Introduction
The IUCN Red List is the most widely used and authoritative conservation assessment of the world's flora (Mace et al., Reference Mace, Collar, Gaston, Hilton-Taylor, Akcakaya and Leader-Williams2008). However, the disparity between the available information and the magnitude of the task means that assessments will always be subject to improvement and few species are assessed based on comprehensive surveys of their native populations (Brummitt et al., Reference Brummitt, Bachman, Griffiths-Lee, Lutz, Moat and Farjon2015). Habitat loss (Krauss et al., Reference Krauss, Bommarco, Guardiola, Heikkinen, Helm and Kuussaari2010) and invasion of exotic species (Pyšek et al., Reference Pyšek, Jarosik, Hulme, Pergl, Hejda and Schaffner2012) are threats to plant species. Although the decline of some species has been inferred from historical and current collecting records (Robbirt et al., Reference Robbirt, Roberts and Hawkins2006; Brummitt et al., Reference Brummitt, Bachman, Griffiths-Lee, Lutz, Moat and Farjon2015), and decline from disease has been estimated (Schwartz et al., Reference Schwartz, Hermann and van Mantgem2000), to our knowledge there have not been any published attempts to quantify the response of individual plant species to past declines, or to estimate future threats to habitat quality. This is a major omission, given the importance of this process for assessing the conservation status of the world's flora under the Red List criteria (Mace et al., Reference Mace, Collar, Gaston, Hilton-Taylor, Akcakaya and Leader-Williams2008).
Conservation biologists recognize that rarity alone does not determine extinction risk and the Red List criteria have low thresholds for population size and extent of occurrence (EOO) for species that have not declined (IUCN, 2012). The species of greatest concern are those that were once common but are subject to ongoing threatening processes (McIntyre & Lavorel, Reference McIntyre and Lavorel1994; Burgman, Reference Burgman2002). However, accurately assessing the magnitude of historical and continuing declines is difficult and requires a clear understanding of species’ responses to potentially threatening processes. Systematic surveys and assessments are necessary to quantitatively evaluate species’ conservation status using the IUCN Red List criteria.
The conservation status of most plant species is not usually derived by a systematic assessment of population density across the area of occupancy (AOO; Keith, Reference Keith2000) and conservative extrapolations that take into account available habitat (see Silcock et al., Reference Silcock, Healy and Fensham2014). This study provides a methodological standard for assessing the conservation status of plant species where habitat loss and decline of habitat quality can be evaluated. Our aim is to generate adequate survey data to assess the conservation status of four Solanum species associated with the forest type of eastern Australia dominated by brigalow Acacia harpophylla. This forest has been reduced to c. 10% of its former area by clearing, and the remnants are being further degraded by the invasion of exotic grasses (Fensham et al., Reference Fensham, Biggs, Butler, MacDermott and Keith2017). We employ herbarium specimen records to identify habitat associations and to direct field surveys, and use mapping of habitat loss as a surrogate for population decline. We demonstrate how the ongoing advance of invasive grasses affects Solanum species, and integrate this information to estimate the historical and current populations. We quantify threatening processes to evaluate the conservation status of the species using the IUCN Red List criteria, and provide management recommendations for the habitat of the Solanum species.
Study area and study species
The Brigalow Belt consists of two biogeographical regions spanning a subcoastal area in eastern Australia typified by brigalow-dominated forest on clay-rich substrates, but occurring with a range of other vegetation and soil types (Fig. 1; Fensham et al., Reference Fensham, Biggs, Butler, MacDermott and Keith2017). The climate is semi-humid, with rain falling predominantly in the summer and a mean annual rainfall of 521–716 mm throughout the ranges of the Solanum species (modelled data from Jeffrey et al., Reference Jeffrey, Carter, Moodie and Beswick2001). The brigalow forest generally has a dense tree canopy and a sparse ground layer of herbaceous vegetation; where the canopy is intact it is only rarely subject to fire (Fensham et al., Reference Fensham, Biggs, Butler, MacDermott and Keith2017). The overstorey is dominated by Acacia harpophylla, but other tree species also occur and the understorey consists of shrubs, grasses and herbs (Fensham et al., Reference Fensham, Biggs, Butler, MacDermott and Keith2017).

Fig. 1 The EOO (convex polygons) of the four Solanum species in relation to the north-east coast of Australia (top right); and the location of sites used to define the distribution of Solanum adenophorum (Sa), Solanum dissectum (Sd), Solanum elachophyllum (Se) and Solanum johnsonianum (Sj). Triangles represent historical herbarium records (grey, population not located during current surveys; black, population located during current surveys); circles represent survey searches for this study (grey, population not located; black, population located). Some absence records from the current study have been excluded for clarity.
The once extensive brigalow forest was mostly converted to pastures and crops in the latter half of the 20th century (Seabrook et al., Reference Seabrook, McAlpine and Fensham2006; Fensham et al., Reference Fensham, Biggs, Butler, MacDermott and Keith2017). The remaining forest is fragmented and surrounded by productive pastures for cattle farming, which are mostly dominated by the African buffel grass Cenchrus ciliaris L. Exotic grasses also invade remnant brigalow forest, outcompeting native species (Fairfax & Fensham, Reference Fairfax and Fensham2000) and rendering the normally fire retardant brigalow ecosystems prone to fire. The resulting open canopy further facilitates buffel grass invasion, leading to a destructive grass–fire cycle (Butler & Fairfax, Reference Butler and Fairfax2003). Roadside corridors provide important habitat for remnants of the brigalow ecosystem but are often heavily infested with exotic grasses (Butler, Reference Butler2008; Butler et al., Reference Butler, McAlpine, Fensham and House2014). The brigalow ecological community (Acacia harpophylla dominant and co-dominant) has a threatened status under the Australian Government Environment Protection and Biodiversity Conservation Act 1999.
Plant species associated with brigalow forest are potentially threatened and the magnitude of this potential threat has been ranked (Fensham et al., Reference Fensham, Laffineur and Silcock2018). The genus Solanum exhibits substantial radiation in the brigalow ecosystem (Bean, Reference Bean2004), and four species, namely Solanum adenophorum F. Muell., Solanum dissectum Symon, Solanum elachophyllum F. Muell. and Solanum johnsonianum A.R. Bean (Plate 1), were second, third, sixth and ninth on the ranked list of threatened species (Fensham et al. Reference Fensham, Laffineur and Silcock2018).
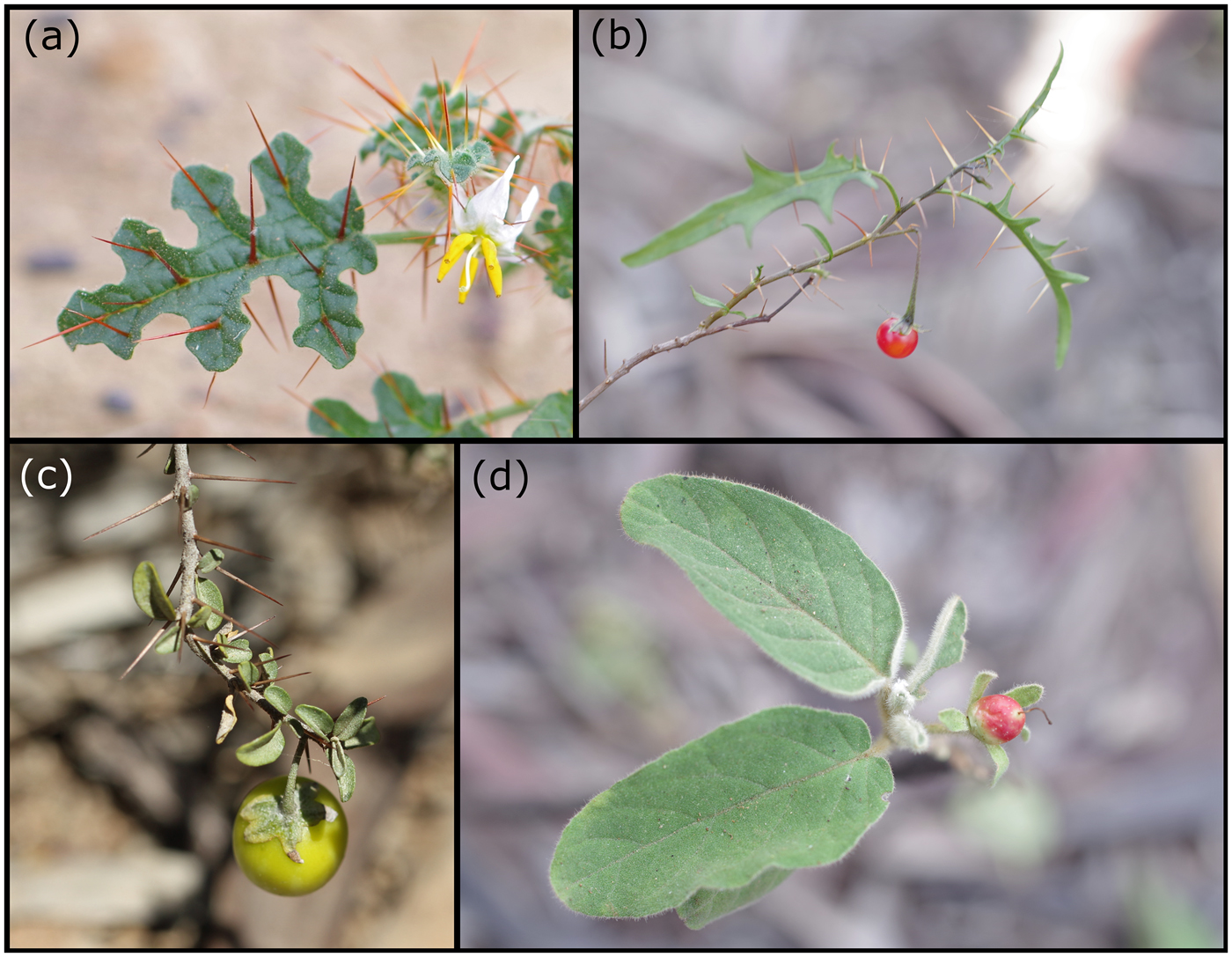
Plate 1 The four species of Solanum forming the target of this study: (a) S. adenophorum, (b) S. dissectum, (c) S. elachophyllum, (d) S. johnsonianum.
Solanum is the largest genus in the family Solanaceae, with > 1250 species (Weese & Bohs, Reference Weese and Bohs2007). On the IUCN Red List Solanum is mostly represented by species from South and Central America, and Australian species are yet to be assessed (Table 1). The spiny Solanum clade (subgenus Leptostemonum) is the largest subgenus, with c. 450 species (Bohs, Reference Bohs, Hollowell, Keating, Lewis and Croat2005) and includes all four target species. A significant share of this diversity occurs within the north-eastern Australian state of Queensland, with 88 indigenous (50 endemic) and two naturalized species (Bean, Reference Bean2004). Several other species in the subgenus Leptostemonum also occur in brigalow forest within the geographic range of the target species, but they are not considered rare because they are more widely distributed or have broader habitat preferences (Fensham et al., Reference Fensham, Laffineur and Silcock2018). All four target species are categorized as Endangered under the Queensland Government Nature Conservation Act 1992 (Table 1). Solanum adenophorum, S. dissectum, and S. johnsonianum are the only Solanum species categorized as Endangered under Australian Government legislation and S. elachophyllum is not categorized as threatened even though it has an Endangered status under Queensland state legislation (Table 1). As for the majority of plant species assessed using the Red List criteria (IUCN, 2012) for international, national or provincial listings, the assessment of these species has relied on expert opinion rather than on quantitative information. Discrepancies in the status of Solanum species between national and state jurisdictions (Table 1) provide an imperative to update categorizations with current information, such as the systematic surveys presented here, and to align categorizations, including an update of the IUCN Red List for Solanum from Australia.
Table 1 Red List status of Solanum species (Sa, Solanum adenophorum; Sd, Solanum dissectum; Se, Solanum elachophyllum; Sj, Solanum johnsonianum) in various countries and regions (with authorities in parentheses).

1 EPBC, Environment Protection and Biodiversity Conservation Act 1999; NCA, Nature Conservation Act 1992.
Methods
Regional ecosystem mapping
This study establishes associations between Solanum species and native habitats delineated on regional ecosystem maps in Queensland, Australia (Queensland Herbarium, 2016). We use these maps to document habitat decline because they provide a complete coverage of regional ecosystems prior to extensive forest clearance (Queensland Herbarium, 2016). Regional ecosystems are defined by combinations of biogeographical region, geological substrate and dominant vegetation type that are then grouped into broad vegetation groups. Broad vegetation groups are a higher-level grouping that combine regional ecosystems, and the brigalow forests are unit 25: Acacia harpophylla (brigalow) sometimes with Casuarina cristata Miq. (belah) open forests to woodlands on heavy clay soils (Neldner et al., Reference Neldner, Niehus, Wilson, McDonald and Ford2015). Another broad vegetation group dominated by species related to brigalow is unit 26: Acacia cambagei R.T. Baker (gidgee)/Acacia georginae F.M. Bailey (georgina gidgee)/Acacia argyrodendron Domin (blackwood) dominated associations, and occurs in similar edaphic environments to brigalow. This broad vegetation group has only small areas within the EOO of the target species and is combined here with unit 25, and the two broad vegetation groups are referred to as ‘brigalow’ hereafter. Another mapping layer represents regional ecosystems as ‘remnant’ (uncleared) in 2017 (Queensland Herbarium, 2016) and the proportion of ‘remnant’ relative to ‘pre-clearing’ is used here as a measure of habitat loss. We allocated our search effort to different areas based on the regional ecosystem mapping. Previous models suggest that on average buffel grass cover reaches 30% when forest canopy is reduced to 50% of the original canopy cover (Butler et al., Reference Butler, McAlpine, Fensham and House2014). We assumed that areas with < 50% canopy cover will be subject to increased fire frequency and further invasion by exotic grasses (Butler & Fairfax, Reference Butler and Fairfax2003), and will not provide habitat for Solanum species in the long term. We conducted additional mapping to distinguish intact (≥ 50% tree cover) and degraded remnant habitat (< 50% tree cover) by visually assessing high-resolution imagery (Department of Environment and Science, 2017). We used this to assess the likely magnitude of ongoing decline according to the IUCN Red List criteria.
Habitat association, distribution and density
We used historical records from the Queensland Herbarium database to quantify previous search effort within the EOO of the four species from collecting locations in 2012. Records with the same geographical coordinates and survey date were aggregated as a single record. We assigned all records to a broad habitat category (see Table 1) using search terms from the habitat notes (Supplementary Table 1), and, where the habitat notes were inadequate, by intersecting the collecting localities with the regional ecosystem mapping (Queensland Herbarium, 2016). This data collation also contributes to the evaluation of the habitat specificity of the four Solanum species.
Field surveys
We conducted field surveys in February–October 2012, November 2013–May 2014 and May 2017. These surveys aimed to confirm historical records, search for new populations within and up to 80 km from the geographic range as it was known from collecting locations in 2012, and assess the habitat association of the species following the procedures of Keith (Reference Keith2000). Rainfall during the 6 months prior to the survey was above average for 2012 and 2017, and slightly below average in 2013 and 2014. Historical records suggested that the target species have a strong association with brigalow (Table 2), directing most of the survey effort to remnants of this ecosystem. However, non-remnant brigalow (cleared pasture dominated by buffel grass) and other vegetation types were also included (Table 3). We surveyed 741 locations to determine the EOO, habitat requirements and population density of the Solanum species. Of these locations 645 were within the EOO of the four species. We conducted surveys in all designated conservation reserves within the species’ EOO. To estimate population densities at each location, we measured search distance using GPS tracking along linear transects, counted the number of plants within a width of either 4 or 6 m around the transect line, and calculated the search area by multiplying the search distance by the transect width. Surveyed areas for individual species within remnant brigalow were 47.5–195.4 ha (Table 3). We also observed the life history and regeneration of the species during the surveys and found that vegetative shoots can be connected by underground suckers (forming a single genet); we treated each emergent shoot (ramet) as an individual plant.
Table 2 Number of specimen records of the four target Solanum species within their respective EOO (records of other vascular plants in parentheses), by broad vegetation group (Neldner et al., Reference Neldner, Niehus, Wilson, McDonald and Ford2015). Numbers include all records from the Queensland Herbarium with a unique location and date.

Table 3 Summary of survey, habitat and population characteristics within the EOO of the four target species including assessment of decline. The combined search area is provided in brackets after the number of searches. Habitat references are pre-clearing (prior to clearing), remnant (uncleared in 2017), non-remnant (cleared in 2017) and intact (remnant brigalow with ≥ 50% canopy cover).

Population assessment
We estimated population sizes using the regional ecosystem mapping and population densities from field surveys. The EOO of each species was calculated from a minimum convex polygon containing all historical and current records. Within the EOO of each species, where plants were present in remnant brigalow, we used a weighted average to calculate densities (in plants per ha):
where x is the number of plants recorded at each search plot where x > 0, n is the sample size and w′ is the proportion of the total search areas represented by each search plot where x > 0. A measure of the standard error of the weighted mean (SEM) is given by:
 $${\rm SE}{\rm M}_w = \sqrt {\displaystyle{n \over {(n-1){\bar{w}}^2}}\mathop \sum \limits_{}^{} w_i^2 {(x_i-\bar{x})}^2} $$
$${\rm SE}{\rm M}_w = \sqrt {\displaystyle{n \over {(n-1){\bar{w}}^2}}\mathop \sum \limits_{}^{} w_i^2 {(x_i-\bar{x})}^2} $$To provide an estimate of the total population of each species, we multiplied the total pre-clearing, remnant and intact areas by the proportion of the presences at surveyed sites (P in Table 3) and the respective densities of species. The historical population estimates assume that historical plant densities were the same as current densities. We used the proportion of remnant habitat relative to pre-clearing habitat to assess past decline, and the proportion of intact habitat relative to remnant habitat to assess the magnitude of ongoing decline. We visually assessed the average canopy cover using DMC-3 imagery (2017) and determined the edge-to-area ratio of remnant brigalow habitat for the current localities of the target species. Remnant habitat with an edge-to-area ratio > 0.15 km/ha or a canopy cover < 50% was considered inviable in the long term, and we used the proportion of populations occurring in these areas to assess the magnitude of ongoing decline under the IUCN Red List criteria.
Association with tree canopy and exotic grass cover
We collected data to establish relationships between tree cover and the occurrence of the four Solanum species and perennial grasses. Within populations (S. adenophorum, n = 5; S. dissectum, n = 5; S. elachophyllum, n = 23; S. johnsonianum, n = 16), a 50 m tape was laid out within representative habitat. At every metre along the tape we recorded the presence or absence of a tree canopy and/or a perennial grass tussock and a Solanum colony (i.e. a group of plants whose stems were less than 1 m apart).
Assessment of conservation status
We assessed conservation status using the IUCN Red List criteria (IUCN, 2012), following the guidelines in IUCN Standards & Petitions Subcommittee (2017). Estimated plant density is here based on a replicated sample of subpopulations. Population size and decline are inferred in that they are indirectly derived from estimated plant density and mapped areas of available habitat. We calculated AOO using a 4 km2 grid (IUCN Standards & Petitions Subcommittee, 2017), but used the regional ecosystem mapping as high resolution habitat maps to determine decline in population size. This variation in procedure is justified because it represents a modelling of subpopulations occurring in past and present suitable habitat that has not been surveyed.
Reservation status and land tenure
We collated the occurrence of populations of the four Solanum species in conservation reserves and investigated the tenure of other large brigalow remnants within the EOO of the four species.
Results
Life history and habit observations
During our surveys we did not observe seedlings (cotyledons or small-statured plants without fertile parts) of any of the Solanum species. The stems of S. adenophorum are not woody and populations that are abundant in the wet season disappear or dwindle dramatically during the dry season. Excavation of S. adenophorum plants revealed no robust underground structures, suggesting the species regenerates only from seed and is short-lived but is not an annual (RF, pers. obs.). Excavation of the other three species revealed lateral stems regenerating clonal shoots from rhizomes. These species are perennial subshrubs with woody stems, although the number of aerial shoots growing from rhizomes varies depending on seasonal conditions, and all shoots can flower and fruit rapidly in response to rain.
Habitat association, distribution and density
Herbarium records indicate considerable botanical collections within the EOO of the four target species and confirm the strong association of all species with brigalow forest (Table 2). The targeted surveys also confirm the association of the four species with brigalow forest. However, 8% of S. elachophyllum historical records are from eucalypt-dominated vegetation. We also observed it in eucalypt forest during the surveys, but always near brigalow forest (Table 3).
Solanum adenophorum had the largest EOO and S. johnsonianum the smallest (Table 3, Fig. 1). Solanum adenophorum occurred in 26% (18/70), S. dissectum 32% (18/57), S. elachophyllum 56% (52/93) and S. johnsonianum 78% (56/78) of sites in remnant brigalow. For the sites where the species were present, population densities in remnant brigalow have high standard error estimates, and in the case of S. adenophorum this is higher than the weighted average. The weighted average is relatively low for S. adenophorum, moderate for S. dissectum and S. elachophyllum and high for S. johnsonianum (Table 3). Estimates for pre-clearing population sizes are in the millions and tens of millions (Table 3). Within remnant brigalow habitat all species have undergone > 93% reductions in population size, and decline is even greater if remnants with < 50% canopy are considered inviable habitat. Assuming future decline in inviable habitat, the decrease in population size will be > 98% for S. dissectum and S. johnsonianum, and > 95% for S. adenophorum and S. elachophyllum. Solanum elachophyllum occurs in eight non-brigalow habitat sites of a total of 65 sites and five sites of cleared brigalow (Table 3), thus the assumption that persistence requires remnant brigalow forest is least valid for this species.
Of the confirmed locations, five sites (28%) for S. adenophorum, 10 sites (53%) for S. dissectum, 28 sites (48%) for S. elachophyllum and 30 sites (58%) for S. johnsonianum are in remnants with a high edge-to-area ratio (> 0.15 km/ha) and low tree canopy cover (< 50%) (Fig. 2). The proportion of degraded habitat (< 50% canopy cover) of S. dissectum and S. johnsonianum, both of which occur in the southern portion of the study area, is generally lower than for the other two species (Fig. 1).

Fig. 2 Histogram of habitat condition for the current populations of (a) S. adenophorum, Sa; (b) S. dissectum, Sd; (c) S. elachophyllum, Se; and (d) S. johnsonianum, Sj. The x-axis categories are the edge-to-area ratio of habitat remnants and the bars are shaded according to tree canopy cover (light grey, 0–20%; medium grey, 21–50%; dark grey, ≥ 50%). Viable remnants are those with edge-to-area ratio < 0.15 km/ha and ≥ 50% canopy cover.
Solanum elachophyllum occurred in 17% (8/48) and S. adenophorum in 9% (3/32) of the searches in cleared brigalow, whereas S. johnsonianum and S. dissectum did not occur in these searches (0/21, 0/19 respectively; Table 3). Solanum elachophyllum occurred in 49% and Solanum adenophorum in only 2% of the surveyed cleared brigalow area (Table 3).
Association with tree canopy and exotic grass cover
Buffel grass was the main invasive species in the target species’ habitat but green panic Megathyrsus maximus (Jacq.) B.K. Simon & S.W.L. Jacobs was also common. At the line-intercept level mean exotic grass cover was 14.8% for S. adenophorum, 9.2% for S. dissectum, 8.9% for S. elachophyllum and 16.4% for S. johnsonianum. Out of 49 line-intercepts only three (one for S. elachophyllum, two for S. johnsonianum) had > 40%, and none had > 50% exotic grass cover. Mean tree canopy cover was 44.6% for S. elachophyllum, 52.4% for S. adenophorum, 53.6% for S. dissectum and 59.6% for S. johnsonianum.
Assessment of conservation status
The categorization of S. adenophorum as Vulnerable under criteria B2,b,c is predicated on a decline in association with an AOO of < 500 km2 (Table 4). Based on current records the AOO calculated using the prescribed methodology is 12 × 4 km2 = 48 km2, but 94 km2 based on the area of intact brigalow forest and the proportion of this occupied by the species (Table 3). This species is subject to continuing decline (B2b), and extreme fluctuations (B2c; Table 4). Solanum adenophorum is regarded here as a short-lived ephemeral (< 3 years), and therefore assessment of decline defers to an estimated 10-year period, and it does not qualify as Vulnerable (population decline by > 50% over 10 years) under category A2.
Table 4 Species assessment using the IUCN Red List criteria (IUCN, 2012) with justification using evidence from the current study. Only the criteria for the most threatened status are included. Unless otherwise indicated statistics are presented from Table 3.

Solanum dissectum and S. johnsonianum qualify for categorization as Critically Endangered under criterion A2c (Table 4). It is assumed here that the generation time of the clonal colonies of S. dissectum, S. elachophyllum and S. johnsonianum is at least 20 years (see below), and thus the assessment period is at least 60 years. This period coincides with the decimation of the brigalow ecosystem during 1950–2010, when this habitat declined by > 80% (Table 3).
Solanum elachophyllum persists in cleared and degraded brigalow habitat and also in non-brigalow habitat with low exotic grass cover (Table 4). Thus, it is not accurate to assume that the decline of brigalow habitat is a direct reflection of population reduction. Furthermore, the species has the largest EOO of all target species and an estimated population of > 23 million plants (Table 3). Solanum elachophyllum is categorized here as Near Threatened (Table 4).
Reservation status and land tenure
All species have < 1% of their habitat in conservation reserves, assuming S. adenophorum is no longer present in Dipperu National Park (Table 5). It is also known from Taunton National Park and these two reserves include the largest areas of brigalow forest in the region (Table 5). Solanum elachophyllum is known from five reserves and three of these have > 1,000 ha of habitat. Solanum dissectum and S. johnsonianum are both only reserved in Roundstone Conservation Park and Overdeen State Forest (Table 5).
Table 5 Conservation reserves containing known populations of the four Solanum species and the area of remnant brigalow habitat in each reserve. Solanum adenophorum was collected in Dipperu National Park in 1971 but has not been located there since, despite considerable survey effort.

1 The proportion of reserved habitat assuming S. adenophorum is still present at Dipperu is indicated in parentheses.
The largest known populations of S. dissectum and S. johnsonianum occur in a 264 ha remnant of brigalow forest on freehold land (24°2'S, 150°20'E). Mining leases contain considerable areas of remnant brigalow forest within the geographical range of the four Solanum species, particularly within the range of S. adenophorum and S. elachophyllum (Supplementary Table 2).
Discussion
Our study supports the presumption that S. adenophorum, S. dissectum, S. elachophyllum and S. johnsonianum have a strong association with brigalow forest. However, S. elachophyllum also occurs in Eucalyptus forest, where brigalow and eucalypts co-occur in narrow banded zones on mixed sediments. Solanum adenophorum has been recorded in gidgee Acacia cambagei forest, an ecological analogue of brigalow forest in drier environments.
Extent of occurrence and population estimates varied substantially between species (Table 3). Solanum elachophyllum and S. adenophorum both had broad distributions and historical populations estimated as c. 6 and 50 million individuals, respectively (Table 3). Solanum dissectum and S. johnsonianum have relatively small EOOs but the latter is estimated to have had a much higher historical population than the former because it occurs more often within its EOO and at twice the density (Table 3). The brigalow forest has been extensively cleared during the last 60 years, having diminished in area by 93.5–98.0% for the four species (Table 3).
The current population estimates are generated by multiplying plant densities from extensive surveys by available habitat area. This is not the conventional method for estimating population size, which is usually done by direct survey (e.g. Shapcott et al., Reference Shapcott, Lamont, Conroy, James and Shimizu-Kimura2017). Our search effort was 0.19, 1.06, 0.44 and 2.22% of the available remnant brigalow within their EOOs for S. adenophorum, S. dissectum, S. elachophyllum and S. johnsonianum, respectively. The population sizes in remnant brigalow forest have declined to an estimated 100,000–2,000,000 (Table 3). However, the high standard errors (Table 3) indicate large variations in density. Although we surveyed only a limited proportion of available habitat and generated approximate population estimates, the method presented here is more realistic for Red List assessments than assuming population size based on direct observations and counts in situations where there are large areas of un-surveyed habitat. We recommend that this method be adopted more widely in conservation status assessments.
Perennial forb species richness declines sharply when brigalow forest is cleared (Fairfax & Fensham, Reference Fairfax and Fensham2000). The four target Solanum species were not recorded in areas with > 50% exotic grass cover, and this level of cover is typically exceeded in cleared brigalow pastures. Both S. elachophyllum and S. adenophorum occur in cleared areas (Table 3), although the latter occurs only in areas where exotic grasses are sparse (RF & CH, pers. obs.). The populations that persist in cleared areas are unlikely to do so in the long term without intensive management to restore canopy cover for suppression of exotic grasses. Much of the remnant brigalow has been disturbed by clearing, drought or fire, and tree cover has been reduced, allowing exotic grasses to invade (Butler et al., Reference Butler, McAlpine, Fensham and House2014). The loss of these remnants as viable habitat will result in further decline. Solanum adenophorum and S. elachophyllum are likely to be more affected than the other two species (Table 3). A high proportion of the known locations of the four Solanum species is degraded, with an edge-to-area ratio > 0.15 km/ha or canopy cover < 50% (Fig. 2). These habitats are unlikely to be viable in the long term because of the destructive feedback between invasive grasses and fire. Reducing fuel loads resulting from exotic grasses can be most readily achieved by livestock grazing (Melzer, Reference Melzer2015), but the impact of grazing on Solanum is yet to be evaluated.
Some historical populations could not be relocated in our survey despite the brigalow habitat remaining intact since they were originally located. A population of Solanum adenophorum known from a specimen collected at Dipperu National Park in 1971 was not located after searching of 3.8 ha in 2014 and 1.8 ha in 2017. However, populations of S. adenophorum were relocated at Taunton National Park where it had not been recorded for > 15 years despite substantial search effort (R. Melzer, pers. comm.). Solanum adenophorum is almost certainly ephemeral and was abundant at Taunton 1 month after 120 mm of rain in March 2017. The population sizes of S. adenophorum (Table 3) have probably been underestimated because most field surveys were conducted when antecedent rainfall had been slightly below average and the ephemeral populations had not reached their full potential because of dry conditions.
Security of existing remnants
Current Queensland state legislation prohibits the clearance of existing brigalow remnants on freehold land, but illegal clearing is an ongoing problem (Queensland Department of Science, 2016) and no protection is provided against mining and gas developments. There is an ongoing threat of exotic grass invasion accompanied by the incursion of fire, which degrades tree canopies and results in further exotic grass invasion (Butler & Fairfax, Reference Butler and Fairfax2003).
Securing the single large remnant of brigalow forest on freehold land (24°02'S, 150°20'E) that contains the largest known populations of the Critically Endangered S. dissectum and S. johnsonianum would make a major contribution to their conservation.
Remnant brigalow forest on mining leases (Supplementary Table 2) are under threat as coal mines expand, but remnants that do not overlie extractable coal have potential for perpetual security as conservation reserves. Negotiated agreements over remnant vegetation within mining leases are an important priority for brigalow conservation, particularly for the preservation of S. adenophorum and S. elachophyllum (Supplementary Table 2).
Assessment of conservation status
The dataset accumulated in this study (Table 3; IUCN, 2012) facilitated quantitative assessment of the Solanum species using the IUCN Red List criteria. Determination of AOO is contingent on scale (Willis et al., Reference Willis, Moat and Paton2003; Keith et al., Reference Keith, Butchart, Regan, Harrison, Akçakaya, Solow and Burgman2017) and determining AOO with the recommended 2 km2 grid (IUCN Standards & Petitions Subcommittee, 2017) generates results that differ from those determined by fine-scale mapping (Table 3). If the grid method is applied to current known records, it underestimates the AOO of S. adenophorum, S. dissectum and S. elachophyllum, and overestimates that of S. johnsonianum relative to the mapping of remnant or intact brigalow habitat (Table 3). Underestimation will result from methods that fail to model undetected subpopulations in unsurveyed habitat, and overestimation using the 2 km2 grid will occur when habitat occupies only a portion of the grid cells. Both the grid-based and mapped habitat method of determining AOO for Solanum adenophorum result in estimates < 500 km2 and justify a categorization as Vulnerable. The difference between the grid-based and mapped habitat assessment of AOO does not affect the categorization of S. dissectum, S. elachophyllum and S. johnsonianum, which do not qualify under category B (geographical range size, and fragmentation, decline or fluctuations) because they only satisfy one of the subcriteria (b, continuing decline). Assigning S. dissectum and S. johnsonianum under criterion A (declining population, past, present and/or projected) requires an estimation of time frame based on the generation time of the species. Calculations of generation time can be derived from survival and fecundity rates of different aged individuals (IUCN Standards & Petitions Subcommittee, 2017), all of which are poorly defined for semi-woody clonal plant species. Therefore, we have assumed that generation time is > 20 years based on apparently low rates of germination and the potential for survival by regeneration of ephemeral ramets from perennial rootstocks. Additional tools for determining the generation time of plant lifeforms would be of considerable assistance for assessing species under categories A and C of the IUCN Red List criteria.
Conclusion
Solanum adenophorum, S. dissectum and S. johnsonianum are threatened because of their close association with an ecosystem that has been decimated over the past 60 years. These species and S. elachophyllum will decline further because they are extirpated where exotic grass cover is high, and many remnants of brigalow are already degraded by fire and invasion of exotic grasses. Many existing populations of the Solanum species will probably become extinct because they occur in inviable remnants, such as roadsides, that will be degraded by the same processes. The highest priority for conservation of these threatened Solanum species is the preservation of the last large remnants of brigalow forest and the management of exotic grasses around these remnants to avoid the destructive effects of the grass–fire cycle.
This study presents an example of a Red List assessment of poorly known plant species by (1) supplementing historical records with systematic surveys to accurately determine EOO, (2) identifying the habitat association of the target species, (3) establishing persistence in transformed habitat, (4) identifying the factors that limit distribution in transformed habitat, (5) inferring population size from density and available habitat, and (6) employing habitat mapping to define past declines and predict ongoing threats.
Acknowledgments
We thank Ian Menkins for his passion for solanums and insights into their ecology and distribution; Rhonda Melzer, Derek Johnson, Ralf Regeer and the students of the 2017 Ecology and Management of Invasive Species course at the University of Queensland for the contribution of survey data; the landholders who allowed access to their properties, particularly Shane Edminstone and Dennis Pittman; John Dwyer for advice on the procedure for estimating density; and the reviewers and editor for their critiques. This study was supported by funding from the Australian Government's National Environmental Science Programme through the Threatened Species Recovery Hub. We acknowledge the taxonomy of Tony Bean, which alerted us to the plight of threatened Solanum species.
Author contributions
Study design: RF; fieldwork: RF, JH and CH and BW; analysis: BW; writing: RF, BW.
Conflicts of interest
None.
Ethical standards
This research complies with the Oryx Code of Conduct.










