At the macrostructural level, brain asymmetry features, such as differences in the volume, shape and size of lobes, sulci, gyri or subcortical structures, can be investigated with in vivo imaging, such as X-ray computed tomography (CT), or structural magnetic resonance imaging (MRI) (Amunts, Reference Amunts2010). In contrast, the study of microstructural features, such as the number of spines or the degree of arborization of dendrites and axons, requires postmortem histological sectioning, as these features escape the resolution of current imaging technologies (Amunts, Reference Amunts2010). Furthermore, some aspects of brain organization at the molecular level, such as interhemispheric differences in gene and protein expression, can be analyzed in vivo with techniques such as receptor positron emission tomography (PET), or in vitro with immunohistochemistry, quantitative receptor autoradiography, in situ hybridization, etc. (Amunts, Reference Amunts2010).
The first section of this review will briefly cover brain-wide asymmetries such as the petalia and the Yakovlevian torque, followed by classical interhemispheric differences around the perisylvian region and the central sulcus, which have long been linked to the lateralization of language and handedness, respectively. Studies of cortical and subcortical regions, and their gender-dependent and handedness-dependent effects, will also be reviewed. Posterior sections of the review are dedicated to exploring the factors that influence cerebral asymmetry, including both genetic and environmental factors, and to present findings from studies in neurodevelopmental and psychiatric patients. Although the focus is on structural cerebral asymmetries in humans, findings from studies comprising functional, cognitive, and behavioral analyses are cited throughout the text.
Prominent Brain Asymmetries: Yakovlevian Torque and Petalia
The Yakovlevian anticlockwise torque is a geometric distortion of the brain hemispheres, in which the frontal cortex is wider in the right hemisphere, and the left occipital pole is wider and protrudes further posteriorly (Kertesz et al., Reference Kertesz, Polk, Black and Howell1990; LeMay & Kido, Reference Le May and Kido1978). Recent morphometry studies have consistently found handedness-related effects on the extent of the torque, mainly due to differences in the anatomy of frontal regions. (Good et al., Reference Good, Johnsrude, Ashburner, Henson, Friston and Frackowiak2001; Herve et al., Reference Herve, Crivello, Perchey, Mazoyer and Tzourio-Mazoyer2006; Lancaster et al., Reference Lancaster, Kochunov, Thompson, Toga and Fox2003; Watkins et al., Reference Watkins, Paus, Lerch, Zijdenbos, Collins, Neelin, Taylor, Worsley and Evans2001). The right frontal and left occipital petalias are protrusions of the surface of one hemisphere relative to the other, and constitute two of the most prominent examples of interhemispheric asymmetry (LeMay, Reference LeMay1976). The petalias create impressions on the inner skull, leaving a negative of the outer shape of the brain, a characteristic that has also been observed in nonhuman primates (Zilles et al., Reference Zilles, Dabringhaus, Geyer, Amunts, Qu, Schleicher, Gilissen, Schlaug and Steinmetz1996). A recent review discusses the evolutionary theories of brain lateralization (Corballis, Reference Corballis2009).
Perisylvian Region and Language
One of the first observations in the field of cerebral asymmetry was made by French anatomist Paul Broca, who noticed that disturbances in speech production resulted from lesions of the posterior part of the inferior frontal gyrus in the left hemisphere (Broca, Reference Broca1861). This finding was soon complemented by that of German physicians Karl Wernicke and Ludwig Lichtheim, who discovered that damage to the posterior superior temporal gyrus, also in the left hemisphere, produced deficits in language comprehension (Wernicke, Reference Wernicke1874).
Figure 1 shows a diagrammatic representation of the perisylvian region. Both Wernicke's language comprehension area and Broca's speech area are functionally defined regions located in the vicinity of the lateral sulcus, also known as the sylvian fissure. The fissure is commonly shorter and runs less horizontal in the right hemisphere. Asymmetries within this region are detectable early in development (LeMay & Culebras, Reference LeMay and Culebras1972).

FIGURE 1 A side view illustration of the brain, showing the location of (1) Sylvian fissure (lateral fissure), (2) central sulcus, (3) Broca's area, (4) Wernicke's area, (5) primary motor cortex and (6) sensory cortex.
The planum temporale is a triangular region which forms the heart of Wernicke's language comprehension area. The planum temporale displays pronounced leftward asymmetry in most humans. Early studies reported that this feature was also present — although to a lower degree — in chimpanzees and other great apes (Cantalupo & Hopkins, Reference Cantalupo and Hopkins2001). Later, Taglialatela et al. (Reference Taglialatela, Russell, Schaeffer and Hopkins2008) discovered that Broca's area homolog activates in chimpanzee brains when they communicate with gestures or vocalizations, which led to the theory that enlargement of Broca's area, and its role in communication, began before the evolutionary split between humans and chimpanzees. However, more recent studies have challenged this theory, by showing an apparent lack of significant asymmetry of Broca's area homolog (Schenker et al., Reference Schenker, Hopkins, Spocter, Garrison, Stimpson, Erwin, Hof and Sherwood2010). This difference could be explained by differences in methods and experimental design (e.g., targeting different regions within Broca's area, or differences in the measurement of white matter (WM) and gray matter (GM). In either case, the precise role of the left planum temporale in other great apes remains unknown (Gannon et al., Reference Gannon, Holloway, Broadfield and Braun1998; Gannon et al., Reference Gannon, Kheck, Braun and Holloway2005).
Several studies have investigated possible correlations between asymmetry within the perisylvian region and brain function. For instance, the length of the sylvian fissure correlates with handedness consistency (Witelson & Kigar, Reference Witelson and Kigar1992), and with individual performance during the consonant–vowel–consonant (CVC) task (Heilige et al., Reference Heilige, Taylor, Lesmes and Peterson1998), in which trigrams (three letter combinations which do not form a known word, e.g., V-O-M, L-A-T) are presented in vertical alignment to each hemisphere separately, and also together. The basic assumption is that the left and right hemispheres may each have their own characteristic way of processing CVC syllables. The right hemisphere processes letter by letter, vertically downward, whereas the left hemisphere has the ability to process the entire CVC syllable as a unit (Levy et al., Reference Levy, Heller, Banich and Burton1983). A weaker leftward asymmetry of the planum temporale is a common characteristic of the brains of left-handed individuals, possibly indicating the presence of handedness-dependent hemispheric dominance differences for language (Steinmetz, Reference Steinmetz1996).
In healthy right-handed individuals, atypical hemispheric dominance of language was thought to be remarkably rare. However, Knecht et al. (Reference Knecht, Deppe, Drager, Bobe, Lohmann, Ringelstein and Henningsen2000) measured language lateralization in 188 individuals, finding that the natural distribution of language lateralization occurs along a bimodal continuum, with 7.5% of subjects displaying right hemispheric dominance in language lateralization. No significant differences between males and females were observed. This study showed that atypical hemispheric dominance in language lateralization might be considerably more common than originally suspected.
Broca's region encompasses Broadmann's Areas 44 [BA44] and 45 [BA45]. A histological study of the brain of Emil Krebs, a language genius who knew more than 60 languages, found cytoarchitectonic differences within these regions. When compared against 11 control brains, BA44 was significantly more asymmetrical in the brain of Emil Krebs, whereas BA45 was significantly more symmetrical (Amunts et al., Reference Amunts, Schleicher and Zilles2004). This study linked exceptional language competence and cytoarchitectonic differences in asymmetry within Broca's region, highlighting the region-dependent nature of (a)symmetry.
Central Sulcus and Handedness
The central sulcus (see Figure 1) divides the primary motor cortex from the sensory cortex and has long been regarded as a potential anatomical correlate of handedness. In the population, the proportion of consistently right-handed, mixed and consistently left-handed subjects is approximately 64%, 33%, and 4% respectively (Amunts et al., Reference Amunts, Schlaug, Schleicher, Steinmetz, Dabringhaus, Roland and Zilles1996). Initially, it was hypothesized that the dominant hand would have a larger cortical region at its disposal, compared to the non-preferred hand, and that this functional anatomy would be reflected in the shape of the central sulcus. While an early study reported deeper left central sulcus (Amunts et al., Reference Amunts, Schlaug, Schleicher, Steinmetz, Dabringhaus, Roland and Zilles1996), a later study found that central sulcus was deeper and larger on the right hemisphere for both males and females (Davatzikos & Bryan, Reference Davatzikos and Bryan2002). Such contradictory results may have resulted from differences in sample composition, methods, and parameters applied to estimate central sulcus asymmetry. However, several recent studies have reported leftward asymmetry of the central sulcus in right-handed individuals (Cykowski et al., Reference Cykowski, Coulon, Kochunov, Amunts, Lancaster, Laird, Glahn and Fox2008; Herve et al., Reference Herve, Crivello, Perchey, Mazoyer and Tzourio-Mazoyer2006; Luders, Thompson et al., Reference Luders, Thompson, Narr, Toga, Jancke and Gaser2006), supporting the dominant-hemisphere hypothesis.
Studies in non-human primates have shown that the dorsal-ventral location of the motor-hand region is comparable between humans and chimpanzees, though the depth of the central sulcus is significantly greater in humans, suggesting greater cortical folding and gyrification within the motor-hand area. (Hopkins et al., Reference Hopkins, Coulon and Mangin2010). Interestingly, the central sulcus area corresponding to the motor-hand area was larger in the hemisphere contralateral to the chimpanzee's preferred hand (Hopkins et al., Reference Hopkins, Coulon and Mangin2010). Whether the development of these asymmetries in chimpanzees or humans is genetically determined, or due to experience and training, is not clear.
Heschl's Gyrus and Hearing
The transverse temporal gyrus, also known as Heschl's gyrus, is located in the superior temporal gyrus and comprises the primary auditory cortex. In right-handers, Heschl's gyrus is generally greater on the left side (Penhune et al., Reference Penhune, Zatorre, MacDonald and Evans1996). An interesting study by Golestani and Zatorre (Reference Golestani and Zatorre2004) analyzed structural MRI and diffusion tensor imaging (DTI) images from a group of faster and slower phonetic learners, finding significantly higher WM densities in the left Heschl's gyrus of faster compared with slower learners. Furthermore, faster learners displayed increased leftward asymmetry in parietal lobe volumes, whereas the right insula and Heschl's gyrus of slower learners were more superiorly located compared with faster learners. The results of Golestani and Zatorre provided evidence that the anatomy and asymmetry of the primary auditory cortex partly predicts the learning of foreign speech sounds.
Asymmetry in the Anterior Cingulate Cortex
The anterior cingulate cortex is thought to play a major role in executive processes. Some theories have proposed the fissurization of the cortex to be a product of gross mechanical processes related to cortical growth and local cytoarchitectural characteristics. Huster et al. (Reference Huster, Westerhausen, Kreuder, Schweiger and Wittling2007) used a voxel-based morphometry (VBM) approach to quantify fissurization of the anterior cingulate cortex in left- and right-handers of both genders by recording the presence and extension of the paracingulate sulcus. Overall, their results showed that the paracingulate sulcus occurred more often and was more pronounced in the left as compared to the right hemisphere. However, this was less evident in male left-handers and female right-handers (Huster et al., Reference Huster, Westerhausen, Kreuder, Schweiger and Wittling2007).
Rightward asymmetry of the anterior cingulate region appears to be common in females, but not so in males (Pujol et al., Reference Pujol, Lopez, Deus, Cardoner, Vallejo, Capdevila and Paus2002). A study found a significant association between anterior right cingulate asymmetry and behavioral style, namely a temperamental disposition to fear and anticipatory worry. Both males and females with a larger anterior right cingulate described themselves as experiencing greater worry about possible problems, fearfulness in the face of uncertainty, shyness with strangers, and fatigability (Pujol et al., Reference Pujol, Lopez, Deus, Cardoner, Vallejo, Capdevila and Paus2002).
Other Cortical Asymmetries
Recently, Lyttelton et al. (Reference Lyttelton, Karama, Ad-Dab'bagh, Zatorre, Carbonell, Worsley and Evans2009) developed an automated method to analyze cortical surface area asymmetries in 112 right-handed young adults. Their method helped reveal two novel prominent cortical surface asymmetries: (i) a leftward difference in the supramarginal gyrus which could be over twice as big as the areal asymmetry of the left Heschl's gyrus and planum temporale regions (Lyttelton et al., Reference Lyttelton, Karama, Ad-Dab'bagh, Zatorre, Carbonell, Worsley and Evans2009), and (ii) a rightward surface asymmetry in a band around the medial junction between the occipital, parietal, and temporal lobes (Lyttelton et al., Reference Lyttelton, Karama, Ad-Dab'bagh, Zatorre, Carbonell, Worsley and Evans2009).
Cortical thickness displays gross leftward asymmetry, which is slightly more pronounced in males, albeit a few regions display greater asymmetry in females compared to males (Luders, Narr et al., Reference Luders, Narr, Thompson, Rex, Jancke and Toga2006). A number of significant regional asymmetries in cortical structure have been reported. For instance, leftward asymmetries were identified in the precentral gyrus, middle frontal, anterior temporal, and superior parietal lobes, while rightward asymmetries were detected in the inferior posterior temporal lobe and inferior frontal lobe (Luders, Narr et al., Reference Luders, Narr, Thompson, Rex, Jancke and Toga2006).
The cytoarchitecture of the primary visual cortex [V1/Brodmann area 17 (BA17)], its neighboring area V2 (BA18), and the cytoarchitectonic correlate of the motion-sensitive complex (V5/MT+/hOc5) are sexually dimorphic (Amunts et al., Reference Amunts, Armstrong, Malikovic, Homke, Mohlberg, Schleicher and Zilles2007). These dimorphisms are different among the areas. Such differences may potentially give males more space to process additional information, a finding which is consistent with better male processing in particular visuospatial tasks, such as mental rotation (Amunts et al., Reference Amunts, Armstrong, Malikovic, Homke, Mohlberg, Schleicher and Zilles2007). Interestingly, these gender differences in hOc5 exist even when there are comparable volume fractions of cell bodies in both genders, which indicates that, overall, the visual neural circuitry is similar in males and females (Amunts et al., Reference Amunts, Armstrong, Malikovic, Homke, Mohlberg, Schleicher and Zilles2007).
Finally, a study by Whittle et al. (Reference Whittle, Yap, Yucel, Fornito, Simmons, Barrett, Sheeber and Allen2008) linked decreased leftward asymmetry of orbitofrontal cortex volume with increased reciprocity of dysphoric behavior in adolescents. The same study also found an association between prefrontal cortex volume and affective behavior in adolescent males, with amygdala volume and volumetric asymmetry of the anterior paralimbic cortex showing significant connection with duration of aggressive behavior (Whittle et al., Reference Whittle, Yap, Yucel, Fornito, Simmons, Barrett, Sheeber and Allen2008).
Subcortical Asymmetries
Subcortical structures of the limbic system, such as the hippocampus, the amygdala, and the habenula, also seem to display some degree of asymmetry. Early studies had reported inconsistent results, presumably as a result of differences in sample composition, and in the imaging segmentation protocols used. A very important factor to consider is the methodological constraints related to spatial resolution, since the hippocampus and other subcortical structures are not easily distinguished from neighboring structures in routine MRI scans of 1 mm isotropic resolution. This often results in the inclusion or exclusion of different portions of a given structure during automated segmentation.
A recent meta-analysis of MRI studies found that hippocampal volume consistently displays a rightward asymmetry pattern (Shi et al., Reference Shi, Liu, Zhou, Yu and Jiang2009), and that this is also true, although to different degrees, for patients with conditions such as mild cognitive impairment and Alzheimer's disease (Shi et al., Reference Shi, Liu, Zhou, Yu and Jiang2009). However, due to the segmentation complexities mentioned above, and given the small sample sizes of most published studies, it is not possible to establish the actual patterns of (a)symmetry in healthy individuals for most subcortical structures.
Factors That Influence Cerebral Asymmetry
Brain asymmetry is influenced by a number of genetic and environmental factors. In this section, we will review findings from twin studies of brain asymmetry (genetics), and a number of examples that implicate experiential and environmental effects in (a)symmetry plasticity.
Relation with Body Asymmetry
Firstly, an obvious question in the field has been whether the same molecular mechanisms that regulate body asymmetry are also responsible for directing brain asymmetry. Individuals with a rare congenital condition known as situs inversus totalis, in which the major visceral organs are reversed or mirrored from their normal position, exhibit reversed frontal and occipital petalia. However, the left-hemisphere dominance for language (Kennedy et al., Reference Kennedy, O'Craven, Ticho, Goldstein, Makris and Henson1999), lateralization of auditory processing (Tanaka et al., Reference Tanaka, Kanzaki, Yoshibayashi, Kamiya and Sugishita1999), and asymmetry of regions such as the planum temporale and the inferior-frontal gyrus (Ihara et al., Reference Ihara, Hirata, Fujimaki, Goto, Umekawa, Fujita, Terazono, Matani, Wei, Yoshimine, Yorifuji and Murata2010), are not always different from those of normal subjects. This indicates that, although the developmental mechanisms that underlie visceral organ asymmetry are related to those responsible for the formation of the petalia, these may be different from the processes that direct other structural and functional asymmetries (Kennedy et al., Reference Kennedy, O'Craven, Ticho, Goldstein, Makris and Henson1999; Sun & Walsh, Reference Sun and Walsh2006; Tanaka et al., Reference Tanaka, Kanzaki, Yoshibayashi, Kamiya and Sugishita1999).
Genetics and Twin Studies
Table 1 summarizes twin MRI studies looking at brain asymmetries published to date. Interestingly, according to these studies, there seems to be a significant additive genetic component contributing to variance in brain structure, with several brain regions displaying interhemispheric differences in their heritability estimates.
TABLE 1 Twin Studies of Brain Asymmetry

MZ = monozygous, DZ = dizygous, FA = Fractional anisotropy, GA = geodesic anisotropy and MD = mean diffusivity.
A study involving 54 monozygotic (MZ) and 58 dizygotic (DZ) twin pairs, and 34 of their siblings, estimated heritabilities for focal GM and WM areas of the brain using a VBM approach (Hulshoff Pol et al., Reference Hulshoff Pol, Schnack, Posthuma, Mandl, Baare, van Oel, van Haren, Collins, Evans, Amunts, Bürgel, Zilles, de Geus, Boomsma and Kahn2006). Hemispheric differences in proportional genetic contributions were found for a number of structures. For instance, GM heritabilities in the left and right amygdala were .80 and .55 respectively, whereas WM heritabilities of the optic radiation were .69 and .79 (Hulshoff Pol et al., Reference Hulshoff Pol, Schnack, Posthuma, Mandl, Baare, van Oel, van Haren, Collins, Evans, Amunts, Bürgel, Zilles, de Geus, Boomsma and Kahn2006).
One of the largest asymmetry MRI twin studies published to date used diffusion tensor images from 374 healthy young adults, including 60 MZ and 45 same-sex DZ twin pairs, to compute voxel-based maps of significant asymmetries in fiber integrity: fractional anisotropy (FA), geodesic anisotropy (GA), and mean diffusivity (MD) (Jahanshad et al., Reference Jahanshad, Lee, Barysheva, McMahon, Zubicaray, Martin, Wright, Toga and Thompson2010). Frontal and temporal regions showed significant asymmetries in FA. While frontal lobe FA was greater in the right hemisphere, temporal lobe FA was greater on the left. Genetic factors accounted for between 37% and 10% of the variance in observed asymmetry for the following regions: inferior fronto-occipital fasciculus (.33), anterior thalamic radiation (.37), forceps major (.20), and uncinate fasciculus (.20). The study also found gender-related differences in asymmetry which were greatest in regions with prominent FA asymmetry (Jahanshad et al., Reference Jahanshad, Lee, Barysheva, McMahon, Zubicaray, Martin, Wright, Toga and Thompson2010). Recently, a follow-up of this study was published, incorporating DTI data for 705 twins and their siblings, to investigate whether the genetic control over WM architecture depends on age, sex, socioeconomic status (SES), and intelligence quotient (IQ) (Chiang et al., Reference Chiang, McMahon, de Zubicaray, Martin, Hickie, Toga, Wright and Thompson2011). The study found that genetic influences were greater in adolescence versus adulthood, and greater in males than in females. Moreover, in people with above-average IQ, genetic factors explained over 80% of the observed FA variability in the thalamus, genu, posterior internal capsule, and superior corona radiata, while in those with below-average IQ, however, only around 40% of FA variability in the same regions was attributable to genetic factors.
Another large study, comprising a sample of 600 twins, siblings, and singletons, used a combination of classical quantitative genetic methodologies for variance decomposition, and novel semi-multivariate algorithms for high-resolution measurement of phenotypic covariance across cortical regions (Schmitt et al., Reference Schmitt, Lenroot, Ordaz, Wallace, Lerch, Evans, Prom, Kendler, Neale and Giedd2009). Correlational maps of genetic and environmental relationships between regions of interest and the cortical surface were produced, providing support for the role that genetics plays in global brain patterning of cortical thickness. Furthermore, the consistent finding of a pattern of high bilateral genetic correlations between structural homologues suggests that interhemispheric covariance is largely genetically mediated (Schmitt et al., Reference Schmitt, Lenroot, Ordaz, Wallace, Lerch, Evans, Prom, Kendler, Neale and Giedd2009).
While twin studies indicate that the degree of cerebral asymmetry at the macrostructural level for most brain regions displays modest heritability estimates, current studies are still limited by small sample sizes and the current resolution of imaging technologies. However, these relatively low heritability estimates come as no surprise as, according to the largest meta-analysis to date for handedness, only 24% of the variance is explained by additive genetic effects (Medland et al., Reference Medland, Duffy, Wright, Geffen and Martin2006), which highlights the complex nature of functional laterality and structural asymmetry.
Experiential and Environmental Factors Influencing Plasticity
Evidence suggests that brain asymmetry is plastic and can be modulated by environmental and experiential factors. For instance, an analysis of the planum temporale in professional musicians with and without perfect pitch, and non-musician controls (Schlaug et al., Reference Schlaug, Jancke, Huang and Steinmetz1995) found that musicians with perfect pitch displayed up to twice as much asymmetry compared with musicians without perfect pitch and controls, primarily due to a smaller volume on the right hemisphere (Keenan et al., Reference Keenan, Thangaraj, Halpern and Schlaug2001). A prior study had found that right-handed male professional keyboard players exhibited greater symmetry in the central sulcus, as compared to age-matched controls (Amunts et al., Reference Amunts, Schlaug, Jancke, Steinmetz, Schleicher, Dabringhaus and Zilles1997). In addition, the depth of the sulcus was negatively correlated with the age at which musicians began bimanual training (Amunts et al., Reference Amunts, Schlaug, Jancke, Steinmetz, Schleicher, Dabringhaus and Zilles1997). This study was recently replicated with success in a group of Chinese musicians (Li et al., Reference Li, Han, Wang, Yang, Fan, Lv, Tang, Gong, Zang and He2010). Altogether, these studies seem to suggest that the anatomy and (a)symmetry of the planum temporale and the central sulcus are susceptible to training-induced plasticity processes. However, in the absence of longitudinal data, the possibility still remains that these observations could reflect a natural predisposition of the subjects to become musicians.
Cortical thickness appears to be sensitive to substance use. Decreased cortical thickness and altered thickness asymmetry were reported in cocaine-dependent individuals compared to age-matched controls, with more prominent thinning around regions involved with executive regulation of reward and attention. The authors suggested that the observed differences could be in part attributable to drug use and in part to predisposition toward addiction (Makris et al., Reference Makris, Gasic, Kennedy, Hodge, Kaiser, Lee, Kim, Blood, Evins, Seidman, Iosifescu, Lee, Baxter, Perlis, Smoller, Fava and Breiter2008). However, in the absence of longitudinal data and sufficient statistical power, further research is needed to confirm causal relations. Moreover, caudate nucleus asymmetry has been proposed as a possible neurobiological marker of moderate prenatal alcohol exposure in young adults, suggesting that asymmetry of subcortical regions may also be affected by substance exposure (Willford et al., Reference Willford, Day, Aizenstein and Day2010).
Brain asymmetries are also influenced by lateralized sensory stimulation in prenatal and postnatal development. Experience-dependent plasticity and asymmetrical behaviors may induce different changes in the two hemispheres. Klöppel et al. (2010) looked at how inborn motor preferences and educational standards during childhood impact the structure of the adult human brain by examining structural MRI scans of ‘converted’ left-handers who had been forced as children to become dextral writers. Compared to consistent right-handers and left-handers, who displayed greater central sulcus surface area contralateral to the dominant hand, the converted group displayed a reversed pattern, with a larger surface of the central sulcus in their left, non-dominant hemisphere, demonstrating the plasticity of the primary sensory-motor cortex. Similarly, the asymmetrical use of one forelimb in the post-weaning period in rats has been shown to result in interhemispheric structural asymmetry in the motor cortex (Diaz et al., Reference Diaz, Pinto-Hamuy and Fernandez1994).
Gender Effects
Sex hormones may also contribute to modulate brain lateralization (Sommer et al., Reference Sommer, Aleman, Bouma and Kahn2004). The Geschwind–Galaburda hypothesis was proposed by Norman Geschwind and Albert Galaburda to explain gender differences in cognitive abilities by relating them to lateralization of brain function. The basic idea is that differences in maturation rates between the cerebral hemispheres are mediated by circulating testosterone levels, and that sexual maturation acts to fix the hemispheres at different relative stages of development after puberty (Geschwind & Galaburda, Reference Geschwind and Galaburda1985). According to this theory, male brains mature later than females, and the left hemisphere matures later than the right. Likewise, the hypothesis of progesterone-mediated interhemispheric decoupling (Hausmann & Gunturkun, Reference Hausmann and Gunturkun2000) adduces that functional cerebral asymmetries, which are stable in men and change during the menstrual cycle in women, result from inter-hemispheric inhibition by the dominant over the non-dominant hemisphere. This change of lateralization during the menstrual cycle in women might indicate that sex hormones contribute to modulate functional lateralization. A recent fMRI study used a word-matching task to study the role of estradiol in determining cyclic changes of interhemispheric inhibition and found that the inhibitory influence of left-hemispheric over right-hemispheric language areas is strongest during the menses, which results in enlarged lateralization (Weis et al., Reference Weis, Hausmann, Stoffers, Vohn, Kellermann and Sturm2008). On the other hand, during the follicular phase, due to rising estradiol levels, inhibition and lateralization are reduced (Weis et al., Reference Weis, Hausmann, Stoffers, Vohn, Kellermann and Sturm2008). This analysis revealed a powerful neuromodulatory role of sex hormones over functional brain organization.
Previous studies involving patients with sex chromosomes anomalies, and the related atypical sex-hormone concentrations, provide additional evidence for a role of sex hormones in regulating brain asymmetry. Dichotic listening studies with X Turner syndrome patients, found increased symmetry in performance, presumably as a result of the feminizing role of early estrogen exposure during key development stages (Gordon & Galatzer, Reference Gordon and Galatzer1980; Netley & Rovet, Reference Netley and Rovet1982). Another study involved boys with XXY Klinefelter syndrome, who displayed reduced asymmetry in verbal left-hemispheric and larger asymmetries in nonverbal right-hemispheric tasks (Netley & Rovet, Reference Netley and Rovet1984). However, it is not clear whether these effects were due to the androgen deficit during prenatal development, or to androgen supplementation later in life, or even due to direct, non-hormonal effects of sex chromosomes on brain maturation.
Overall hemispheric volume is a sexually dimorphic trait, with males usually showing higher asymmetry in the volumes of their two hemispheres and women displaying volumetric symmetry. Some volumetric data suggest that these differences are already detectable in the human fetus (Chi et al., Reference Chi, Dooling and Gilles1977; de Lacoste et al., Reference de Lacoste, Horvath and Woodward1991), although other studies failed to detect them (Gilmore et al., Reference Gilmore, Lin, Prastawa, Looney, Vetsa, Knickmeyer, Evans, Smith, Hamer, Lieberman and Gerig2007; Hering-Hanit et al., Reference Hering-Hanit, Achiron, Lipitz and Achiron2001). A recent study tested whether this trait is influenced by sexual orientation, by comparing interhemispheric volumes in heterosexual and homosexual males and females. The study found that while homosexual men showed more symmetric hemispheric volumes than heterosexual men, homosexual women showed asymmetry in hemispheric volumes as heterosexual men do (Savic & Lindstrom, Reference Savic and Lindstrom2008). Given that no differences in sex hormonal levels were shown between individuals of the same gender but different sexual orientation, the authors speculate that the observed gender-atypical asymmetries could have resulted from either one or a combination of factors, such as hormonal effects during early development, genetic predisposition, and environmental and social factors during cerebral maturation, which may also affect males and females differently.
The studies reviewed in this section provide illustrative examples of how cerebral symmetry varies as a function of behavior and gender-related factors. It is particularly interesting that, whilst structurally the brains of females are generally more symmetric than those of males, functional lateralization is generally more stable in men, and dynamically modulated by sex hormone levels across the menstrual cycle in women (Hausmann & Gunturkun, Reference Hausmann and Gunturkun2000). These findings underline the importance of accounting for these aspects, when possible, during brain asymmetry studies. Furthermore, given the relatively small sample size and diversity of age ranges in the cohorts of these studies (listed in Table 2), further investigation and replication may be needed in order to validate some of their conclusions. For instance, the greater symmetry of the central sulcus in professional keyboard players, originally reported by Amunts et al. (Reference Amunts, Schlaug, Jancke, Steinmetz, Schleicher, Dabringhaus and Zilles1997) was independently replicated 13 years later in a group of Chinese musicians by Li et al. (Reference Li, Han, Wang, Yang, Fan, Lv, Tang, Gong, Zang and He2010). Longitudinal studies and larger datasets will be crucial to shedding light on the mechanisms that mediate interhemispheric structural plasticity in the brain.
TABLE 2 Factors that Influence Cerebral Asymmetry
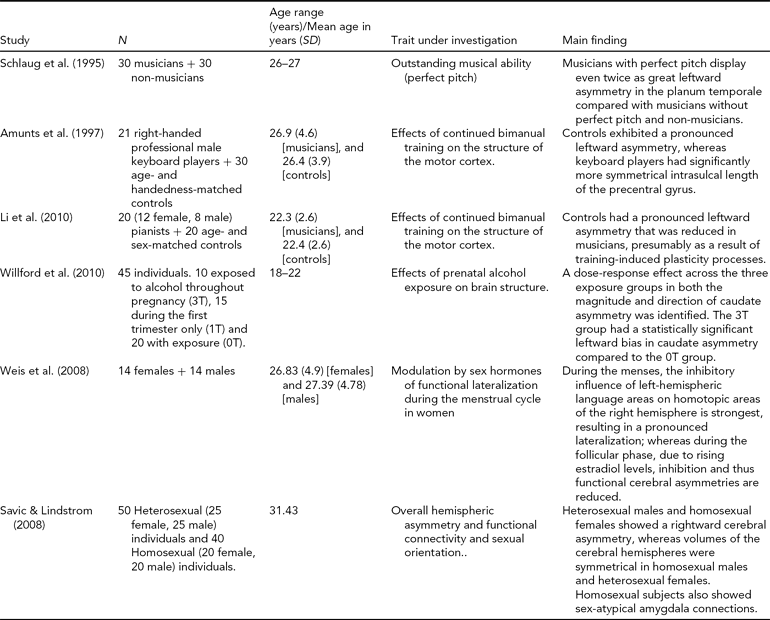
Asymmetry in Neurodevelopmental Disorders
Attention Deficit Hyperactivity Disorder (ADHD)
In 2002, Schrimsher et al. reported that the degree of rightward caudate nucleus volumetric asymmetry predicted the cumulative parent-reported severity ratings of inattentive behaviors in children with ADHD symptoms. Greater rightward asymmetry may therefore be linked to subclinical manifestations of attention deficits (Schrimsher et al., Reference Schrimsher, Billingsley, Jackson and Moore2002).
More recently, a longitudinal MRI study involving children with and without ADHD described a developmental shift in asymmetry during typical development that is lost in children with ADHD. A relatively thicker left anterior and right posterior cortex in childhood develops into the well-established adult asymmetries of a thicker right anterior and left posterior cortex. Presumably, this pattern of evolving asymmetry may contribute to the disruption of prefrontal function characteristic of the disorder (Shaw et al., Reference Shaw, Lalonde, Lepage, Rabin, Eckstrand, Sharp, Greenstein, Evans, Giedd and Rapoport2009). Additionally, the authors reported that, overall, age-related change in asymmetry is less extensive and locates to different cortical regions in typically developing non-right-handers as compared to right-handers.
Dyslexia
Dyslexic children have difficulties reading at an appropriate level for their chronological age, despite having normal intellectual ability, normal hearing and vision, and adequate environmental resources to develop reading skills (Snowling et al., Reference Snowling, Muter and Carroll2007). Postmortem analyses have reported decreased asymmetry of the planum temporale in brains of dyslexic subjects, due to a larger right-hemisphere planum than normal (Galaburda, Reference Galaburda1989; Galaburda et al., Reference Galaburda, Sherman, Rosen, Aboitiz and Geschwind1985). Imaging analyses involving dyslexic children have found structural abnormalities that differ in both directions around a normal degree of asymmetry, with some children displaying increased symmetry of the plana and others demonstrating atypical increased asymmetry. This may indicate that a lower specialization of the left hemisphere for language may be one of the causes of the condition, presumably because information flow between the speech and language comprehension areas would be impaired.
Leonard et al. examined neuroanatomical correlates of reading and language in children with learning disabilities, finding that these individuals may fall into subgroups along a continuum (Leonard et al., Reference Leonard, Eckert, Given, Virginia and Eden2006; Lerch et al., Reference Lerch, Worsley, Shaw, Greenstein, Lenroot, Giedd and Evans2006). Overall size measurements of the cerebellum and primary auditory cortex were analyzed in conjunction with asymmetry indices for planum temporale, planum parietale, cerebrum, and cerebellum. Children with a low brain volume and normal degrees of leftward asymmetry were found to have multiple deficits, including specific language impairment and reading comprehension difficulties, whereas children with higher brain volume, large Heschl's gyrus, asymmetrical planum temporale, and reversed cerebral and cerebellar asymmetries were more often characterized by dyslexia or phonological deficits. Altogether, the authors argue, these results highlight the significance of the underlying relationships between anatomy and comprehension deficits, and reveal differences that may help distinguish developmental dyslexia from specific language impairment (Leonard et al., Reference Leonard, Eckert, Given, Virginia and Eden2006; Lerch et al., Reference Lerch, Worsley, Shaw, Greenstein, Lenroot, Giedd and Evans2006).
Besides the consistent finding of abnormalities in the planum temporale, abnormal asymmetries in dyslexia include other structures such as the cerebellum and the anterior lobes (Eckert et al., Reference Eckert, Lombardino, Walczak, Bonihla, Leonard and Binder2008; Kibby et al., Reference Kibby, Fancher, Markanen and Hynd2008). A previous study with dyslexic adults had found reduced phonological decoding abilities among individuals with more symmetrical cerebella (Rae et al., Reference Rae, Harasty, Dzendrowskyj, Talcott, Simpson, Blamire, Dixon, Lee, Thompson, Styles, Richardson and Stein2002). This is consistent with the original ideas of the cerebellar deficit hypothesis (Nicolson et al., Reference Nicolson, Fawcett, Berry, Jenkins, Dean and Brooks1999), which was much criticized for failure to establish strong correlations with core phonological processing deficits in children with dyslexia. Recently, Nicolson et al. theorized that dyslexia may originate from deficiencies in a procedural learning system that depends upon multiple interconnections in a network that includes cerebellar structures as well as the basal ganglia and parietal regions (Nicolson & Fawcett, Reference Nicolson and Fawcett2007).
In summary, atypical patterns of structural and functional asymmetries involving different brain regions have been reported in children with neurodevelopmental disorders such as ADHD and dyslexia. In ADHD, the disruption of a normal developmental shift in asymmetry involving the anterior and posterior cortices seems to contribute to the characteristic prefrontal dysfunction of the condition (Shaw et al., Reference Shaw, Lalonde, Lepage, Rabin, Eckstrand, Sharp, Greenstein, Evans, Giedd and Rapoport2009). On the other hand, dyslexic children show deviations from normal asymmetry within temporal lobe regions and the cerebellum, coupled with functional deficits in a variety of left-sided temporal, occipitotemporal and frontotemporal regions, but it is not clear at which point these impairments develop (Moncrieff, Reference Moncrieff2010). Further structural and functional MRI research involving patients with neurodevelopmental disorders may help expand our knowledge of how these developmental disorders differ, so that differential diagnoses can be properly established and appropriate targeted techniques can be applied for remediation (Moncrieff, Reference Moncrieff2010).
Psychiatric Disorders
Disturbances in hemispheric lateralization have been reported for a number of psychiatric conditions. For instance, the brains of bipolar disorder patients exhibit dysfunction predominantly in the right hemisphere, which is associated with motor, perceptual, and cognitive disturbances (Caligiuri et al., Reference Caligiuri, Brown, Meloy, Eyler, Kindermann, Eberson, Frank and Lohr2004). Also, a recent study found that male patients with a first episode of major depression had a significantly smaller hippocampal volume than male control subjects. However, the strongest evidence of cerebral asymmetry in a psychiatric disorder has been found in schizophrenia. This includes behavioral (Loberg et al., Reference Loberg, Hugdahl and Green1999), electrophysiological, structural MRI (Gruzelier et al., Reference Gruzelier, Wilson and Richardson1999; Schlaepfer et al., Reference Schlaepfer, Harris, Tien, Peng, Lee, Federman, Chase, Barta and Pearlson1994), and functional MRI (Pearlson et al., Reference Pearlson, Petty, Ross and Tien1996; Ross & Pearlson, Reference Ross and Pearlson1996).
Schizophrenia
In Reference McGuire, Shah and Murray1993, McGuire et al. reported that schizophrenia patients had increased blood flow in Broca's area during the hallucinating phase relative to the recovery state, with blood flow also increased in the left anterior cingulate cortex and left temporal lobe. The authors concluded that auditory hallucinations are associated with increased activity in the network of areas normally specialized for language. Following such observations, a post-mortem structural MRI analysis of schizophrenia patients and controls consistently found a reduction in the volume of planum temporale and Heschl's gyrus, with a relative increase in the right hemisphere, hence resulting in reduced asymmetry in patients (Chance et al., Reference Chance, Casanova, Switala and Crow2008).
Recently, Swanson et al. (Reference Swanson, Eichele, Pearlson, Kiehl, Yu and Calhoun2011) investigated hemispheric differences in the intrinsic fluctuations of the default mode network (DMN) in 28 patients with schizophrenia and 28 healthy controls, both at rest and during an auditory oddball (AOD) task. The DMN comprises regions such as the ventral medial prefrontal cortex, posterior cingulated-retrosplenial cortex, inferior parietal lobule, and dorsal medial prefrontal cortex (Buckner et al., Reference Buckner, Andrews-Hanna and Schacter2008). While the DMN is primarily observed during resting state, it also manifests itself with systematic task-related or event-related deactivations that are related to behavioral variability (Eichele et al., Reference Eichele, Debener, Calhoun, Specht, Engel, Hugdahl, von Cramon and Ullsperger2008; Li et al., Reference Li, Yan, Bergquist and Sinha2007; Weissman et al., Reference Weissman, Roberts, Visscher and Woldorff2006). Swanson et al. (Reference Swanson, Eichele, Pearlson, Kiehl, Yu and Calhoun2011) reported within-group asymmetries in the inferior parietal lobule, and activation asymmetries in the posterior cingulated gyrus in schizophrenia patients. When comparing asymmetries between groups, several areas showed significant cross-group differences, such as the inferior parietal lobule and posterior cingulate. Likewise, their results suggest a reduction in activity in language-related areas for schizophrenic patients compared to healthy controls during rest (Swanson et al., Reference Swanson, Eichele, Pearlson, Kiehl, Yu and Calhoun2011). These changes may underlie structural connectivity differences in between the two hemispheres among patients and controls. Further investigation with technologies such as DTI may shed light on these differences.
Perspectives
Although acknowledged as a prominent feature of the organization of the brains of humans and other species (Vallortigara & Rogers, Reference Vallortigara and Rogers2005), the biological mechanisms that underlie the establishment of normal brain lateralization and brain asymmetry remain largely to be discovered. Greater investigation in this field will be crucial not only to advance our knowledge of normal brain functioning, but it could also contribute to our understanding of neurodevelopmental and psychiatric diseases.
The availability of larger sample sizes and automated segmentation methods will allow imaging researchers to test hypotheses derived from previous studies. Furthermore, accessible genotyping is facilitating the integration of imaging and genetics research. Hence, genome-wide association studies will be valuable to start revealing the molecular basis of brain (a)symmetry. Furthermore, as demonstrated by Shaw et al. (Reference Shaw, Lalonde, Lepage, Rabin, Eckstrand, Sharp, Greenstein, Evans, Giedd and Rapoport2009) in their longitudinal study in developing children, changing cerebral asymmetry could play an important role as part of the process of brain maturation. Hence, the proportional genetic and environmental contributions to brain asymmetry may vary during the lifetime of an individual, and this will also be a topic worth investigating.
Finally, it is important to acknowledge that current imaging technologies only allow for the study of gross macrostructural features; a combination of other approaches may be needed in order to unravel how some of these interhemispheric differences contribute to and interact with cognition, behavior, and disease.
Acknowledgments
M.E.R. is supported by an Endeavour International Postgraduate Research Scholarship (IPRS) and by a University of Queensland Research Scholarship (UQRS). Special thanks to Ms. Coppelia Cerda for graphic design support.





