Partridge A. davidi (Vy et al. Reference Vy, Ngoprasert, Browne and Savini2017) in southern Vietnam. Based on the habitat use model we predicted that density of A. cambodiana should be highest in the CCNP, followed by PSWS and lowest in BKNP (Figure 2, D). However, our survey data showed the opposite (Table1). This contradictory finding is most likely the result of human disturbance (e.g. Hiller et al. Reference Hiller, Jarvis, Lisa, Paulson, Pollard and Stanley2004, Rimbach et al. Reference Rimbach, Link, Heistermann, Gómez-Posada, Galvis and Heymann2013). We observed both small (targeted valuable timber) and large-scale (land clearance for agricultural purpose) logging almost everywhere within CCNP, including numerous paths crossing the area used to export timber from the CCNP through Phnom Aural Wildlife Sanctuary.
In addition, there were numerous ELCs and less government patrolling in areas controlled by private companies. Illegal logging increases hunting, as loggers opportunistically target small terrestrial animals including galliforms (Samnang et al. Reference Samnang, Sary and Browne2009, Poulsen et al. Reference Poulsen, Clark and Bolker2011, Rimbach et al. Reference Rimbach, Link, Heistermann, Gómez-Posada, Galvis and Heymann2013). Arborophila species have been shown to actively avoid or occur at lower densities in areas with human disturbance (Nijman Reference Nijman2003, Liao et al. Reference Liao, Hu and Cao2007a, Reference Liao, Cao, Jin-chu and Xin2007b, Vy et al. Reference Vy, Ngoprasert, Browne and Savini2017). The low-density estimates for A. cambodiana may also result from the presence of other species such as T. chloropus in the area. The effect of potential competitors was also predicted for A. davidi, which was found at lower densities in the presence of T. chloropus in South Vietnam (Vy et al. Reference Vy, Ngoprasert, Browne and Savini2017). A. cambodiana did not extend through much of the Southern Cardamom National Park south to the Gulf of Thailand, where the topography is mostly flat and thus less suitable for the species. Human disturbance such as logging, hunting and land clearance may also be the cause of low A. cambodiana and T. chloropus densities (Samnang et al. Reference Samnang, Sary and Browne2009).
Habitat loss
A. cambodiana is vulnerable to habitat loss and disturbance, as it occurs mostly in well-developed forest with deep litter and complex ground structure (Nijman Reference Nijman2003, BirdLife International 2016). Logging and hunting remain a cause for concern, despite the protected status of most of its remaining habitat (Samnang et al. Reference Samnang, Sary and Browne2009). Addressing these issues is largely a legal matter, although developing and implementing less harmful forestry practices may prove beneficial. The threat from unregulated and unplanned development remains. For example, there are five ELCs in BKNP (Open Development Cambodia 2014). Within PSWS two giant pepper farm ELCs cover 10% of its total area. The concern is that as the revenue from the area increases, the ELCs will be enlarged (e.g. Sodhi et al. Reference Sodhi, Posa, Lee, Bickford, Koh and Brook2010)
Approximately 47% (Table 3) of the remaining A. cambodiana habitat is in CCNP. Unfortunately, CCNP has the lowest A. cambodiana densities which is likely to be due to high fragmentation and human disturbance. Protecting A. cambodiana in the CCNP is also hampered by a lack of human capacity and protected area management planning (Conservation International 2016).
Reassessment of A. cambodiana’s Red List assessment
We have shown that A. cambodiana is range- and habitat-restricted (Brickle et al. Reference Brickle, Duckworth, Tordoff, Poole, Timmins and McGowan2008), its habitat has been fragmented (Figure 3), and human disturbance and development activities negatively affect the species. We also believe that these threats and pressures will only increase across the species’ range with time. Under Red List criterion A2c there has been an inferred reduction in population size with its extent of occurrence having declined by 11% over 20 years. This is less than the 30% decline required over 10 years for the species to be classified as ‘Vulnerable’ (IUCN Standards and Petitions Subcommittee, 2017). Under criterion B1, A. cambodiana’s 2,308 km2 extent of occurrence meets the ‘Endangered’ criterion (< 5,000 km2) along with the condition (bi) as the extent of occurrence continues to decline. However, this study does not provide information to adequately address either of the other conditions under B1, relating to severe fragmentation (a) or extreme fluctuations (c). Under criterion C the number of mature individuals is estimated at 2,800–11,000, therefore possibly exceeding ‘Vulnerable’ requirement for < 10,000 individuals. As A. cambodiana appears to approach the thresholds for threatened status under criteria A, B and C, we feel that this informed analysis warrants a revision of its status to ‘Near Threatened’ from ‘Least Concern’. A more detailed analysis of A. cambodiana’s extinction risk should be undertaken given its restricted extent of occurrence and the continuing threats to its survival.
Acknowledgements
We would like to express our gratitude to the General Department of Administration for Nature Conservation and Protection of the Ministry of Environment and Forestry Administration of the Royal Government of Cambodia for their permissions to fulfil access the study sites. We wish to thank Fauna and Flora International and the Rufford Small Grants Foundation for financial support, and to the Centre for Biodiversity Conservation of the Royal University of Phnom Penh, Wildlife Alliance-Cambodia and Wildlife Conservation Research Unit of Oxford University for permission of using camera trap data, the Department of Biodiversity of General Secretariat for Sustainable Development for logistical support. We deeply thank Dr George A. Gale and the people in the Conservation Ecology Group for their prompt help in sorting out a range of urgent issues. Thanks also to Mr Yav Net, Birdlife International-Cambodia for providing technical support on GIS.








