Ependymomas are rare tumors of neuroectodermal origin arising from the central canal of spinal cord, filum terminale, choroid plexus and ependymal cells of the cerebral ventricles. The World Health Organization (WHO) classification of central nervous system (CNS) tumors stratifies ependymomas according to grade. Grade I lesions include myxopapillary ependymoma and subependymoma, grade II lesions include cellular, papillary, clear cell and tanycytic ependymoma and anaplastic ependymomas are grade III.Reference Louis, Ohgaki and Wiestler 1 Intracranial ependymomas represent 6-9% of primary CNS neoplasms and account for 30% of primary CNS tumors in children younger than 3 years.Reference McGuire, Sainani and Fisher 2 In the spinal cord, ependymomas are the most common neuroepithelial neoplasms, comprising 50-60% of spinal gliomas.Reference Louis, Ohgaki and Wiestler 1 , Reference Oh, Ivan and Sun 3
The management of these patients is controversial and the identification of prognostic and predictive factors for survival and recurrence is an area of ongoing research. Most clinicians accept that maximal safe surgical resection is optimal where possible. At present, no chemotherapeutic regimen consistently increases survival.Reference Vaidya, Smee and Williams 4 , Reference Wright and Gajjar 5 There is clinical equipoise regarding the role of radiotherapy (RT) with some studies suggesting benefitReference Oh, Ivan and Sun 3 - Reference McGuire, Sainani and Fisher 7 and others reporting no clear advantage.Reference Venkatramani, Dhall and Patel 8 , Reference Benesch, Weber-Mzell and Gerber 9 Specifically, it is unclear whether the addition of RT can improve the survival of a patient with an subtotal resection (STR) to levels similar to patients who have had a gross total resection (GTR). Furthermore, it is not clear if other factors such as age, gender, location, extent of surgical resection, and pathological grade influence the ability of radiation to improve survival.Reference Vaidya, Smee and Williams 4 , Reference Jung, Choi and Ahn 10 - Reference Cage, Clark and Aranda 13
In this study, we report the results of a large population-based study including all cases of ependymoma diagnosed over a 32 year period in the province of Alberta, Canada. Our two objectives were to (1) identify clinical and treatment factors that affect recurrence and survival in patients diagnosed with ependymoma and (2) determine if the addition of postoperative radiotherapy can make up for incomplete surgery and improve the survival of patients treated with STR to levels comparable to those who have GTR.
Methods
This retrospective population based study included every patient diagnosed with ependymoma between December/1975 and July/2007 in the province of Alberta, Canada. One hundred eighty patients were identified from the Alberta Cancer Registry and clinicopathological data was retrieved from the patients’ charts (Figure 1). In ten cases where the pathological description was ambiguous with respect to tumor classification, a neuropathologist (JC) performed secondary review of the specimen (six cases) or pathological report (four cases) if the specimen was not available. From this initial cohort, 158 patients were included for the final analysis. Seventeen patients were excluded because the diagnosis was made radiologically in the absence of histopathology or tumors that would not be classified as ependymoma according to the WHO classification of tumors of the CNS.Reference Louis, Ohgaki and Wiestler 1 Five additional patients were excluded due to the lack of clinical data. Study approval was obtained from the University of Calgary Conjoint Health Research Ethics Board.
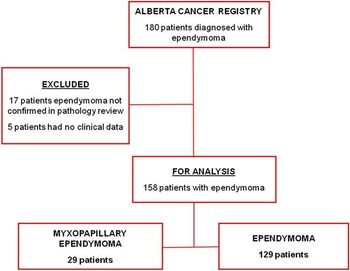
Figure 1 Flowchart of patients diagnosed with ependymoma in Alberta, from 1975 to 2007.
Clinical and Statistical Considerations
Age ranges were defined as 0-6, 7-17, 18-50 and greater than 50 years old. Tumor location was catergorized as spinal, supratentorial or infratentorial. The extent of resection was based on postoperative imaging (n=85; 71%) or the surgeon’s intraoperative impression (n=35; 29%) where imaging was not available. In nine cases the extent of resection could not be determined. Gross total resection was classified as complete resection of the mass. Any resection with residual lesion was classified as STR. Progression-free survival (PFS) was defined as the interval between diagnosis and time to when the patient recurred/progressed, died (events) or was lost to follow up (censored data point). Overall survival (OS) was calculated from date of diagnosis to death or lost to follow-up. Results were tabulated and analyzed with STATA/OC 12. Two-tailed Chi-squared or Fisher’s exact tests were used to determine the significance of associations between proportions. Clinical variables were evaluated for association with survival using Cox proportional hazards model and Kaplan-Meier survival analysis with log-rank test. Variables with significance in univariate analysis (p<0.05) were included in multivariate regression.
Results
Myxopapillary ependymomas
Myxopapillary ependymomas comprised 18% of the patients (29/158). The median age at diagnosis was 33 years and 14 were female (48%). Five patients were younger than 18 years (17%) and 24 were adults (83%). All but one tumor (28/29) was located in the spine, except for one case of concurrent supratentorial and spinal lesions (both resected and confirmed to be myxopapillary ependymomas). Eleven (38%) were subtotally resected and 14 had GTR. The extent of resection was unknown in four cases. Postoperative radiotherapy was administered in 73% (8/11) and 14% (2/14) of the patients with STR and GTR, respectively. Recurrent disease was noted in five patients. The majority of these patients (n=4) had STR of which two received postoperative RT. Only two patients with myxopapillary tumors died in this series after survivals of 66 and 158 months; median progression-free survival (PFS) and overall survival (OS) were 95 and 108 months, respectively. Neither age, location, nor initial treatment was associated with progression-free or overall survival in this group. Patients with myxopapillary ependymomas had longer survival compared to the 129 patients with other ependymoma in this series (p=0.017 and p=0.0082 for PFS and OS, respectively).
Relationship Between Clinical Factors (Gender, Age, Grade, Tumor Location) to Progression and Survival
Excluding patients with myxopapillary ependymoma, 129 patients were included for subsequent analysis. Patient characteristics are summarized in Table 1. Patient gender did not correlate with any clinical/prognostic variable, although most patients under the age of 18 (69%) were male (31/45; p=0.037).
Table 1 Characteristics of 129 patients with ependymoma diagnosed in Alberta between 1975 to 2007
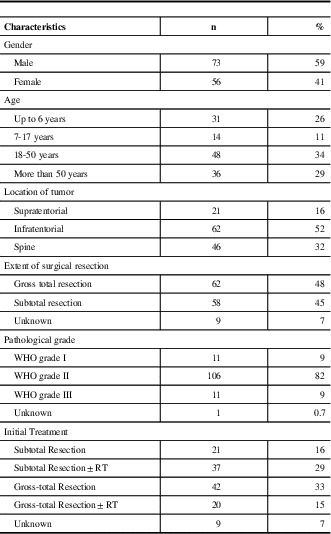
WHO=World Health Organization; RT=Radiotherapy
Patients up to the age of six years were more likely to progress (23/51 total recurrences; p<0.0001) and die (18/45 total deaths; p=0.001) compared with older patients. Age was associated with tumor location (p<0.0001) as children up to six years old had tumors supra- or infratentorially (90%; 28/31) while only three patients (10%) in this age range had spinal tumors. Patients between the age of 7-17 were more likely to have infratentorial tumors (n=8/14). In adults, 26 of the 48 patients between ages of 18 and 50 years old (54%) had spinal tumors and those older than 50 usually had infratentorial and spinal-located tumors (17/36 and 15/36, respectively).
Grade I (n=11) and III tumors (n=11) were more likely to be distributed either supra- or infratentorially but rarely in the spinal cord whereas Grade II lesions (n=106) were present throughout the CNS comprising 40% of all supratentorial lesions, 85% of all infratentorial lesions and 98% of spinal tumors. In this series histological grade was associated with tumor location (p<0.0001) but the predominance of Grade II tumors, as described above, may bias the analysis.
We identified a statistically significant association between age group and histological grade. The youngest patients (0-6 years old) were more likely to be diagnosed with grade III lesions whereas every incidence of grade I tumors were seen in adults (p=0.003). In this series, age was not associated with differences in either the extent of resection or the addition of postoperative radiotherapy.
Radiation Therapy
Sixty-three patients (49%) did not receive postoperative radiotherapy as part of their initial management, whereas 57 patients (44%) received RT following surgery. Radiation therapy was administered to the local tumor in 42 cases and to the tumor and cranio-spinal axis in 15 cases. There was evidence of leptomeningeal spread in pre- or post-operative images in only five patients, of which two received cranio-spinal RT. In ten cases, RT was given after the first recurrence and one patient received RT twice. Chemotherapy was administered only in six patients; all of them had grade II infratentorial lesions and median age was 1.5 years years-of-age (range; 0.5 to 7.7 years old).
Sixty-two patients underwent GTR of the tumor (48%), 58 had a STR (45%) and the extent of tumor resection was unknown in 9 patients. The extent of initial surgery was not associated with age, location or histological grade.
Following STR, 64% (37/58) of patients received radiotherapy. The decision to deliver radiotherapy was dependent on the extent of surgery and grade. Tumor location did not seem to impact the delivery of radiotherapy, however, tumor grade was important with only 9% of grade I tumors receiving adjuvant RT compared with 72% and 60% of grade II and III tumors, respectively (p=0.029). After GTR, only 11% (1/9) supratentorial ependymoma and 4% (1/22) spinal tumor received radiation, compared with 58% (18/31) of infratentorial tumors (p=0.002). Radiotherapy was administered after GTR in 31% (16/51) of patients with grade II tumors and 67% (4/6) patients with grade III tumors.
Progression Free Survival
Median progression-free survival was 55 months for all patients. Table 2 includes univariate analysis for PFS with Hazard Ratio (HR) (95% CI) and p values for each variable. For those patients younger than seven years, the median PFS was 20 months. This was significantly worse compared with 58, 83 and 63 months for patients between 7-17 years (p=0.034), 18-50 years (p<0.0001) and greater than 50 years of age (p<0.0001), respectively.
Table 2 Univariate analysis for Progression Free Survival (PFS) and Overall Survival (OS) of 129 patients with ependymoma (excluded myxopapillary)

OS=overall survival; PFS=Progression Free Survival; HR=Hazard Ratio; CI=Confidence Interval; WHO=World Health Organization; STR=Subtotal resection; GTR=Gross total resection; RT=Radiotherapy
a Statistically significant p value
Tumor location and grade impacted PFS
Patients with supratentorial tumors had a decreased median PFS of 19 months compared with infratentorial (55 months) and spinal (77 months) lesions (p=0.074 and p<0.0001, respectively). Histological grade I and II tumors had median PFS of 75 months and 56 months, respectively (p=0.256). Not unexpectedly, PFS for grade I tumors was significantly better than those with grade III lesions (15 months) (p=0.005).
Patients with GTR had an improved median PFS (71 months) compared with 37 months for those receiving subtotal resection (p=0.0161). Importantly, the addition of radiotherapy did not improve PFS following STR (p=0.62) or GTR (p=0.67). Patients with GTR alone had a better median progression-free survival (64 months) compared with those treated with STR and radiotherapy (31 months) (p=0.046).
Overall survival
The overall median survival was 90 months. Univariate analysis is shown in Table 2. Median survival was significantly different by age ranges with median survival of 62 months, 95 months, 138 months and 92 months for patients up to 6 years old, 7 to 17, 18 to 50 and more than 50 years old, respectively.
Patients with spinal tumors lived a median of 127 months compared with 86 months for infratentorial and 75 months for patients with supratentorial tumors (p=not significant). When dichotomizing patients by spinal vs cerebral location (supratentorial and infratentorial tumors together) the difference in survival was suggestive but not statistically significant (p=0.08). Patients with grade I tumors lived a median of 123 months compared with 90 months and 78 months for patients with grade II and III tumors, respectively but the differences were not statistically significant (p>0.2).
We observed a statistically significant difference in overall survival between patients receiving GTR versus STR with median OS of 123 months and 79 months, respectively (p=0.0027) (Figure 2). In this series the addition of radiotherapy to STR or GTR did not improve overall survival (p=0.178 and p=0.618, respectively). Furthermore, those with GTR had significantly improved median overall survival (122 months) as compared with patients who had STR and radiotherapy (82 months [p=0.0022) (Figure 3).

Figure 2 Overall survival of 129 patients with ependymoma (excluding myxopapillary) diagnosed in Alberta from 1975 to 2007, according to extent of initial resection. HR=Hazard Ratio; CI=Confidence Interval; STR=Subtotal resection; GTR=Gross total resection; RT=Radiotherapy

Figure 3 Overall survival of 129 patients with ependymoma (excluding myxopapillary) diagnosed in Alberta from 1975 to 2007 comparing subtotal resection followed by radiotherapy and gross-total resection. HR: Hazard Ratio, CI: Confidence Interval, STR: Subtotal resection, GTR: Gross total resection, RT: Radiotherapy
In multivariate analysis which included age, location, grade and extent of resection, only GTR was independently associated with an improvement in progression-free survival. An independent prognostic factor was not identified for overall survival (Table 3).
Table 3 Multivariate analysis for PFS and OS of 129 patients with ependymoma (excluded myxopapillary) diagnosed in Alberta between 1975 and 2007
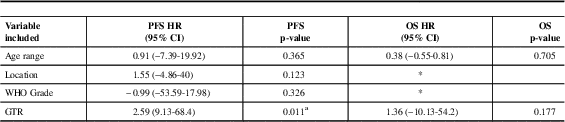
CI: Confidence Interval, WHO: World Health Organization, GTR: gross-total resection, OS: overall survival, PFS: Progression Free Survival, HR: Hazard Ratio
* Variable not included as not statistically significant in univariate analysis
a Statistically significant p value
Discussion
To our knowledge, this is the first population based analysis of patients with ependymoma including adults and children in Canada without any age limitation. This study included 158 patients with 29 tumors classified as myxopapillary ependymoma. The remaining 129 patients were mostly adult patients (63%) usually with either infratentorial (52%) or spinal tumors (32%). Most patients had grade II lesions (82%) and a similar proportion of patients had GTR versus STR (48% to 45 %). Amirian et al.Reference Amirian, Armstrong, Aldape, Gilbert and Scheurer 14 published a large American retrospective review from 1973-2007 and reported, similar to our study, that the majority of were adults (71%) with grade II (88%) lesions similarly distributed throughout the CNS.
When analyzing our data to identify prognostic factors in patients with ependymoma, age was significant in univariate analysis for both progression-free and overall survival but was not an independent prognostic factor. Similar to other reports, we observed a bimodal association of age with death as both the youngest patients and those over the age of 50 had the worst prognosis.Reference Armstrong, Vera-Bolanos, Bekele, Aldape and Gilbert 12 , Reference Amirian, Armstrong, Aldape, Gilbert and Scheurer 14 However, tumor location was not associated with better overall survival in our series. Patients with anaplastic ependymomas had worse PFS compared with those ependymomas grade I, but histological grade was not associated with OS in our study. However, other series have showed the prognostic value of both location and tumor anaplasia.Reference Oh, Ivan and Sun 3 , Reference Swanson, Amdur and Morris 11 , Reference Armstrong, Vera-Bolanos, Bekele, Aldape and Gilbert 12
Gross-total resection has been identified as a prognostic factor in manyReference Cage, Clark and Aranda 13 - Reference Reni, Gatta, Mazza and Vecht 15 , Reference Merchant, Li, Xiong, Kun, Boop and Sanford 16 - Reference Metellus, Barrie and Figarella-Branger 20 but not all pediatric and adult ependymoma series.Reference Guyotat, Signorelli and Desme 21 - Reference Moreno, Bautista and Zacharoulis 23 . In our study, GTR was associated with statistically significant improved PFS and OS compared with STR in univariate analysis but this did not hold up in multivariable analysis. However, the relationship between extent of surgery and survival is inconsistent within the literature. Our results corroborate the findings of Reni et al.Reference Reni, Brandes and Vavassori 24 who observed that GTR was associated with improved progression-free but not overall survival but not those of Amirian et al.Reference Amirian, Armstrong, Aldape, Gilbert and Scheurer 14 who identified GTR as an independent prognostic factor for overall survival. Importantly, the definition of GTR varies depending on whether surgical opinion or a specific postoperative imaging modality is used. Regardless, maximal safe resection continues to be the standard of care.
One of the goals of this study was to analyze if the addition of radiotherapy to patients with subtotal resection could improve PFS/OS to levels similar to gross-total resection. In our series, postoperative radiotherapy after STR did not compensate for suboptimal surgery. Specifically, overall survival in patients with GTR was statistically superior compared with patients who had STR followed by radiotherapy. In a recently published retrospective review that included 348 patients who underwent STR of spinal cord ependymomas, the addition of radiotherapy was associated with prolonged progression-free survival (p=0.047) but not overall survival.Reference Oh, Ivan and Sun 3 In another series of 88 patients with spinal ependymomas the addition of RT to GTR or STR did not improve progression-free or overall survival.Reference Lee, Chung and Kim 25 A subanalysis of 193 children with infratentorial tumors by McGuire et al.Reference McGuire, Sainani and Fisher 7 showed that postoperative radiotherapy improved survival (p=0.033) but the extent of surgery in these patients was not reported. A review of 45 patients with posterior fossa ependymomas found that progression-free survival was similar between patients treated with GTR and STR followed by radiotherapy. However, in univariate analysis, the addition of RT to GTR improved PFS (p=0.018) without an effect in overall survival.Reference Rogers, Pueschel and Spetzler 26 Also, Amirian et al.Reference Amirian, Armstrong, Aldape, Gilbert and Scheurer 14 compared extent of surgery with and without surgery in a manner similar to our study and reported that the addition of radiotherapy to less than GTR did not improve survival. A randomized control trial could assess the role of radiotherapy on outcome in patients with ependymoma. However, such a study may be difficult given that this is a rare tumor and a clinical trial randomization process would need, at a minimum, to control for age, tumor location, histological grade and extent of initial surgical resection.
The limitations of this study are those common to registry-based studies. Incomplete report or coding errors are possible. The pathologic classification was by report only in most cases, with a minority of cases, where the pathology report did not allow initial assignment into one of the diagnostic categories, requiring formal pathology review. Although little has changed in the histopathologic guidelines for classification of ependymomas over the time period of the study, it is possible that formal pathology review for every case may have changed some diagnoses. The extent of resection was based on the surgeon’s intraoperative impression in about one third of the cases where radiological confirmation was not possible. This is particularly true for patients who had surgery during the early years of the study period. We recognize surgeons are not reliable at estimating degree of resection, and in more recent years the accuracy of this evaluation has improved with advances in neuro-imaging.
This series describes the characteristics and outcomes of unselected patients diagnosed with ependymoma. In our study, the addition of radiotherapy to STR did not improve overall survival compared with patients with GTR. We highlight the importance of maximum safe resection with GTR identified as the main significant prognostic factor for better patient outcome.
Disclosures
JE does not have anything to disclose.








