Gestational anaemia (GA) can be defined as Hb levels <6·82 mmol/l (or <11·0 g/dl). Anaemia affects approximately 29 % of women worldwide and 38 % of pregnant women(1). Fe-deficiency anaemia is the most common type of anaemia, accounting for approximately 50 % of cases. Serum ferritin (SF), an Fe-binding protein, can be used to assess the body’s Fe storage and support diagnosis; normal ferritin levels in women can range from 15 to 150 µg/l, although literature indicates cut-off values at <12 µg/l when speaking of Fe deficiency(2). Previous studies have reported a relationship between anaemia and low birth weight (LBW)(Reference Figueiredo, Gomes-Filho and Batista3–Reference Col Madendag, Eraslan Sahin and Madendag6), and children born to anaemic and/or Fe-deficient mothers are at risk for anaemia; anaemia and more specifically Fe-deficiency anaemia may lead to multiple health issues including cognitive impairment, pregnancy complications and reduced immunity, amongst others(Reference Suitor7,Reference Friedrisch and Friedrisch8) .
During pregnancy, Fe need increases to supply the growing fetus and placenta. Several physiological adaptive changes during pregnancy, such as increase in Fe absorption, also help to maintain Fe homoeostasis(Reference Suitor7). In fact, a decrease in the concentration of erythrocytes does not necessarily imply a real anaemic state, much less Fe-deficiency anaemia(Reference Fisher and Nemeth9). Recent studies have raised questions about the value of Fe supplementation in women who are Fe-replete and non-anaemic and suggested that excess Fe intake may paradoxically increase the risk of reproductive disorders(Reference Brannon and Taylor10,Reference Ng, Norwitz and Norwitz11) and also rather appears to be associated with significantly more adverse pregnancy events(Reference Ziaei, Norrozi and Faghihzadeh12,Reference Shastri, Mishra and Dwarkanath13) and that a high Fe status in late pregnancy is associated with a significantly smaller birth weight(Reference Dewey and Oaks14). The WHO recommends routine Fe supplementation of 30–60 mg/d throughout pregnancy to prevent Fe deficiency(15), but these policies could lead to prescription of Fe supplementation in pregnant women who, without deficiencies and assured intake, receive an excess of this nutrient, in view of the fact that physiologically, pregnant women undergo a haemodilution process due to expansion of blood volume(Reference Friedrisch and Friedrisch8,Reference Gonzales, Olavegoya and Gonzales16) . The nutritional status of women, before and during pregnancy, is a fundamental factor for the health of herself and her product. This situation could be affected because Latina women are a vulnerable group from a nutritional point of view(Reference Taipe-Ruiz17). Colombia, a middle-income country of high social inequality, presents moderate rates of GA (26·2 %) and Fe deficiency (44·5 %), being geographic location, wealth index and ethnicity its main determining factors(Reference Forero, Galindo and Hernández18). According to WHO guidelines(19), national public health policies and clinical guidelines include mandatory Fe supplementation (60 mg/d) to all pregnant women(20). However, studies from low-income African and Asian countries have also raised the concern of Fe excess even when the risk of developing anaemia is much higher(Reference Oaks, Jorgensen and Baldiviez21,Reference Hwang, Lee and Kim22) . There is an unmet need of evidence in Latin American countries, where many of them are improving socio-economic conditions, but social inequality persists. This study aimed to evaluate the relationship between GA and perinatal outcomes in a sample of women representative of low socio-economic level population in Cartagena, Colombia.
Materials and methods
Study design/population
Participants of this prospective analytical cohort study were recruited from ‘E.S.E. Clínica Maternidad Rafael Calvo C’, a reference hospital in Cartagena, Colombia, which provides obstetric care for the entire department of Bolívar. A total of 1218 women and their infants born between 1 December 2018 and 28 February 2019 were enrolled in this study. The study was designed to create a community-based birth cohort for a prospective follow-up and collection of epidemiological data and biological samples to evaluate the influence of sociodemographic and biological factors on LBW and stunting. The current data result from a cross-sectional analysis at the baseline of mothers and their infants.
Study location
Cartagena is located at sea level in the North Coast of Colombia (10° 23′ 59″ North, 75° 30′ 52″ West). Most inhabitants are poor according to a governmental index that classifies households by assessing type of housing, overcrowding (three or more people per bedroom), water and sanitation conditions, income, level of urbanisation of each home and school attendance. This stratification is carried out mainly to assign subsidies and collect contributions differentially by strata(23). This socio-economic stratification (SES) ranges from 1 to 6, and 90 % of the population is grouped in the lowest strata, 1–3.
Eligibility criteria and enrolment procedures
Pregnant women attending this medical institution for parturition were screened for eligibility by nurses of the research staff. Inclusion criteria incorporated women in their last trimester of pregnancy who were in the first stage of labour before expulsive, residing in the Department of Bolivar, aged between 18 and 45 years. Exclusion criteria were extended to mothers diagnosed with HIV, mothers suffering from autoimmune diseases, diabetes mellitus and/or chronic kidney disease. Newborns with congenital defects and/or TORCH syndrome and children born with chromosomal abnormalities were not included in this study.
Mothers were interrogated during admission to the delivery room, and informed consent was obtained. A questionnaire was conducted on sociodemographic characteristics and medical conditions of interest during pregnancy and consumption of nutritional supplements (ferrous sulphate, folic acid and Ca). Blood samples for haemogram were collected from participants before delivery. SF determination was an additional exam included in the research protocol.
Collection of baseline data and follow-up
This study was based on multidimensional surveys, blood work, stool sample collection and anthropometric data documentation.
Surveys
A risk factor identification questionnaire was conducted during the last trimester of pregnancy based on medical history, prior to and during pregnancy. Infant anthropometric data at birth were provided by official registration documents from the National Administrative Department of Statistics (DANE).
Blood samples
Two blood samples in different collector tubes were obtained and processed. Total IV generation blood count (Mindray Bc 3000) was performed in the Clinical laboratory of the hospital. Quantitative determination of circulating ferritin concentrations in human serum was established by immunoenzymometric sequential assay (AccuBind Elisa Microwells; Monobind Inc.).
Stool samples and parasitological examination
Soil-transmitted helminthiases are frequent in tropical and vulnerable communities and are also known to be associated with anaemia. A faecal sample was obtained during delivery or up to 2 d postpartum. Parasitological analysis was carried out using 0·85 % saline solution and Lugol staining; helminth eggs were counted using the Kato Katz technique (Copro Kit; C&M Medical). The presence of eggs from geohelminths or parasite visualisation was considered diagnosis of active infection.
Definition of exposure and outcomes
Exposures
Maternal anaemia
Participants were diagnosed with anaemia when the Hb level was below 6·82 mmol/l (11 g/dl)(1).
Serum ferritin depletion
Subjects with SF levels were below <12 µg/l(2).
Iron deficiency anaemia
Participants with GA and SF depletion(25).
Birth outcomes
Preterm birth
An infant is considered preterm when born alive before 37 weeks of pregnancy are complete(28).
Small for gestational age
Neonate born with a birth weight below the 10th percentile according to INTERGROWTH-21st project definitions(Reference Villar, Ismail and Victora29).
Data analysis
Frequency rates and their 95 % CI were obtained with Epidat 3.1 (Xunta de Galicia, PAO/WHO). Most variables were not normally distributed, and they were therefore reported as the median value and its interquartile range.
Inferential analyses were done with Statistical Package for Social Sciences (SPSS ver. 25.0; IBM). Multivariate generalised linear models were applied to evaluate the relationship between Hb or SF levels and birth weight as a continuous outcome. An exploratory analysis was first performed to select covariates and factors to be included in the model. Continuous variables were identified by Spearman correlation test. Results are shown in correlograms generated in ‘corrplot’ R package(Reference Wei, Simko and Levy31). In addition, potential associated factors (binary predictors) were explored by comparing birth weight between groups by using the non-parametric Mann–Whitney U test. Predictors with a P-value <0·1 entered the multivariate model. Living in the urban or rural area was selected a priori as a confounding factor irrespective of its crude association with the outcome.
To assess the association between binary birth-related outcomes and predictors, univariate analysis was first performed by calculating crude OR with 95 % CI. The following factors were considered as independent variables: maternal age, socio-economic status, place of residency (urban/rural), health care scheme (contributive, subsidised or another(28)), number of prenatal care visits, previous pregnancies, method of delivery and infant sex. Multivariate binary logistic regression models were built for each outcome by including as covariates, predictors that showed a P-value <0·10 in the univariate analysis and potential confounders determined a priori (maternal age, SES and neonate sex). GA was also explored as an outcome using a similar approach to birth outcomes, except for including neonate sex as a predictor. Adjusted OR with 95 % CI were estimated. P-value < 0·05 was considered significant for all tests.
Results
Anaemia is associated with poverty-related sociodemographic conditions
A total of 1218 women in labour were recruited and fulfilled the eligibility criteria for inclusion in the study; however, Hb assessment could be performed in 930 mothers (Table 1). Prevalence of GA was 41·6 % (95 % CI 38·4, 44·8). SF levels were evaluated in 701 mothers, observing SF depletion in 41·1 % (95 % CI 37·4, 44·8) women. SF depletion was more common among pregnant women with anaemia than in the non-anaemic group (Fe-deficiency anaemia: 55·6 % v. 27·6 %, P < 0·0001). Median ferritin level was 14·7 µg/l (IQR: 8·5–24·9), and median Hb level was 11·2 g/dl (IQR 10·3–12·0). Cases of soil-transmitted helminthiases were scarce. Six women out of 424 with stool exam tested positive for helminths: four with Trichuris trichiura, four with Ascaris lumbricoides and one with Strongyloides stercoralis. No cases of hookworm infections were found. Due to the low prevalence of soil-transmitted helminthiases, its relationship with anaemia was not determined.
Table 1 Descriptive of mothers and newborns
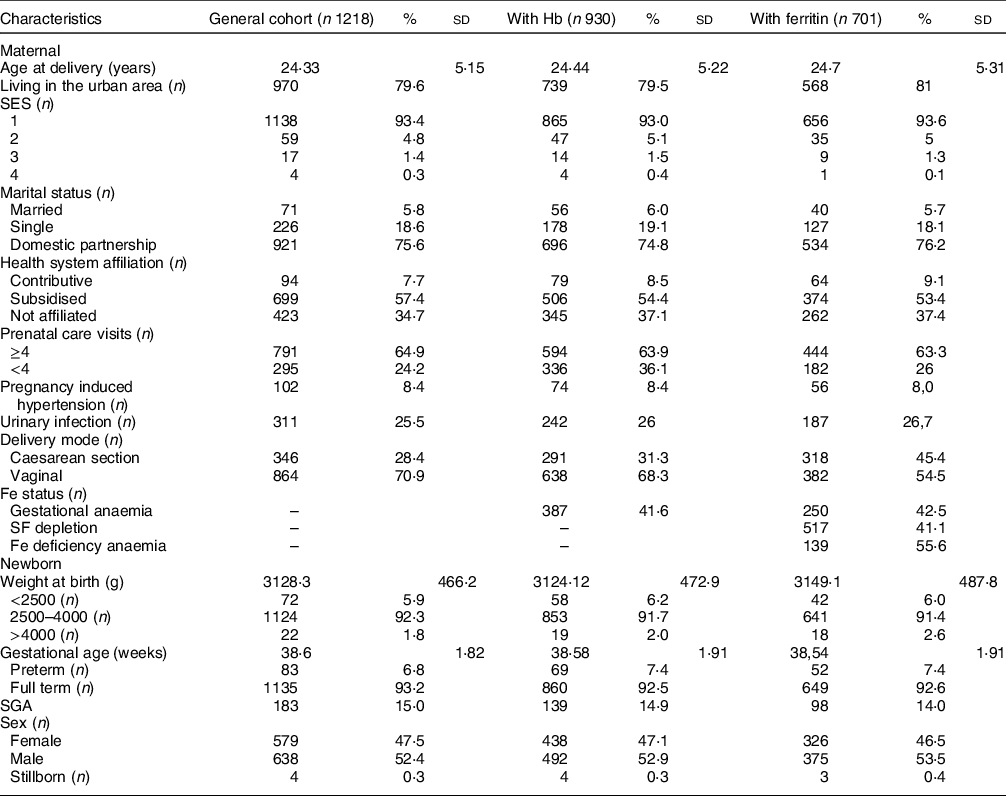
SES, socio-economic stratification; SF, serum ferritin; SGA, small for gestational age.
The effect of several sociodemographic conditions on GA was assessed by logistic regression. Crude OR indicate that sociodemographic factors such as affiliation to the contributive health care scheme (OR: 0·59, 95 % CI 0·36, 0·97, P = 0·04), belonging to the lowest social class (OR: 1·94, 95 % CI 0·11, 3·40, P = 0·02) and living in the urban area (OR: 1·59, 95 % CI 1·13, 2·22, P = 0·01) were associated with this outcome (see online Supplemental Table 1). However, in the multivariate analysis, only living in the urban area remained associated with anaemia presentation. Maternal age was included as a potential confounder without a significant association in the univariate analysis, but its relationship with GA became significant after adjustment (Table 2). History of previous pregnancies was also a significant predictor for GA (Table 2). Due to the strong association of low SES with health care scheme affiliation and prenatal care access, to avoid the effect of collinearity, in an independent multivariate model, healthcare-related variables were excluded, observing that belonging to the lowest SES was directly associated with this outcome (aOR: 1·83, 95 % CI 1·03, 3·25, P = 0·04).
Table 2 Predictors of gestational anaemia
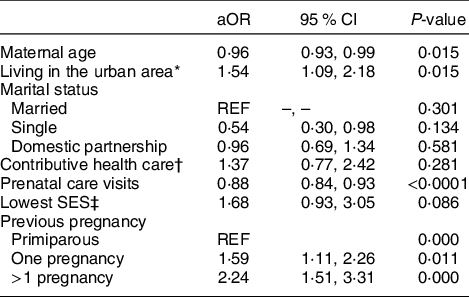
REF = reference; SES, socio-economic stratification.
* Living in the urban area (Cartagena) v. rural municipalities.
† Affiliation to the contributive health care (mother or partner affiliated to health care due to employment) v. subsidiary or no affiliation.
‡ Mothers belonging to the strata 1 v. others (range: 2–4).
Adjusted model included maternal age and all covariates if significantly associated with the outcome in the former univariate analysis.
The number of prenatal care visits correlated positively with Hb (rho: 0·25, P < 0·0001) and SF levels (rho: 0·21, P < 0·0001) (Fig. 1). Higher attendance to prenatal care was associated with lower risk of anaemia (Fig. 2) independent of age and SES, with observable significant risk reduction from 4 to 5 visits (aOR: 0·62, 95 % CI 0·40, 0·95, P = 0·028) and lowest risk when attending six or more (aOR: 0·40, 95 % CI 0·27, 0·60, P < 0·0001).

Fig. 1 Correlogram of maternal variables and birth outcomes. The scale indicates the Spearman coefficient (Rho) from −1 to 1. Positive correlations are indicated in the blue scale, and inverse correlations are indicated in the orange scale. *Significant correlations (P < 0·05)

Fig. 2 Forest plot for prenatal care attendance and the risk of gestational anaemia
Birth weight is associated with Hb and serum ferritin
The effects of Hb and SF on birth weight were explored by generalised linear models. Other variables that correlated with birth weight (Fig. 2) and potential confounders were included in the multivariate model. Birth weight was associated with Hb independent of gestational age. There was a −36·8 g decrease (P < 0·0001) in the weight of the infant per 1 g/dl (0·62 mmol/l) of maternal Hb (Fig. 3 and Table 3). SF levels and birth weight did not show a linear relationship. However, compared with mothers with SF levels in the highest quartile (Quartile 4), those in lower quartiles gave birth to higher weight babies (see online Supplemental Table 2).

Fig. 3 Relationship between birth weight and Hb. Dots and regression lines are coloured to discriminate between term (black) and preterm (grey) neonates
Table 3 Multivariate generalised linear regression model: maternal Hb and birth weight

REF, reference value; SES, socio-economical strata.
* After evaluation the goodness of fit, gestational age was included as a binary variable in the final model.
A linear model with identity link function was run. It included all predictors specified in this table.
Anaemia is inversely associated with adverse birth outcomes
The rate of LBW in the whole sample study was 5·8 % (n 71) and 2·7 % among term neonates (n 31). By multivariate logistic regression, it was observed that GA and SF depletion behaved as protective factors for LBW (Tables 4 and 5). SF depletion (OR: 0·45, 95 % CI 0·24, 0·87, P = 0·02), but not anaemia (OR: 0·90, 95 % CI 0·54, 1·47, P = 0·89), was associated with preterm birth (PB) in the univariate analysis. Association of SF depletion with this outcome remained significant after adjustment for several covariates. Small for gestational age (SGA) outcome showed a significant association with GA (aOR: 0·52, 95 % CI 0·33, 0·80) but no significant association with SF depletion (aOR: 0·66, 95 % CI 0·42, 1·05, P = 0·08). The number of prenatal care visits during pregnancy was negatively associated with PB and LBW, but not with SGA outcome. Living in the urban area was inversely associated with LBW and SGA presentation in multivariate models assessing the effects of GA or SF depletion.
Table 4 Effect of gestational anaemia on adverse birth outcomes: logistic regression

aOR, adjusted OR; LBW, low birth weight; SES, socio-economic stratification; SGA, small for gestational age.
* All models included potential confounders as covariates (maternal age, SES and neonate sex), except for SGA because INTERGROWTH21 calculates sex-adjusted centiles.
Table 5 Effect of maternal serum ferritin (SF) depletion on adverse birth outcomes: logistic regression analysis
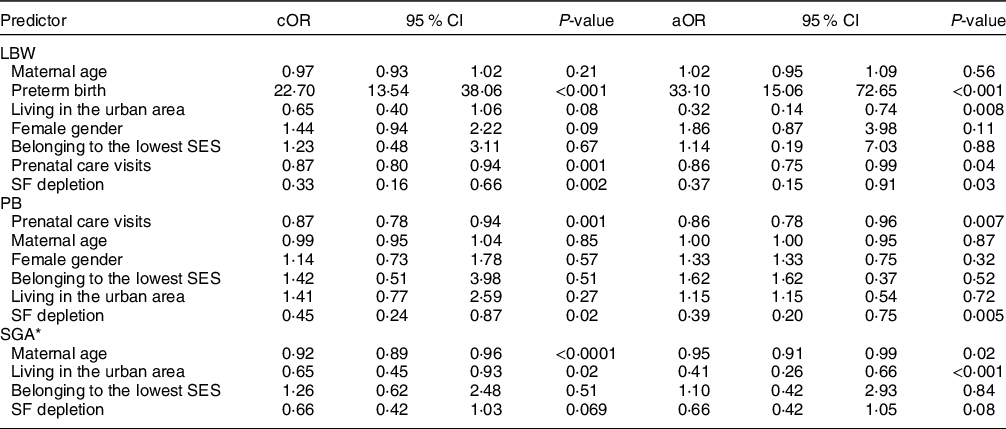
cOR, crude OR; aOR, adjusted OR; LBW, low birth weight; SES, socio-economic stratification; PB, preterm birth; SGA, small for gestational age.
* All models included potential confounders as covariates (maternal age, SES and neonate sex), except for SGA because INTERGROWTH21 calculates sex-adjusted centiles.
Discussion
In this study, we have found that GA and SF depletion are prevalent conditions, associated with poor prenatal care attendance in a study sample representative of a deprived community from a low-to-middle-income Latin American country. However, anaemia or SF depletion was inversely associated with different birth-related outcomes such as LBW and PB.
Our results agree with the national findings of frequency rate of GA (26·2 %) presented in 2015 by the Demographic and Health Survey and National Nutritional Survey (ENSIN)(Reference Forero, Galindo and Hernández18). The overall prevalence of low SF was 44·5 %(Reference Forero, Galindo and Hernández18). As expected from previous studies, the presence of GA tended to be associated with sociodemographic conditions associated with poverty(Reference Mahamoud, Mwambi and Oyet33,Reference Okia, Aine and Kiiza34) . Mothers with other children also had a higher risk of GA, probably due to a higher risk of food insecurity. Although poor socio-economic conditions may also increase the risk of LBW, it was found that GA showed an inverse relationship with LBW and other poor birth outcomes, which raises different concerns on the impact of Fe supplementation and medical decisions based on this nutritional variable.
There is strong evidence supporting that in early pregnancy, Fe deficiency or anaemia increases the risk of PB and LBW(Reference Rahman, Abe and Rahman35); however, their relationship in late pregnancy with poor birth outcomes is still controversial. As reviewed by Dewey et al. (Reference Dewey and Oaks14), in the third trimester, few studies demonstrated a link between a low Hb concentration and a higher risk of LBW(Reference Mohamed, Ahmad and MacRi36) and PB(Reference Zhang, Ananth and Rhoads37,Reference Meng Lu, Goldenberg and Cliver38) ; meanwhile, others have found greater occurrence of adverse birth outcomes in mother with high Hb levels(Reference Zhang, Ananth and Rhoads37,Reference Chang, O’Brien and Nathanson39,Reference Maghsoudlou, Cnattingius and Stephansson40) . Our results are similar to other reports supporting that anaemia or SF depletion during the third trimester is inversely associated with poor birth outcomes(Reference Xiong, Buekens and Alexander41–Reference Symington, Baumgartner and Malan43). In a prospective study of 250 pregnant women in South Africa, Symington et al. found that anaemia and SF depletion at 22 and 36 weeks were associated with higher birth weight. Women in the lowest ferritin quartile gave birth to babies weighing more than those in the highest quartile(Reference Symington, Baumgartner and Malan43). Also, in a cohort of pregnant women in Papua New Guinea (n 279), lower ferritin concentrations at enrolment were associated with higher mean birth weights and Fe deficiency women gave birth to heavier newborns when compared with Fe-replete women(Reference Fowkes, Moore and Opi42). In a recent publication based on a large retrospective study of Chinese women, SF depletion was inversely associated with LBW, PB and SGA birth. Others have also found that maternal SF was negatively associated with neonatal birth weight and length in the third trimester and at delivery(Reference Yuan, Hu and Zhang44,Reference Hsu, Wu and Hsieh45) . Furthermore, in a Taiwanese population, severe anaemia was found to be protective against SGA products(Reference Chu, Shaw and Lo46). In contrast, Mohamed et al. found that, in US, anaemia – defined by Hb measurement at delivery – was associated with LBW and PB, especially in African Americans(Reference Mohamed, Ahmad and MacRi36). Reasons for these disparities are unknown, but it is important to highlight that differences in genetic and sociodemographic conditions of population as well as in the Fe supplementary programmes may influence results and reinforce the importance of studying locally these relationships(Reference Oaks, Jorgensen and Baldiviez21). Few studies have been published in Latin American countries about Fe-related nutritional status and birth outcomes. A recent study by Figueiredo et al. reported that maternal anaemia at any time during pregnancy was associated with low/insufficient birth weight in a Brazilian population. However, since this study involved pregnant women from 8 to 32 weeks of gestational age, its results are not comparable to ours(Reference Figueiredo, Gomes-Filho and Batista3). A Chinese cohort study (n 511) of non-anaemic pregnant women receiving Fe supplements as part of routine antenatal care also found a significantly higher birth weight in the lowest compared with the highest ferritin quartile(Reference Lao47). Also, in a South Korean cohort (n 337), Hwang et al. found that excessive maternal Fe intake at mid-pregnancy was associated with reduced fetal growth(Reference Hwang, Lee and Kim22).
The biological plausibility of the inverse association between LBW and anaemia/low Fe status may take into consideration several factors. First, there is a physiological reduction of Hb levels throughout pregnancy, possibly related to plasmatic volume expansion(Reference Gonzales, Olavegoya and Gonzales16). This implies that a low Hb or SF concentration may not be equivalent to depleted Fe stores(Reference De Haas, Ghossein-Doha and Van Kuijk48). Since volume expansion may also be affected or determined by pathological situations such as undernutrition and hypertensive disorder, we cannot conclude that anaemia and SF depletion had a real protective effect on these birth outcomes(Reference Gernand, Christian and Schulze49). However, studies analysing other markers such as soluble transferrin receptor concentration, which increases with Fe deficiency and thus is less biased by volume expansion, also support the direction of our results(Reference Dewey and Oaks14,Reference Oaks, Jorgensen and Baldiviez21) . Hypothesis explaining the inverse link between Hb or Fe levels and adverse birth outcomes have been postulated by several authors(Reference Dewey and Oaks14,Reference Ng, Norwitz and Norwitz50) . Higher Hb concentrations may increase blood viscosity, ultimately compromising placental blood flow(Reference Ziaei, Norrozi and Faghihzadeh51). Excessive non-transferrin bound Fe may contribute to oxidative stress, lipid peroxidation and DNA damage in placental cells(Reference Casanueva and Viteri52). It has also been found that SF levels correlate with higher superoxide concentration in placenta, which in turn affects microvascular endothelial function and promote conditions leading to PB(Reference Mannaerts, Faes and Cos53). It is also possible that Hb levels may be related to birth weight independently of gestational age, as we and others have found by determining its relationship with SGA outcome. Stangret et al. also reported that maternal Hb may affect fetus development by influencing placental angiogenesis, finding that it was inversely correlated with placental expression of the flt-1 receptor (placental growth factor receptor) which, at the same time, was positively correlated with birth weight(Reference Stangret, Wnuk and Szewczyk54).
It is also important to highlight that elevated Fe levels during pregnancy could have an impact on maternal and neonatal outcomes, as it may lead to a potentially harmful inflammatory state, and is associated with: premature rupture of membranes(Reference Valappil, Varkey and Areeckal55), pregnancy-induced hypertension, pre-eclampsia(Reference Rayman, Barlis and Evans56) and gestational diabetes(Reference Rawal, Hinkle and Bao57,Reference Soheilykhah, Mojibian and Moghadam58) . Also, an Fe overload may impair the systemic response to inflammation and infection, which could be associated with adverse birth outcomes(Reference Martins, Maier and Gorki59–Reference Drakesmith and Prentice61). Finally, there is also the potential for excess Fe to alter the maternal gut microbiome(Reference Jaeggi, Kortman and Moretti62) as well as increase the risk of Cu and Zn deficiency(Reference Ziaei, Janghorban and Shariatdoust63), which may have implications for birth outcomes(Reference Pathak and Kapil64).
This study also found that poor prenatal care may increase the risk of GA and LBW. Similar results have been reported in studies conducted in other developing countries. A 2015 study carried out in South Nigeria suggested that good-quality prenatal care appears to be a valuable preventive intervention against anaemia(Reference Ikeanyi and Ibrahim65). In another study published in 2019, Zhou et al. assessed LBW and its relationship with prenatal care in the poor counties of Western China and reported that LBW was associated with poor attendance to prenatal care (<5 visits), not receiving any prenatal care during the first trimester, and not having access to assess certain prenatal care content (i.e. weight, blood pressure, blood tests, urine test, B-scan ultrasound and folic acid supplement)(Reference Zhou, Wang and Huang66). In Colombia, Pinzón-Rondón et al. have also found this inverse relationship between prenatal care and LBW, independent of the health care insurance system providing this service(Reference Pinzón-Rondón, Gutiérrez-Pinzon and Madriñan-Navia67). Since GA and LBW are inversely associated in our study, we propose that the relationship of prenatal care with lower presentation of adverse birth outcomes is mediated by several factors in addition to promote a healthy Fe status on pregnancy.
As mentioned previously, soil-transmitted helminthiasis is frequent in tropical and poor communities and is also known to be associated with anaemia(Reference Gyorkos and Gilbert68,Reference Gopalakrishnan, Eashwar and Muthulakshmi69) . According to the Centers for Disease Control and Prevention, as of 2013, a large part of the world’s population was infected with one or more soil-transmitted helminths (i.e. ascaris, whipworm and hookworm). A meta-analysis published in 2008 showed that even light intensity (1–1999 eggs per gram) hookworm infection is associated with a significant decrease in blood Hb(Reference Brooker, Hotez and Bundy70). The low frequency of intestinal parasitosis in our population is most likely a reflection of adequate sanitation strategies and better access to potable water. In fact, 90 % of people living in urban Cartagena have access to drinking water and 60 % to a sewage system(Reference Acevedo, Sánchez and Zakzuk71). Furthermore, the Colombian Ministry of Health recommends preventive deworming every 6 months in people living in rural areas or where sufficient sanitary and hygienic standards are not met, and every year for people living in cities(72), a series of measures which have helped keep this health hazard under control and which are mirrored in our results.
This study is the first report on the relationship between late GA and perinatal outcomes in a prospective cohort of pregnant women in Colombia. Its results have implications on the evaluation of national public health policies related to nutritional supplementation during pregnancy, motivating further studies. Stored biological samples may also led to analyse other nutritional markers that led to have a better understanding of this relationship. Of note, monitoring of child growth and development will also continue up to 2 years in this study, and this will permit to evaluate the association of Fe status during pregnancy with nutritional parameters at early infancy. Limitations of this study include that women who participated in this cohort came from the most vulnerable socio-economic background and did not recruit teenage women; thus, our results may not reflect the general population. Other underlying genetic factors, nutrient and/or mineral deficiency that may be related to LBW were not assessed. Also, C-reactive protein levels were not determined, which would have been useful to identify inflammatory states in women with elevated SF levels. However, most samples have SF values lower than 30 ng/ml which suggest that bias of including mothers with active infection/inflammation is low. Haematocrit, red cell distribution width and mean corpuscular volume were not taken into consideration in this particular context; thus, classification of anaemia and its underlying causes and possible outcomes could not be evaluated and correlated during data analysis. Nevertheless, these data are accessible thanks to sample storage and could be analysed a posteriori.
Conclusions
GA and SF depletion were inversely associated with LBW in a Colombian population. Prenatal care attendance lowered the risk of GA and LBW. Our results are representative of women from vulnerable socio-economic background and provide initial information to question the real benefit of indiscriminate Fe supplementation during pregnancy.
Acknowledgements
Acknowledgements: We thank all women who voluntarily participated in this study and the staff of the ‘ESE Clínica Maternidad Rafael Calvo C’ for their administrative support to obtain the study population and get the necessary data from the participants. We also express deep gratitude to Alejandra Castilla, Bacteriologist of Clinibac Ltda, who collaborated with the preparation of necessary laboratory exams and important information to carry out this manuscript and Leung Hon for helping to insert information into the database. Financial support: This study was funded by the Instituto de Nutrición y Salud Kellogg’s® award 2018 – Agreement INSK-ALZAK 02-10-2018 lead by ALZAK Foundation and Universidad de Cartagena grant 112-2018. Conflict of interest: There are no conflicts of interest. Authorship: Conceived of the presented idea (J.Z. and N.A.Z.), consecution of the grant (J.Z., N.A.Z., N.A.G. and F.E.), collected the data (A.P., A.T. and A.A.), sample processing and analysis (A.A.), Data analysis (J.Z., A.P. and A.T.), coordination of fieldwork (A.P.) data interpretation (A.P., A.T. and J.Z.), writing (A.P., J.Z. and A.T.), study design (J.Z., N.A.Z. and R.L.S.) or critical revision (N.A.G., N.A.Z., F.E. and R.L.S.). All authors read and approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research participants were approved by the Ethics Committee of the ‘E.S.E. Clinica Maternidad Rafael Calvo C.’ (Authorisation number: Acta 001-18). Written informed consent was obtained from all subjects.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S136898002100166X











