Impact statement
Coastal wetlands, including mangrove forests, tidal marshes and seagrass meadows, can take carbon out of the atmosphere and store it in plant tissue and soil at the highest rates of any ecosystem. Because of this unique feature, coastal wetland restoration can act as a natural climate solution (NCS), helping to mitigate climate change by having a net cooling benefit compared to pre-restoration conditions. However, uncertainty remains in when and where coastal wetland restoration acts as effective NCS. This manuscript synthesizes the fundamental requirements for restoration to act as effective NCS: additionality, permanence and feasibility. We highlight the minimum data required to understand these requirements, which are less robust than the data needed for carbon crediting or accounting. Many of these data are spatial and widely available. We also highlight future perspectives that may help address uncertainty in restoration as NCS, by taking a landscape-scale approach and incorporating methane emissions. Ultimately, reducing uncertainty in when and where coastal wetland restoration acts as NCS supports the broader effort to mitigate climate change most effectively.
Coastal wetlands as natural climate solutions
Climate change is causing cascading impacts to human and natural systems globally, and all possible mitigation and adaptation actions will be needed to keep warming below critical thresholds over the next decade (United Nations Framework Commission on Climate Change (UNFCCC), 2015; Intergovernmental Panel on Climate Change (IPCC), 2022; Diffenbaugh and Barnes, Reference Diffenbaugh and Barnes2023). For coastal landscapes, sea-level rise is among the greatest drivers of change, impacting coastal communities through increased flooding and salinization risks (Intergovernmental Panel on Climate Change (IPCC), 2021; Sweet et al., Reference Sweet, Hamlington, Kopp, Weaver, Barnard and Bekaert2022). Natural climate solutions (NCS), or those actions that mitigate climate change using ecosystem management, can remove greenhouse gases from the atmosphere, complementing efforts to reduce fossil fuel emissions (Fargione et al., Reference Fargione, Bassett, Boucher, Bridgham, Conant, Cook-Patton, Ellis, Falcucci, Fourqurean, Gopalakrishna, Gu, Henderson, Hurteau, Kroeger, Kroeger, Lark, Leavitt, Lomax, McDonald, Megonigal, Miteva, Richardson, Sanderman, Shoch, Spawn, Veldman, Williams, Woodbury, Zganjar, Baranski, Elias, Houghton, Landis, McGlynn, Schlesinger, Siikamaki, Sutton-Grier and Griscom2018; Macreadie et al., Reference Macreadie, Costa, Atwood, Friess, Kelleway, Kennedy, Lovelock, Serrano and Duarte2021; United Nations Environment Programme (UNEP) and International Union for Conservation of Nature (IUCN), 2021). Although we explicitly focus on NCS as actions that remove greenhouse gases here (without concurrent negative impacts; Ellis et al., Reference Ellis, Page, Wood, Fargione, Masuda, Carrasco Denney, Moore, Kroeger, Griscom, Sanderman, Atleo, Cortez, Leavitt and Cook-Patton2024), restoration of coastal ecosystems comes with a host of additional co-benefits (Hagger et al., Reference Hagger, Waltham and Lovelock2022; Krauss et al., Reference Krauss, Lovelock, Chen, Berger, Ball, Reef, Peters, Bowen, Vovides, Ward, Wimmler, Carr, Bunting and Duberstein2022a; Rogers et al., Reference Rogers, Lal, Asbridge and Dwyer2023b; Novick et al., Reference Novick, Keenan, Anderegg, Normile, Runkle, Oldfield, Shrestha, Baldocchi, Evans, Randerson, Sanderman, Torn, Trugman and Williams2024).
Coastal wetlands, including mangrove forests, tidal marshes and seagrass meadows (among all other tidal wetlands; Adame et al., Reference Adame, Kelleway, Krauss, Lovelock, Adams, Trevathan-Tackett, Noe, Jeffrey, Ronan, Zann, Carnell, Iram, Maher, Murdiyarso, Sasmito, Tran, Dargusch, Kauffman and Brophy2024), are highly productive ‘blue carbon’ ecosystems connecting terrestrial and marine realms globally. These ecosystems are unique in their ability to mitigate climate change as they continually absorb and store carbon from the atmosphere, leading to a climate cooling benefit (Figure 1; Neubauer, Reference Neubauer2021) that grows over time if they continue to add carbon within the accommodation space created by sea-level rise (Rogers et al., Reference Rogers, Kelleway, Saintilan, Megonigal, Adams, Holmquist, Lu, Schile-Beers, Zawadzki, Mazumder and Woodroffe2019a; Buffington et al., Reference Buffington, Janousek, Dugger, Callaway, Schile-Beers, Borgnis Sloane and Thorne2021). Present day coastal wetlands initiated development when relative sea-level rise decelerated sufficiently for coastal wetlands to maintain their position within the tidal frame; the timing of this development varies globally due to differences in glacio-isostatic adjustment of coastlines (Woodroffe, Reference Woodroffe, Perillo, Wolanski, Cahoon and Hopkinson2019). Global distribution of blue carbon ecosystems is variable as well and largely determined by climate constraints (McKenzie et al., Reference McKenzie, Nordlund, Jones, Cullen-Unsworth, Roelfsema and Unsworth2020; Jia et al., Reference Jia, Wang, Mao, Ren, Song, Zhao, Wang, Xiao and Wang2023; Worthington et al., Reference Worthington, Spalding, Landis, Maxwell, Navarro, Smart and Murray2024); these ecosystem types vary in how they store and cycle carbon to mitigate climate change.

Figure 1. Key terms as defined in this manuscript. Conceptual comparison is of the radiative balance of a coastal wetland in pre-restored (black) and post-restored (gray) states (modified from Neubauer, Reference Neubauer2021). In this example, the pre-restored and post-restored states both have positive radiative balances, adding energy to Earth’s energy budget. After restoration, there is a change in radiative balance (i.e., a radiative forcing); restoration action led to a reduction in radiative balance. Because the radiative forcing is negative, this example indicates a cooling benefit from restoration actions; the project has additionality.
At the regional scale, hydrogeomorphic setting (i.e., landscape configuration) constrains the occurrence of blue carbon ecosystem types and their ability to store carbon. Hydrogeomorphic setting influences the dominance of water forcings (e.g., wind, wave, tide; Boyd et al., Reference Boyd, Dalrymple and Zaitlin1992), sediment availability and deposition (Hupp et al., Reference Hupp, Kroes, Noe, Schenk and Day2019), connectivity to other habitats (Noe et al., Reference Noe, Hupp, Bernhardt and Krauss2016; Woo et al., Reference Woo, Davis, De La Cruz, Windham-Myers, Drexler, Byrd, Stuart-Haëntjens, Anderson, Bergamaschi, Nakai, Ellings, Hodgson, Krauss, Zhu and Stagg2022), and freshwater availability and timing important for sulfate concentrations and methane production (Poffenbarger et al., Reference Poffenbarger, Needelman and Megonigal2011; Knox et al., Reference Knox, Bansal, McNicol, Schafer, Sturtevant, Ueyama, Valach, Baldocchi, Delwiche, Desai, Euskirchen, Liu, Lohila, Malhotra, Melling, Riley, Runkle, Turner, Vargas, Zhu, Alto, Fluet‐Chouinard, Goeckede, Melton, Sonnentag, Vesala, Ward, Zhang, Feron, Ouyang, Alekseychik, Aurela, Bohrer, Campbell, Chen, Chu, Dalmagro, Goodrich, Gottschalk, Hirano, Iwata, Jurasinski, Kang, Koebsch, Mammarella, Nilsson, Ono, Peichl, Peltola, Ryu, Sachs, Sakabe, Sparks, Tuittila, Vourlitis, Wong, Windham‐Myers, Poulter and Jackson2021). As one example, intermittently connected lakes and lagoons (ICOLLs) or temporarily open/closed estuaries (TOCEs), are coastal wetlands that can undergo state shifts in salinity and water level that drive changes in ecosystem parameters like macrophyte community extent and composition (Riddin and Adams, Reference Riddin and Adams2008), presenting specific challenges in quantifying dynamic climate benefits.
Accounting for the temporal evolution of coastal wetlands can be challenging for practitioners, researchers and policy-makers alike (Neubauer and Megonigal, Reference Neubauer and Megonigal2015; Neubauer, Reference Neubauer2021; Abernethy and Jackson, Reference Abernethy and Jackson2022). Continuous and effectively permanent soil carbon sequestration, a particularly important aspect of coastal wetlands as blue carbon ecosystems, is a long-term additive process (Chmura et al., Reference Chmura, Anisfeld, Cahoon and Lynch2003; Mcleod et al., Reference Mcleod, Chmura, Bouillon, Salm, Björk, Duarte, Lovelock, Schlesinger and Silliman2011). In the context of NCS, however, decadal timescales are of primary interest to assist in meeting climate commitments as soon as possible (United Nations Framework Commission on Climate Change (UNFCCC), 2015; United Nations Environment Programme (UNEP) and International Union for Conservation of Nature (IUCN), 2021). Greenhouse gas fluxes and herbaceous biomass can respond rapidly to management actions in coastal wetlands (Wang et al., Reference Wang, Ho, Flanagan and Richardson2021; Woo et al., Reference Woo, Davis, De La Cruz, Windham-Myers, Drexler, Byrd, Stuart-Haëntjens, Anderson, Bergamaschi, Nakai, Ellings, Hodgson, Krauss, Zhu and Stagg2022), and carbon sequestration rates and woody biomass can also recover within decades in certain situations (Marbà et al., Reference Marbà, Arias‐Ortiz, Masqué, Kendrick, Mazarrasa, Bastyan, Garcia‐Orellana and Duarte2015; Osland et al., Reference Osland, Feher, Spivak, Nestlerode, Almario, Cormier, From, Krauss, Russell, Alvarez, Dantin, Harvey and Stagg2020; Eagle et al., Reference Eagle, Kroeger, Spivak, Wang, Tang, Abdul-Aziz, Ishtiaq, O’Keefe Suttles and Mann2022; Rogers et al., Reference Rogers, Kellway and Saintilan2023a). Regardless, losing millennia of stored carbon simply cannot be regained over short timescales by restoration; preservation of existing carbon stocks and functioning ecosystems is therefore key (Drexler et al., Reference Drexler, Fontaine and Deverel2009; Arias-Ortiz et al., Reference Arias-Ortiz, Masqué, Glass, Benson, Kennedy, Duarte, Garcia-Orellana, Benitez-Nelson, Humphries, Ratefinjanahary, Ravelonjatovo and Lovelock2021a).
Given historical degradation and land conversion of coastal wetlands globally (Friess et al., Reference Friess, Rogers, Lovelock, Krauss, Hamilton, Lee, Lucas, Primavera, Rajkaran and Shi2019; Turschwell et al., Reference Turschwell, Connolly, Dunic, Sievers, Buelow, Pearson, Tulloch, Côté, Unsworth, Collier and Brown2021; Campbell et al., Reference Campbell, Fatoyinbo, Goldberg and Lagomasino2022), under-recognized but tractable opportunities exist to use restoration as NCS to recover carbon sequestration functionality (Macreadie et al., Reference Macreadie, Nielsen, Kelleway, Atwood, Seymour, Petrou, Connolly, Thomson, Trevathan‐Tackett and Ralph2017; United Nations Environment Programme (UNEP) and International Union for Conservation of Nature (IUCN), 2021; Krauss et al., Reference Krauss, Zhu and Stagg2022b; Lovelock et al., Reference Lovelock, Adame, Bradley, Dittmann, Hagger, Hickey, Hutley, Jones, Kelleway, Lavery, Macreadie, Maher, McGinley, McGlashan, Perry, Mosley, Rogers and Sippo2022a). Hydrologic impoundment is a leading cause of stress and degradation for intertidal coastal ecosystems (Montague et al., Reference Montague, Zale and Percival1987; Warren et al., Reference Warren, Fell, Rozsa, Brawley, Orsted, Olson, Swamy and Niering2002; Lewis et al., Reference Lewis, Milbrandt, Brown, Krauss, Rovai, Beever and Flynn2016; Chambers et al., Reference Chambers, Steinmuller and Breithaupt2019). Reconnecting degraded wetlands to their watersheds is therefore a common restoration technique, with documented success in halting oxidative loss of carbon stores or otherwise shifting carbon cycling for a climate cooling benefit (Kroeger et al., Reference Kroeger, Crooks, Moseman-Valtierra and Tang2017; Dittmann et al., Reference Dittmann, Mosley, Beaumont, Bestland, Guan, Sandhu, Clanahan, Baring, Quinn, Sutton, Thomson, Shepherd, Whalen, Marschner and Townsend2019; Cormier et al., Reference Cormier, Krauss, Demopoulos, Jessen, McClain-Counts, From, Flynn, Krauss, Zhu and Stagg2022; Eagle et al., Reference Eagle, Kroeger, Spivak, Wang, Tang, Abdul-Aziz, Ishtiaq, O’Keefe Suttles and Mann2022; Windham-Myers et al., Reference Windham-Myers, Oikawa, Deverel, Chapple, Drexler and Stern2023). Sediment augmentation is also commonly used to increase resilience to relative sea-level rise in coastal wetlands that have deteriorated from increased flooding stress (Stagg and Mendelssohn, Reference Stagg and Mendelssohn2010; Yuan et al., Reference Yuan, Liu, Tian, Yuan, Bo, Ma, Wu, Zhao, Zhang and Keesing2022; Fard et al., Reference Fard, Brown, Ambrose, Whitcraft, Thorne, Kemnitz, Hammond and MacDonald2024), leading to enhanced longevity of carbon sequestration compared to no-action alternatives. Additionally, improving water quality (e.g., eutrophication) and other threats (Turschwell et al., Reference Turschwell, Connolly, Dunic, Sievers, Buelow, Pearson, Tulloch, Côté, Unsworth, Collier and Brown2021) before introducing large numbers of foundation species may be critical for seagrass restoration success (van Katwijk et al., Reference van Katwijk, Thorhaug, Marbà, Orth, Duarte, Kendrick, Althuizen, Balestri, Bernard, Cambridge, Cunha, Durance, Giesen, Han, Hosokawa, Kiswara, Komatsu, Lardicci, Lee, Meinesz, Nakaoka, O’Brien, Paling, Pickerell, Ransijn and Verduin2016).
Regardless of restoration approach, coastal wetlands have been identified as particularly impactful habitats for restoration actions as NCS because of (a) their high rates of carbon sequestration and high densities of carbon storage over centuries to millennia (Bridgham et al., Reference Bridgham, Megonigal, Keller, Bliss and Trettin2006; Mcleod et al., Reference Mcleod, Chmura, Bouillon, Salm, Björk, Duarte, Lovelock, Schlesinger and Silliman2011; Poulter et al., Reference Poulter, Fluet-Chouinard, Hugelius, Koven, Fatoyinbo, Page, Rosentreter, Smart, Taillie, Thomas, Zhang, Wijedasa, Krauss, Zhu and Stagg2022); (b) the potential for management actions that have meaningful impacts on carbon budgets of degraded habitats, leading to climate cooling benefits; and (c) the potential for interventions to have additional social and environmental co-benefits (Lovelock and Duarte, Reference Lovelock and Duarte2019). Given that opportunities for restoration are distributed unevenly across continental scales (e.g., Holmquist et al., Reference Holmquist, Eagle, Molinari, Nick, Stachowicz and Kroeger2023) and resources for restoration activity are limited, there remains a lack of clarity on where coastal wetland restoration is maximally effective as NCS, and under which circumstances action is warranted.
Ultimately, to be effective as NCS, coastal wetland restoration projects must accrue climate cooling benefits that would not occur without management action (Figure 1). Here, we synthesize current understanding to 1) illustrate the fundamental requirements for coastal wetland restoration to be an effective NCS, addressing uncertainty in where restoration maximizes climate benefits, and 2) discuss potential paths forward to overcome current implementation barriers, addressing uncertainty in when restoration action is warranted.
Requirements for coastal wetland restoration as an effective natural climate solution
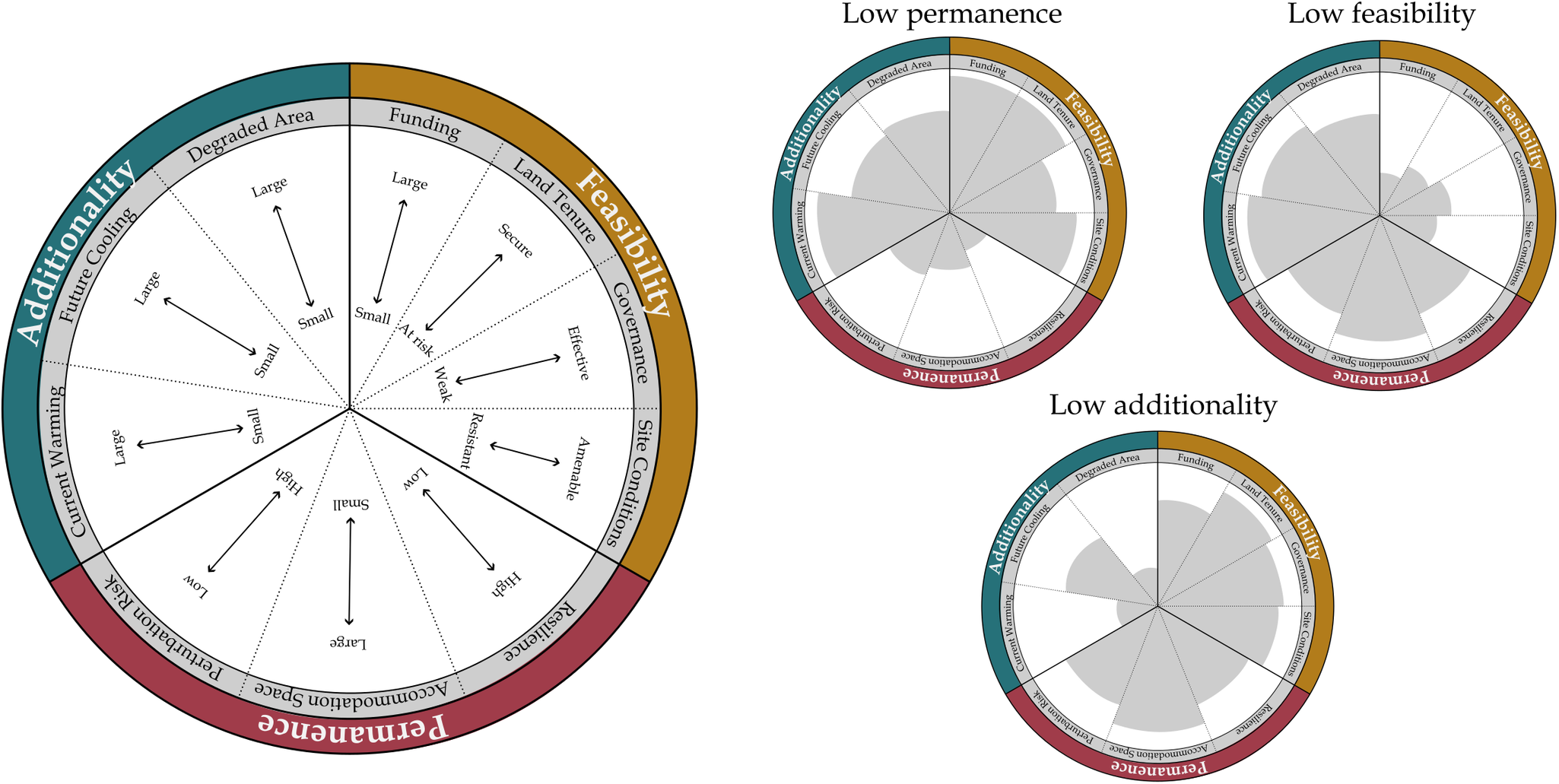
Three fundamental criteria determine the effectiveness of restoration actions as NCS: additionality, feasibility and permanence (Table 1). Below, we discuss requirements in an ecological sense, rather than within the context of a particular carbon finance or accounting framework. Due to their potential to influence site-specific climate benefits, local-scale factors are also considered.
Table 1. The fundamental requirements for coastal wetland restoration to be effective as NCS: additionality, feasibility and permanence

Note: Specific values of these requirements can maximize the cooling benefit of coastal wetland restoration. There are relatively straightforward minimum data needed to quantify if the fundamental requirements are met to address the question, ‘Does this management action lead to a net climate benefit?’
Additionality
Coastal wetland restoration is effective as NCS when actions ‘add’ carbon to the landscape, reducing atmospheric greenhouse gas concentrations and leading to a cooling benefit compared to initial degraded conditions (Figure 1). Maximal cooling benefits occur where the difference in pre-restoration and post-restoration climate impact is large. For example, highly degraded pre-restoration sites with large carbon emissions being converted to productive post-restoration sites with large carbon sequestration maximizes additionality. This cooling benefit can be achieved through restoring areas back to their original ecosystem type (e.g., conversion of shrimp ponds back to mangrove forests; Sidik et al., Reference Sidik, Fernanda Adame and Lovelock2019), enhancing or rehabilitating function within an ecosystem type (e.g., restoring hydrology to impounded marshes, Eagle et al., Reference Eagle, Kroeger, Spivak, Wang, Tang, Abdul-Aziz, Ishtiaq, O’Keefe Suttles and Mann2022), or creating new/novel habitat. Large areas available in degraded condition that can be converted through management action to an enhanced condition equates to large potential cooling benefits. Small estuarine systems therefore may not have the same potential as large deltas/bays (unless aggregated as regional systems; Duarte de Paula Costa et al., Reference Duarte de Paula Costa, Lovelock, Waltham, Moritsch, Butler, Power, Thomas and Macreadie2022), because habitat size (i.e., degraded land that can be restored) was originally small. Beyond size considerations, often ignored but potentially important biophysical changes can occur after restoration, leading to net cooling benefits without changing carbon cycling directly (e.g., changes in albedo, latent/sensible heat flux, roughness; Graf et al., Reference Graf, Wohlfahrt, Aranda-Barranco, Arriga, Brümmer, Ceschia, Ciais, Desai, Di Lonardo, Gharun, Grünwald, Hörtnagl, Kasak, Klosterhalfen, Knohl, Kowalska, Leuchner, Lindroth, Mauder, Migliavacca, Morel, Pfennig, Poorter, Terán, Reitz, Rebmann, Sanchez-Azofeifa, Schmidt, Šigut, Tomelleri, Yu, Varlagin and Vereecken2023; Zhu et al., Reference Zhu, Chen, Wu, Huang, Shao, Dong, Xu and Li2024).
Conditions amenable to quick recovery of carbon storage pools, reduction in greenhouse gas emissions, and/or enhanced carbon sequestration rates are key to maximizing additionality in coastal wetland restoration. While most carbon is stored in coastal wetland soils over the long-term, biomass pools often develop more rapidly and can be the first sign of additionality from restoration (Rogers et al., Reference Rogers, Kellway and Saintilan2023a). Habitat types with large woody vegetation (characteristic of mangrove and tidal forests) contain substantially more biomass carbon than habitat types with herbaceous vegetation (characteristic of tidal marshes and seagrasses) (Adame et al., Reference Adame, Kelleway, Krauss, Lovelock, Adams, Trevathan-Tackett, Noe, Jeffrey, Ronan, Zann, Carnell, Iram, Maher, Murdiyarso, Sasmito, Tran, Dargusch, Kauffman and Brophy2024), and can amass considerable additionality over 15–25 years after restoration (Osland et al., Reference Osland, Feher, Spivak, Nestlerode, Almario, Cormier, From, Krauss, Russell, Alvarez, Dantin, Harvey and Stagg2020; Rogers et al., Reference Rogers, Kellway and Saintilan2023a). Restored sites that have the potential for large gains in biomass carbon after management action may therefore maximize additionality over decadal scales (e.g., Sasmito et al., Reference Sasmito, Taillardat, Clendenning, Cameron, Friess, Murdiyarso and Hutley2019). This additional vegetation biomass can be constrained by regional scale factors (e.g., Rovai et al., Reference Rovai, Twilley, Castañeda‐Moya, Midway, Friess, Trettin, Bukoski, AEL, Pagliosa, Fonseca, Mackenzie, Aslan, Sasmito, Sillanpää, Cole, Purbopuspito, Warren, Murdiyarso, Mofu, Sharma, Tinh and Riul2021 for mangroves). The accommodation space for carbon burial in an estuary also varies regionally, based largely on geologic ‘maturation’ stage (Owers et al., Reference Owers, Woodroffe, Mazumder and Rogers2022; Rogers et al., Reference Rogers, Zawadzki, Mogensen and Saintilan2022). Regionally variable sediment availability for allochthonous carbon burial and freshwater availability to support autochthonous production can drive the potential for adding carbon to the landscape as well (e.g., Thorne et al., Reference Thorne, Jones, Freeman, Buffington, Janousek and Guntenspergen2022) (see the discussion on allochthonous carbon in Section ‘Coastal wetlands as cross-ecosystem linkages’). Additionality after restoration may not follow a linear increase, instead showing rapid initial responses (e.g., for carbon accumulation; Burden et al., Reference Burden, Garbutt and Evans2019). For effective cooling, additionality and general carbon cycling after restoration do not need to match remnant ecosystems; there needs to be enhanced function compared to the initial/alternative degraded state.
Feasibility
Coastal wetland restoration is effective as NCS when actions are feasible to implement. Pinpointing areas on the landscape where restoration actions will have the largest benefits to climate mitigation is inconsequential if the actions themselves cannot be completed. Feasibility is largely set by conditions external to the restoration site, including regional socioeconomic and governance constraints that influence human decision-making (Friess et al., Reference Friess, Rogers, Lovelock, Krauss, Hamilton, Lee, Lucas, Primavera, Rajkaran and Shi2019; Stewart-Sinclair et al., Reference Stewart-Sinclair, Purandare, Bayraktarov, Waltham, Reeves, Statton, Sinclair, Brown, Shribman and Lovelock2020). Restoration can take considerable infrastructure and funding to implement; this funding must be in place or accessible in the region for action to commence, and may use a variety of financial instruments (Friess et al., Reference Friess, Howard, Huxham, Macreadie and Ross2022). Regional and local land tenure is an additional crucial consideration for effective restoration (Lovelock and Brown, Reference Lovelock and Brown2019; Lovelock et al., Reference Lovelock, Barbier and Duarte2022c; Bell-James et al., Reference Bell-James, Fitzsimons and Lovelock2023), as additional co-benefits should be delivered to local communities and stakeholders, who are often direct (and historical) users of coastal ecosystems (Wylie et al., Reference Wylie, Sutton-Grier and Moore2016; Dencer-Brown et al., Reference Dencer-Brown, Shilland, Friess, Herr, Benson, Berry, Cifuentes-Jara, Colas, Damayanti, García, Gavaldão, Grimsditch, Hejnowicz, Howard, Islam, Kennedy, Kivugo, Lang’at, Lovelock, Malleson, Macreadie, Andrade-Medina, Mohamed, Pidgeon, Ramos, Rosette, Salim, Schoof, Talukder, Thomas, Vanderklift and Huxham2022). Existing policies and regulations can vary in scope and purpose across jurisdictional lines, making a complex web that may impede effective coastal management, including restoration activities (Herr et al., Reference Herr, Vegh, Von Unger, Windham-Myers, Crooks and Troxler2019). To maximize feasibility, external conditions will support restoration action through available funding, appropriate land tenure, and effective governance (Stewart-Sinclair et al., Reference Stewart-Sinclair, Purandare, Bayraktarov, Waltham, Reeves, Statton, Sinclair, Brown, Shribman and Lovelock2020; Macreadie et al., Reference Macreadie, Robertson, Spinks, Adams, Atchison, Bell-James, Bryan, Chu, Filbee-Dexter, Drake, Duarte, Friess, Gonzalez, Grafton, Helmstedt, Kaebernick, Kelleway, Kendrick, Kennedy, Lovelock, Megonigal, Maher, Pidgeon, Rogers, Sturgiss, Trevathan-Tackett, Wartman, Wilson and Rogers2022; Windham-Myers et al., Reference Windham-Myers, Oikawa, Deverel, Chapple, Drexler and Stern2023).
Maximizing feasibility also includes ensuring internal site conditions are amenable to region-specific restoration culture and practice. Pre-restoration land use can influence post-restoration vegetation and water quality recovery, through impacts on elevation and initial plant community composition (Janousek et al., Reference Janousek, Bailey and Brophy2020). Restoration activities can also fail when restoration practice does not align with local site conditions. For instance, planting mangrove propagules on mudflats for rehabilitation can have low survival rates if species are used that are unlikely to naturally establish at available elevations (Wodehouse and Rayment, Reference Wodehouse and Rayment2019; Lovelock et al., Reference Lovelock, Barbier and Duarte2022c). Further, the cultural practice of restoration itself, including methods, goals, and rationale, may vary by region (e.g., Hudson and Kenworthy, Reference Hudson, Kenworthy and Best2021; Lovelock et al., Reference Lovelock, Adame, Butler, Kelleway, Dittmann, Fest, King, Macreadie, Mitchell, Newnham, Ola, Owers and Welti2022b) (Table 2). Feasible restoration actions mesh with the regional context of restoration practice and are therefore context specific; creating shared goals across diverse stakeholders can underpin feasibility and successful implementation in this regard (Surgeon Rogers et al., Reference Surgeon Rogers, Kroeger, Gonneea, Abdul-Aziz, Tang, Moseman-Valtierra, Windham-Myers, Crooks and Troxler2019).
Table 2. A non-exhaustive list of example methods and applicable case studies for restoration of coastal wetlands that may lead to climate cooling benefits

(Broome et al., Reference Broome, Seneca and Woodhouse1988; O’Brien and Zedler, Reference O’Brien and Zedler2006; Ray, Reference Ray2007; Zamith and Scarano, Reference Zamith and Scarano2010; Miller and Fujii, Reference Miller, Fujii and Contreras2011; Orth et al., Reference Orth, Moore, Marion, Wilcox and Parrish2012; Kroeger et al., Reference Kroeger, Crooks, Moseman-Valtierra and Tang2017; Masselink et al., Reference Masselink, Hanley, Halwyn, Blake, Kingston, Newton and Williams2017; Gamble et al., Reference Gamble, Debney, Glover, Bertelli, Green, Hendy, Lilley, Nuuttila, Potouroglou, Ragazzola, Unsworth and Preston2021; Hudson and Kenworthy, Reference Hudson, Kenworthy and Best2021; Manning et al., Reference Manning, Scott and Leegwater2021; Sinclair et al., Reference Sinclair, Sherman, Statton, Copeland, Matthews, Waycott, van Dijk, Vergés, Kajlich, McLeod and Kendrick2021; Claasens et al., Reference Claasens, Adams, de Villiers, Wasserman and Whitfield2022; Cormier et al., Reference Cormier, Krauss, Demopoulos, Jessen, McClain-Counts, From, Flynn, Krauss, Zhu and Stagg2022; Eagle et al., Reference Eagle, Kroeger, Spivak, Wang, Tang, Abdul-Aziz, Ishtiaq, O’Keefe Suttles and Mann2022; Mossman et al., Reference Mossman, Pontee, Born, Hill, Lawrence, Rae, Scott, Serato, Sparkes, Sullivan and Dunk2022; van Bijsterveldt et al., Reference van Bijsterveldt, Debrot, Bouma, Maulana, Pribadi, Schop, Tonneijck and van Wesenbeeck2022; Windham-Myers et al., Reference Windham-Myers, Oikawa, Deverel, Chapple, Drexler and Stern2023; Fard et al., Reference Fard, Brown, Ambrose, Whitcraft, Thorne, Kemnitz, Hammond and MacDonald2024)
Permanence
Coastal wetland restoration is effective as NCS when cooling benefits are ‘permanent’ over management-relevant timescales. These timescales should be explicitly defined. Here, we propose decades are the appropriate permanence timescale, to align with 2050 emissions reduction targets (United Nations Framework Commission on Climate Change (UNFCCC), 2015; Intergovernmental Panel on Climate Change (IPCC), 2022). Permanence over management-relevant timescales occurs when restored sites are resilient to relative sea-level rise over the next several decades. Regional controls on sediment type and availability influence the capacity for wetland vertical accretion of allochthonous material, and therefore resilience (Rovai et al., Reference Rovai, Twilley, Castañeda-Moya, Riul, Cifuentes-Jara, Manrow-Villalobos, Horta, Simonassi, Fonseca and Pagliosa2018; Gorham et al., Reference Gorham, Lavery, Kelleway, Masque and Serrano2021; Breithaupt and Steinmuller, Reference Breithaupt and Steinmuller2022). Resilient restoration will balance rates of relative sea-level rise and sediment supply to be successful; restoration at low elevation sites where sediment supply is low risks failure as vegetation may be rapidly overwhelmed by rising sea levels or erosion from wave action. However, where sediment supply is ample, restoration at lower elevations may still be successful as rapid gains in elevation and carbon addition from root biomass may occur (Liu et al., Reference Liu, Fagherazzi and Cui2021; Mossman et al., Reference Mossman, Pontee, Born, Hill, Lawrence, Rae, Scott, Serato, Sparkes, Sullivan and Dunk2022). Permanence can also occur where autochthonous production is high, particularly in more biogenic/organogenic settings (Krauss et al., Reference Krauss, Cormier, Osland, Kirwan, Stagg, Nestlerode, Russell, From, Spivak, Dantin, Harvey and Almario2017; Cahoon et al., Reference Cahoon, McKee and Morris2021; Windham-Myers et al., Reference Windham-Myers, Oikawa, Deverel, Chapple, Drexler and Stern2023). Restored coastal wetlands do not need to depend on vertical processes alone for decadal-scale permanence. Where geomorphic development has led to available accommodation space and land use is amenable, lateral migration into upland or upstream habitats can allow continued cooling benefits of coastal wetland restoration activity even where vertical elevation-building processes are expected to be overwhelmed (Osland et al., Reference Osland, Chivoiu, Enwright, Thorne, Guntenspergen, Grace, Dale, Brooks, Herold, Day, Sklar and Swarzenzki2022; Owers et al., Reference Owers, Woodroffe, Mazumder and Rogers2022; Rogers et al., Reference Rogers, Zawadzki, Mogensen and Saintilan2022; Wang et al., Reference Wang, Krauss, Noe, Dai and Trettin2023).
Perhaps of more immediate concern regarding restored site permanence are short-term disturbances, such as stochastic storm impacts and anthropogenic pressures on restored coastal wetlands (Hanley et al., Reference Hanley, Bouma and Mossman2020; Newton et al., Reference Newton, Icely, Cristina, GME, Turner, Ashan, Cragg, Luo, Tu, Li, Zhang, Ramesh, Forbes, Solidoro, Béjaoui, Gao, Pastres, Kelsey, Taillie, Nhan, Brito, de Lima and Kuenzer2020). Minimizing the risk of such short-term perturbation will support permanence. If short-term perturbation risks can be minimized, sites with high sediment supply, large tide ranges, high rates of foundation species primary productivity, shallow elevation gradients, and harmonious upslope land use may both accumulate carbon rapidly and be resilient to future sea-level rise, retaining carbon in the long term (Cahoon et al., Reference Cahoon, McKee and Morris2021; Osland et al., Reference Osland, Chivoiu, Enwright, Thorne, Guntenspergen, Grace, Dale, Brooks, Herold, Day, Sklar and Swarzenzki2022; Saintilan et al., Reference Saintilan, Kovalenko, Guntenspergen, Rogers, Lynch, Cahoon, Lovelock, Friess, Ashe, Krauss, Cormier, Spencer, Adams, Raw, Ibanez, Scarton, Temmerman, Meire, Maris, Thorne, Brazner, Chmura, Bowron, Gamage, Cressman, Endris, Marconi, Marcum, St. Laurent, Reay, Raposa, Garwood and Khan2022). Overall, restoration may be most successful at achieving permanence when targeting areas where intertidal surfaces can readily adjust vertically and/or laterally through a combination of allochthonous and autochthonous processes, ensuring resilience through 2050. Projects that do submerge from relative sea-level rise after management-relevant timescales can still have important mitigation contributions over the next several decades.
Local factors
Whether or not the fundamental requirements of additionality, feasibility and permanence are met by a restoration action is largely set by global and regional-scale factors. However, local-scale factors including restoration design and the identity/abundance of biota can enhance or detract from site-specific restoration effectiveness as NCS. Restoration design decisions can determine channel density and flow path, wetland elevation and inundation, and vegetation cover and community identity through planting or natural colonization approaches (Lester et al., Reference Lester, Dubel, Hernán, McHenry and Rassweiler2020; Vanderklift et al., Reference Vanderklift, Doropoulos, Gorman, Leal, Minne, Statton, Steven and Wernberg2020; Valach et al., Reference Valach, Kasak, Hemes, Szutu, Verfaillie, Baldocchi, Krauss, Zhu and Stagg2021), all of which can influence the net cooling benefit of restoration compared to initial conditions. Research exploring the impact that coastal wetland restoration design decisions have on restored site effectiveness as NCS could expand on what little is currently known about which designs maximize carbon sequestration. These studies may be especially informative if they focus explicitly on how design options influence additionality, feasibility and permanence.
Local interactions of environmental conditions with biota, including macrophytes, macrofauna and microbes, can influence restoration effectiveness as NCS as well. Vegetation influence on local-scale carbon dynamics is becoming better characterized (Jones et al., Reference Jones, Stagg, Krauss and Hester2018; Mueller et al., Reference Mueller, Mozdzer, Langley, Aoki, Noyce and Megonigal2020; Kennedy et al., Reference Kennedy, Pagès, Lagomasino, Arias‐Ortiz, Colarusso, Fourqurean, Githaiga, Howard, Krause‐Jensen, Kuwae, Lavery, Macreadie, Marbà, Masqué, Mazarrasa, Miyajima, Serrano and Duarte2022; Kong et al., Reference Kong, Ryu, Liu, Dechant, Rey-Sanchez, Shortt, Szutu, Verfaillie, Houborg and Baldocchi2022; Jeffrey et al., Reference Jeffrey, Moras, Tait, Johnston, Call, Sippo, Jeffrey, Laicher-Edwards and Maher2023), although current work is often less clear on the precise mechanisms of plant-mediation of carbon processes (but see Vroom et al., Reference Vroom, van den Berg, Pangala, van der Scheer and Sorrell2022). Foundation plant species often establish quickly after restoration, jump-starting wetland carbon uptake, but this is not universally true where foundation species are large and/or slow growing (Marbà et al., Reference Marbà, Duarte, Cebrian, Gallegos, Olesen and Sand-Jensen1996; Ballanti et al., Reference Ballanti, Byrd, Woo and Ellings2017). Less well known are macrofaunal influences on restoration effectiveness as NCS. A growing body of literature has emphasized the importance of crab bioturbation on carbon loss in tidal marshes and mangroves, for example, via changes in sediment permeability/exchange and microbial communities, among other mechanisms (Gutiérrez et al., Reference Gutiérrez, Jones, Groffman, Findlay, Iribarne, Ribeiro and Bruschetti2006; Guimond et al., Reference Guimond, Seyfferth, Moffett and Michael2020; Xiao et al., Reference Xiao, Wilson, Li, Santos, Tamborski, Smith, Lang, Zheng, Luo, Lu and Correa2021; Qin et al., Reference Qin, Lu, Gan, Zhang, Wu, Sanders, He, Yu, Zhang, Zhou, Ding, Huang, Chen, He, Yu, Li and Wang2024; Smith, Reference Smith2024). Microbial processes, dependent upon the abundance and identity of microbial communities, vary at small spatial scales and are strongly influenced by tidal inundation and associated abiotic factors (e.g., water content, salinity, oxygen and nutrient availability; Cheung et al., Reference Cheung, Wong, Chu and Kwan2018; Rinke et al., Reference Rinke, Maraun and Scheu2022). Following wetland restoration, changes can occur in fungal communities as well, as the ecosystem matures into a marine setting (Walker and Campbell, Reference Walker and Campbell2010; Dini-Andreote et al., Reference Dini-Andreote, Pylro, Baldrian, van Elsas and Salles2016). Microbial communities may have a strong impact on restoration effectiveness as NCS by exerting a key influence on carbon cycling processes important for cooling benefits (e.g., methanogenesis and methane oxidation; Oremland and Polcin, Reference Oremland and Polcin1982; Segarra et al., Reference Segarra, Comerford, Slaughter and Joye2013; Capooci et al., Reference Capooci, Seyfferth, Tobias, Wozniak, Hedgpeth, Bowen, Biddle, McFarlane and Vargas2024). Additional studies that explore how organism presence and abundance impact a site’s capacity to meet the fundamental NCS requirements within the range set by climate and geomorphology would be helpful.
Future perspectives
Coastal wetland restoration will be most effective as NCS where additionality, feasibility and permanence are maximized. Verifying these requirements are met in an ecological context (Table 1) is more straightforward than the complex task of quantifying the magnitude of project-specific cooling benefits for carbon finance or accounting purposes. Issues with quantifying magnitudes of climate benefit are not addressed here, as we focus below on the issues impeding initial deliberation of whether restoration has a net climate benefit, the crucial point for restoration implementation.
Coastal wetlands as cross-ecosystem linkages
One issue in understanding when and where coastal restoration actions are effective NCS is uncertainty in additionality for ecosystems that are interfaces and integrators of terrestrial and aquatic habitats. It is sometimes unclear if a restoration project meets the fundamental requirement of having a net cooling benefit when those cooling benefits can occur in habitats outside of project footprints. For example, connectivity between restored sites and surrounding landscapes can be an important driver of the carbon cycling benefits of restoration (Woo et al., Reference Woo, Davis, De La Cruz, Windham-Myers, Drexler, Byrd, Stuart-Haëntjens, Anderson, Bergamaschi, Nakai, Ellings, Hodgson, Krauss, Zhu and Stagg2022; Mazarrasa et al., Reference Mazarrasa, Neto, Bouma, Grandjean, Garcia-Orellana, Masqué, Recio, Serrano, Puente and Juanes2023). Allochthonous material, in particular, can be buried at substantial rates upon initial restoration in salt marshes (Wollenberg et al., Reference Wollenberg, Ollerhead and Chmura2018; Mossman et al., Reference Mossman, Pontee, Born, Hill, Lawrence, Rae, Scott, Serato, Sparkes, Sullivan and Dunk2022). The reduced water movement through seagrass meadow canopies (Peralta et al., Reference Peralta, van Duren, Morris and Bouma2008) not only facilitates high retention of autochthonous production, but also results in increased deposition of allochthonous carbon (Fonseca and Fisher, Reference Fonseca and Fisher1986; Hendriks et al., Reference Hendriks, Sintes, Bouma and Duarte2008), estimated to contribute to ~50% of the sediment organic C pool in these meadows on average (Kennedy et al., Reference Kennedy, Beggins, Duarte, Fourqurean, Holmer, Marbà and Middelburg2010). Tidal forests can also trap substantial amounts of allochthonous material (e.g., Noe et al., Reference Noe, Hupp, Bernhardt and Krauss2016). However, there remains uncertainty on whether allochthonous carbon removed from the atmosphere upstream or upslope and then buried in a coastal wetland should be considered part of the cooling benefit from the restoration action. Similarly, there remains uncertainty on if autochthonous carbon that is removed from the atmosphere in a coastal wetland restoration area and then exported laterally to the near-shore environment with potential long-term storage (especially as dissolved inorganic carbon or total alkalinity; Santos et al., Reference Santos, Maher, Larkin, Webb and Sanders2019, Reference Santos, Burdige, Jennerjahn, Bouillon, Cabral, Serrano, Wernberg, Filbee-Dexter, Guimond and Tamborski2021; Yau et al., Reference Yau, Xin, Chen, Zhan, Call, Conrad, Sanders, Li, Du and Santos2022; Reithmaier et al., Reference Reithmaier, Cabral, Akhand, Bogard, Borges, Bouillon, Burdige, Call, Chen, Chen, Cotovicz, Eagle, Kristensen, Kroeger, Lu, Maher, Pérez-Lloréns, Ray, Taillardat, Tamborski, Upstill-Goddard, Wang, Wang, Xiao, Yau and Santos2023) should be considered part of the cooling benefit. Ignoring these lateral connections can affect the estimated cooling benefit of a restoration action, potentially influencing if projects meet the fundamental requirement of additionality (Bogard et al., Reference Bogard, Bergamaschi, Butman, Anderson, Knox and Windham‐Myers2020; Schutte et al., Reference Schutte, Moore, Wilson and Joye2020; Correa et al., Reference Correa, Xiao, Conrad, Wadnerkar, Wilson, Sanders and Santos2022).
To address this issue, one approach is to take a landscape/systems view for determining if a specific management action will lead to a net cooling benefit (Figure 2), regardless of the spatial footprint that benefit occurs in (similar to the efforts underway for landscape-scale carbon accounting; Glass et al., Reference Glass, Emmer, Howard and Tonneijck2024). In other words, tracking the response of a landscape (e.g., a watershed) to management actions, not the response of one habitat type to management actions. Lateral export of carbon that is buried (or emitted) outside of the restoration site should contribute to understanding a wetland’s cooling benefit compared to initial conditions, as long as the export would not have occurred without restoration action. In the case of greenhouse gas emissions, particular care must be taken to ensure appropriate baseline comparisons that consider surrounding land uses as potential sources contributing to wetland fluxes (e.g., N2O from prairie pothole wetlands; Tangen and Bansal, Reference Tangen and Bansal2022), to avoid penalizing wetlands as the spot where allochthonous carbon enters the atmosphere even when the land management decisions driving carbon and nutrient export and mineralization are made upslope or upstream. For allochthonous carbon burial, if the accommodation space created by restoration allows the preservation of carbon that would have otherwise been mineralized, that leads to a cooling benefit even if the carbon was removed from the atmosphere offsite (Wollenberg et al., Reference Wollenberg, Ollerhead and Chmura2018). We acknowledge that taking a landscape approach to the cooling benefit of coastal wetland restoration may be difficult in practice, as mass balance approaches are most tractable at site-level scales. However, this approach may allow a more holistic understanding of additionality and cooling benefit from restoration actions in coastal wetlands, incorporating the true connectivity of these habitats as cross-ecosystem linkages.

Figure 2. Land-use types of interest to carbon sequestration and/or GHG mitigation across the relative tidal elevation range in Suisun Bay and Delta lands. Corn indicates conventional row crops. Tidal channel refers to open-water aquatic habitats, whether deep or shallow (such as flooded islands) and which may be populated by submerged or floating aquatic vegetation (SAV and FAV). Permanently flooded wetland refers to wetlands impounded to reverse subsidence. Seasonal wetland refers to wetlands managed via freshwater flooding to benefit wildlife. Credit: Illustrated by Vincent Pascual, California Office of State Publishing, adapted from SFEI.
Coastal wetland migration
Another issue in understanding when and where coastal restoration actions are effective NCS is uncertainty in permanence for ecosystems that are dynamic on the landscape. As they cope with accelerating sea-level rise, coastal wetlands have the potential to migrate both upslope and upstream over management-relevant timeframes (Krauss et al., Reference Krauss, Noe, Duberstein, Conner, Stagg, Cormier, Jones, Bernhardt, Graeme Lockaby, From, Doyle, Day, Ensign, Pierfelice, Hupp, Chow and Whitbeck2018; Gedan and Fernández‐Pascual, Reference Gedan and Fernández‐Pascual2019; Osland et al., Reference Osland, Chivoiu, Enwright, Thorne, Guntenspergen, Grace, Dale, Brooks, Herold, Day, Sklar and Swarzenzki2022; Wang et al., Reference Wang, Krauss, Noe, Dai and Trettin2023). Restored wetlands may therefore move out of the original project footprint over time, making it difficult to estimate the longevity of cooling benefits from management actions. Restored coastal wetlands may submerge under relative sea-level rise rates above ~5–7 mm/year (Saintilan et al., Reference Saintilan, Kovalenko, Guntenspergen, Rogers, Lynch, Cahoon, Lovelock, Friess, Ashe, Krauss, Cormier, Spencer, Adams, Raw, Ibanez, Scarton, Temmerman, Meire, Maris, Thorne, Brazner, Chmura, Bowron, Gamage, Cressman, Endris, Marconi, Marcum, St. Laurent, Reay, Raposa, Garwood and Khan2022; Morris et al., Reference Morris, Langley, Vervaeke, Dix, Feller, Marcum and Chapman2023), converting to unvegetated flats (Haywood et al., Reference Haywood, Hayes, White and Cook2020; Schoolmaster et al., Reference Schoolmaster, Stagg, Creamer, Laurenzano, Ward, Waldrop, Baustian, Aw, Merino, Villani and Scott2022). The fate of wetland carbon cycling with such state change and concurrent potential erosion is not clear (Creamer et al., Reference Creamer, Waldrop, Stagg, Manies, Baustian, Laurenzano, Aw, Haw, Merino, Schoolmaster, Sevilgen, Villani and Ward2024), but cooling benefit losses may be at least partially offset by cooling benefit gains as upslope or upstream habitats are converted to new wetlands (Osland et al., Reference Osland, Chivoiu, Enwright, Thorne, Guntenspergen, Grace, Dale, Brooks, Herold, Day, Sklar and Swarzenzki2022; Wang et al., Reference Wang, Krauss, Noe, Dai and Trettin2023). In certain regions with large areas of accommodation space, in fact, relative sea-level rise may increase the total habitat of coastal wetlands (Schieder et al., Reference Schieder, Walters and Kirwan2018), although newly colonized habitat from upslope migration may initially have lower soil carbon accumulation rates than mature habitat (Sandi et al., Reference Sandi, Rodriguez, Saco, Saintilan and Riccardi2021). The resilience of a restored wetland to submergence over management-relevant decadal scales, including the availability of accommodation space to migrate upslope or upstream, therefore becomes of prime importance.
To address this issue, a landscape/systems approach again may be helpful (Figure 2), but for permanence: not tying permanence of restoration cooling benefit to a specific spatial footprint, unless human constraints on wetland migration preclude movement of carbon benefits across the landscape. This approach incorporates the natural dynamism of coastal wetlands and the reality of complex landscapes. Dealing with disturbance and dynamism is not new for habitats used as NCS: forests also experience disturbances like wildfire that can release stored carbon (Hurteau et al., Reference Hurteau, Koch and Hungate2008). Unlike forests experiencing fire, it is possible that stored carbon from submerging wetlands can continue to be stored with lateral export to shallow ocean shelves (Santos et al., Reference Santos, Burdige, Jennerjahn, Bouillon, Cabral, Serrano, Wernberg, Filbee-Dexter, Guimond and Tamborski2021). Existing remote sensing tools and analyses can help to identify priority areas where restoration could be resilient and extend the lifetime of restored wetlands undergoing relative sea-level rise impacts (e.g., Rogers et al., Reference Rogers, Lal, Asbridge and Dwyer2023b; Ganju et al., Reference Ganju, Ackerman and Defne2024). Taking a landscape approach to permanence provides an opportunity to move beyond single--habitat focused restoration, thus aggregating restoration action influence across the landscape (Thorslund et al., Reference Thorslund, Jarsjo, Jaramillo, Jawitz, Manzoni, Basu, Chalov, Cohen, Creed, Goldenberg, Hylin, Kalantari, Koussis, Lyon, Mazi, Mard, Persson, Pietro, Prieto, Quin, Van Meter and Destouni2017). Framing permanence in a management-relevant context (i.e., the next few decades) addresses the concern that some wetlands will submerge in the future (especially by the end of the century; Saintilan et al., Reference Saintilan, Kovalenko, Guntenspergen, Rogers, Lynch, Cahoon, Lovelock, Friess, Ashe, Krauss, Cormier, Spencer, Adams, Raw, Ibanez, Scarton, Temmerman, Meire, Maris, Thorne, Brazner, Chmura, Bowron, Gamage, Cressman, Endris, Marconi, Marcum, St. Laurent, Reay, Raposa, Garwood and Khan2022); having enhanced uptake in the next few decades can buy time for more robust climate solutions even if additionality somewhat decreases with migration. Ultimately, a landscape approach prevents focusing on the storage of a particular molecule in a particular place, shifting perspective to the overall cooling benefit of a management action.
Methane and management-relevant timeframes
Uncertainty in how to incorporate methane emissions is another issue preventing understanding of when and where coastal restoration actions are effective NCS. Methane is a potent but short-lived greenhouse gas (Neubauer and Megonigal, Reference Neubauer and Megonigal2015), and becomes a crucial component of the cooling benefit of restoration actions given decadal management-relevant timescales. Regardless of methane emissions, wetlands commonly exhibit climate cooling impacts on geologic timescales (Frolking et al., Reference Frolking, Roulet and Fuglestvedt2006; Neubauer and Megonigal, Reference Neubauer and Megonigal2015); on management-relevant timescales, however, methane emissions can significantly influence the efficacy of restoration activities as NCS (Schutte et al., Reference Schutte, Moore, Wilson and Joye2020; Arias-Ortiz et al., Reference Arias-Ortiz, Oikawa, Carlin, Masqué, Shahan, Kanneg, Paytan and Baldocchi2021b). Microbial communities responding to environmental conditions control the balance between methane production (methanogenesis) and consumption (methanotrophy/oxidation) in soils, as they break down organic matter for energy (Oremland and Polcin, Reference Oremland and Polcin1982; Segarra et al., Reference Segarra, Comerford, Slaughter and Joye2013; Capooci et al., Reference Capooci, Seyfferth, Tobias, Wozniak, Hedgpeth, Bowen, Biddle, McFarlane and Vargas2024; Hartman et al., Reference Hartman, Bueno de Mesquita, Theroux, Morgan-Lang, Baldocchi and Tringe2024). There is broad agreement that salinity (often used as a proxy for sulfate concentrations) decreases methane emissions in coastal wetlands, even if mechanisms remain uncertain (Bartlett et al., Reference Bartlett, Bartlett, Harriss and Sebacher1987; Poffenbarger et al., Reference Poffenbarger, Needelman and Megonigal2011; Bridgham et al., Reference Bridgham, Cadillo-Quiroz, Keller and Zhuang2013; Chuang et al., Reference Chuang, Young, Dale, Miller, Herrera-Silveira and Paytan2016; Rosentreter et al., Reference Rosentreter, Al‐Haj, Fulweiler and Williamson2021; Sanders-DeMott et al., Reference Sanders-DeMott, Eagle, Kroeger, Wang, Brooks, O-Keefe Suttles, Nick, Mann and Tang2022). Dominant plant communities can also control methane emissions, through plant-mediated gas fluxes. These fluxes can make up the dominant pathway of methane emissions to the atmosphere in coastal wetlands, as methane vents through herbaceous or woody tissues (Jeffrey et al., Reference Jeffrey, Maher, Johnston, Kelaher, Steven and Tait2019; Mueller et al., Reference Mueller, Mozdzer, Langley, Aoki, Noyce and Megonigal2020; Villa et al., Reference Villa, Ju, Stephen, Rey‐Sanchez, Wrighton and Bohrer2020; Comer-Warner et al., Reference Comer-Warner, Ullah, Ampuero Reyes, Krause and Chmura2022). Finally, it is becoming clear that lateral export of dissolved methane is a potentially important, but under-recognized, methane flux pathway (Santos et al., Reference Santos, Maher, Larkin, Webb and Sanders2019; Schutte et al., Reference Schutte, Moore, Wilson and Joye2020; Chen et al., Reference Chen, Santos, Hu, Zhan, Zhang, Zhao, Hu and Li2022; Wang et al., Reference Wang, Sadat-Noori and Glamore2022b). Especially in low-salinity conditions and/or with high productivity of wetland-adapted plants, methane can complicate understanding if restoration actions meet the basic requirement of NCS of having a net cooling benefit.
Incorporating methane emissions is crucial, but may be most helpful within the context of coastal restoration as NCS when focused on the cooling benefit of specific management actions. Methane emissions are not inherently bad, and productive low-salinity restored sites with high methane emissions may still provide large cooling benefits compared to pre-restoration conditions (Hemes et al., Reference Hemes, Chamberlain, Eichelmann, Anthony, Valach, Kasak, Szutu, Verfaillie, Silver and Baldocchi2019; Günther et al., Reference Günther, Barthelmes, Huth, Joosten, Jurasinski, Koebsch and Couwenberg2020; Arias-Ortiz et al., Reference Arias-Ortiz, Oikawa, Carlin, Masqué, Shahan, Kanneg, Paytan and Baldocchi2021b; Nyberg et al., Reference Nyberg, Black, Ketler, Lee, Johnson, Merkens, Nugent and Knox2022; Adame et al., Reference Adame, Kelleway, Krauss, Lovelock, Adams, Trevathan-Tackett, Noe, Jeffrey, Ronan, Zann, Carnell, Iram, Maher, Murdiyarso, Sasmito, Tran, Dargusch, Kauffman and Brophy2024). Methane emissions are sometimes measured in restored coastal wetlands, but often pre-restoration baseline data or data from analog/alternative land use sites are lacking, preventing an understanding of the net change in methane emissions and overall cooling benefit attributable to restoration actions. Therefore, effective methane monitoring includes data collection at alternative land use sites and begins pre-restoration where possible. Additionally, coordinated synthesis activities can help in gathering, making available, and interpreting the rapidly accumulating greenhouse gas flux datasets from blue carbon ecosystems, especially for marshes and mangroves (Knox et al., Reference Knox, Jackson, Poulter, McNicol, Fluet-Chouinard, Zhang, Hugelius, Bousquet, Canadell, Saunois, Papale, Chu, Keenan, Baldocchi, Torn, Mammarella, Trotta, Aurela, Bohrer, Campbell, Cescatti, Chamberlain, Chen, Chen, Dengel, Desai, Euskirchen, Friborg, Gasbarra, Goded, Goeckede, Heimann, Helbig, Hirano, Hollinger, Iwata, Kang, Klatt, Krauss, Kutzbach, Lohila, Mitra, Morin, Nilsson, Niu, Noormets, Oechel, Peichl, Peltola, Reba, Richardson, Runkle, Ryu, Sachs, Schafer, Schmid, Shurpali, Sonnentag, Tang, Ueyama, Vargas, Vesala, Ward, Windham-Myers, Wolhfahrt and Zona2019; Rosentreter et al., Reference Rosentreter, Laruelle, Bange, Bianchi, Busecke, Cai, Eyre, Forbrich, Kwon, Maavara, Moosdorf, Najjar, Sarma, Van Dam and Regnier2023; Arias-Ortiz et al., Reference Arias-Ortiz, Wolfe, Bridgham, Knox, McNicol, Needelman and Holmquist2024). Seagrasses pose a particular challenge here, as they exchange dissolved inorganic carbon with the water column rather than carbon dioxide directly with the atmosphere. Coordinated synthesis of benthic, air–water, and lateral fluxes in seagrass ecosystems, including methane, can provide needed insight into their restoration benefit as NCS, as with lateral fluxes in blue carbon ecosystems more generally (Santos et al., Reference Santos, Burdige, Jennerjahn, Bouillon, Cabral, Serrano, Wernberg, Filbee-Dexter, Guimond and Tamborski2021). Incorporating methane emissions over management-relevant timeframes (e.g., by using sustained-flux global warming potential for a 20-year time horizon; Neubauer, Reference Neubauer2021) without forgetting that methane emissions do not inherently preclude effectiveness as NCS can help to move the field toward inclusion of all tidal wetlands that may provide climate mitigation benefits (Adame et al., Reference Adame, Kelleway, Krauss, Lovelock, Adams, Trevathan-Tackett, Noe, Jeffrey, Ronan, Zann, Carnell, Iram, Maher, Murdiyarso, Sasmito, Tran, Dargusch, Kauffman and Brophy2024).
Minimum data requirements
Information supporting the likelihood that a project will, at a minimum, lead to a cooling benefit is a prerequisite for taking restoration action as NCS, but it is unclear if the magnitude of cooling benefits also needs to be quantified before action takes place. Modeling is often used as a tool for guiding restoration decision-making, but some projects do not require modeling approaches to understand the binary outcome of whether or not an action will have a cooling benefit (e.g., Twomey et al., Reference Twomey, Nunez, Carr, Crooks, Friess, Glamore, Orr, Reef, Rogers, Waltham and Lovelock2024). Coastal wetland restoration projects are already happening around the world without a modeled estimate of cooling benefit; this lack of carbon accounting does not influence whether or not a real climate benefit is occurring. In landscapes with multiple competing values, or where a high level of precision is needed, complex models are certainly required to understand if an action has a net benefit. In the cases where more complex modeling is required, several biogeochemical models designed for tidal wetlands enable the prediction of organic carbon accumulation, sediment accretion, and other carbon-related processes with changes in relative sea levels (Buffington et al., Reference Buffington, Janousek, Dugger, Callaway, Schile-Beers, Borgnis Sloane and Thorne2021; Morris et al., Reference Morris, Langley, Vervaeke, Dix, Feller, Marcum and Chapman2023; Vahsen et al., Reference Vahsen, Todd‐Brown, Hicks, Pilyugin, Morris and Holmquist2024). This particular scenario may be uncommon when considering all the locations where blue carbon restoration is likely to be successful globally. Where complex models are not required, there remains disagreement on the data necessary to understand project effectiveness as NCS. When plot-level data exist, an additional uncertainty is how best to use spatially explicit information to scale up to footprints relevant for projecting landscape-level response to restoration action (Duarte de Paula Costa et al., Reference Duarte de Paula Costa, Lovelock, Waltham, Moritsch, Butler, Power, Thomas and Macreadie2022; Matthes et al., Reference Matthes, Sturtevant, Verfaillie, Knox and Baldocchi2014; Shahan et al., Reference Shahan, Chu, Windham‐Myers, Matsumura, Carlin, Eichelmann, Stuart‐Haentjens, Bergamaschi, Nakatsuka, Sturtevant and Oikawa2022). Regardless of the complexity of data required, long-term post-implementation monitoring allows evaluating actual restoration project responses and ensures projects are meeting expectations and targets over time (Wortley et al., Reference Wortley, Hero and Howes2013; Cadier et al., Reference Cadier, Bayraktarov, Piccolo and Adame2020; Lovelock et al., Reference Lovelock, Barbier and Duarte2022c). A robust understanding of carbon cycling responses to restoration action is crucial for quantifying the total magnitude of cooling benefit, but where cooling benefit is not predicted to be large, it may be critical for understanding if a cooling benefit exists at all.
It may be useful to explicitly differentiate the minimum data requirements for coastal restoration as effective NCS (i.e., answering ‘does this management action accrue a climate benefit?’) from the data requirements for quantifying the magnitude of cooling benefits for carbon accounting purposes (i.e., answering ‘how much climate benefit does this management action accrue?’). There is a need for widely distributed, standardized minimum data that can be applied to address the former question (Table 1). Much of the minimum data needed is spatial in nature, as spatially explicit data are most useful to land managers and restoration practitioners for on-the-ground prioritization (Lovelock et al., Reference Lovelock, Adame, Butler, Kelleway, Dittmann, Fest, King, Macreadie, Mitchell, Newnham, Ola, Owers and Welti2022b; Rogers et al., Reference Rogers, Lal, Asbridge and Dwyer2023b). These spatial data include up-to-date maps of regional land use/land cover (Sleeter et al., Reference Sleeter, Liu, Daniel, Rayfield, Sherba, Hawbaker, Zhu, Selmants and Loveland2018, Reference Sleeter, Frid, Rayfield, Daniel, Zhu and Marvin2022), land ownership (Lovelock and Brown, Reference Lovelock and Brown2019), and topography (including human alterations that impede wetland migration; Osland et al., Reference Osland, Chivoiu, Enwright, Thorne, Guntenspergen, Grace, Dale, Brooks, Herold, Day, Sklar and Swarzenzki2022; Rogers et al., Reference Rogers, Lal, Asbridge and Dwyer2023b). Vegetation types in particular are often mappable, and may be crucial to up-scale data on climate benefits using remote sensing observations (e.g., Kong et al., Reference Kong, Ryu, Liu, Dechant, Rey-Sanchez, Shortt, Szutu, Verfaillie, Houborg and Baldocchi2022). Other minimum data requirements are not explicitly spatial (but may still vary regionally), including carbon balance or emissions/removal factor estimates for land use/land cover classes (from direct measurements or model outputs; Hagger et al., Reference Hagger, Waltham and Lovelock2022; Windham-Myers et al., Reference Windham-Myers, Oikawa, Deverel, Chapple, Drexler and Stern2023), estimates of restoration cost per area restored given the prevailing restoration culture and financial incentive (Taillardat et al., Reference Taillardat, Thompson, Garneau, Trottier and Friess2020; Hagger et al., Reference Hagger, Waltham and Lovelock2022), and resilience to relative sea-level rise (Holmquist et al., Reference Holmquist, Brown and MacDonald2021; Ganju et al., Reference Ganju, Ackerman and Defne2024). Further, communicating and collaborating with local communities to ensure stakeholder involvement and equitable distribution of restoration co-benefits is key in any project (Surgeon Rogers et al., Reference Surgeon Rogers, Kroeger, Gonneea, Abdul-Aziz, Tang, Moseman-Valtierra, Windham-Myers, Crooks and Troxler2019; Dencer-Brown et al., Reference Dencer-Brown, Shilland, Friess, Herr, Benson, Berry, Cifuentes-Jara, Colas, Damayanti, García, Gavaldão, Grimsditch, Hejnowicz, Howard, Islam, Kennedy, Kivugo, Lang’at, Lovelock, Malleson, Macreadie, Andrade-Medina, Mohamed, Pidgeon, Ramos, Rosette, Salim, Schoof, Talukder, Thomas, Vanderklift and Huxham2022; Lovelock et al., Reference Lovelock, Barbier and Duarte2022c). Beyond these suggested standard data types, additional project-specific considerations that impact additionality, feasibility and permanence will arise. If minimum data requirements are unavailable in a region, that helps prioritize new data collection efforts. One way to fill gaps for areas without the minimum required data is to leverage areas with more intensive data. Using regional-scale data stratified by local-scale gradients like elevation, for example, can provide a path forward for estimating the value of restoration from a carbon perspective (Wang et al., Reference Wang, Dai, Trettin, Krauss, Noe, Burton, Stagg and Ward2022a; Lovelock et al., Reference Lovelock, Adame, Butler, Kelleway, Dittmann, Fest, King, Macreadie, Mitchell, Newnham, Ola, Owers and Welti2022b; Windham-Myers et al., Reference Windham-Myers, Oikawa, Deverel, Chapple, Drexler and Stern2023; Yando et al., Reference Yando, Jones, James, Colombano, Montemayor, Nolte, Raw, Ziegler, Chen, Daffonchio, Fusi, Rogers and Sergienko2023). Understanding the magnitude of cooling benefits for accounting purposes is a crucial, but distinct, second step in the process of addressing effectiveness of coastal wetland restoration as NCS. We posit that confounding these distinct questions can impede implementation of restoration projects that are likely to have a climate benefit.
Conclusion
Here, we synthesize the fundamental requirements of additionality, feasibility and permanence to address the question, ‘when and where is coastal wetland restoration effective as a natural climate solution?’ Maximizing the values underpinning these three key factors can increase the effectiveness of restoration projects, for example, by targeting regions with large areas of degraded habitat that will net a substantial climate cooling benefit when restored (additionality); where socio-economic and governance factors are in place to support action (feasibility); and where there is high resilience to future relative sea-level rise (permanence). Recent work is leading the way for effective site-level prioritization (Rogers et al., Reference Rogers, Macreadie, Kelleway and Saintilan2019b; Moritsch et al., Reference Moritsch, Young, Carnell, Macreadie, Lovelock, Nicholson, Raimondi, Wedding and Ierodiaconou2021; Duarte de Paula Costa et al., Reference Duarte de Paula Costa, Lovelock, Waltham, Moritsch, Butler, Power, Thomas and Macreadie2022; Rogers et al., Reference Rogers, Lal, Asbridge and Dwyer2023b). To move toward successful implementation at scale, we highlight paths forward on several issues impeding confidence in coastal wetland restoration as NCS. First, tracking the cooling benefit of specific management actions across the interconnected coastal landscape, not project-specific spatial footprints. Second, the importance of incorporating methane into restoration considerations, as effective NCS will function over management-relevant decadal timescales. Finally, the minimum data required to understand if an action has a climate benefit is likely more tractable than the data required to understand the separate issue of quantifying the magnitude of climate benefit. Ultimately, for maximal NCS effectiveness, energy and resources will be focused on prioritizing sites with high additionality, where restoration actions are feasible and where permanence is likely. We stress that within this framework, coastal wetland restoration provides immense benefits beyond mitigating climate change (Vegh et al., Reference Vegh, Pendleton, Murray, Troxler, Zhang, Castañeda-Moya, Guannel and Sutton-Grier2019; Pindilli, Reference Pindilli, Krauss, Zhu and Stagg2022; Hambäck et al., Reference Hambäck, Dawson, Geranmayeh, Jarsjö, Kačergytė, Peacock, Collentine, Destouni, Futter, Hugelius, Hedman, Jonsson, Klatt, Lindström, Nilsson, Pärt, Schneider, Strand, Urrutia-Cordero, Åhlén, Åhlén and Blicharska2023). There are strong calls for ecosystem restoration over the next decade (United Nations Environment Programme (UNEP), 2021). Reducing uncertainty can help to ensure that coastal restoration actions deliver climate cooling benefits within the decadal timeframes necessary to function as one climate mitigation strategy among many.
Open peer review
To view the open peer review materials for this article, please visit http://doi.org/10.1017/cft.2024.14.
Data availability statement
No data were used in the preparation of this manuscript.
Acknowledgments
Any use of trade, firm or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. We thank the editor and anonymous reviewers for their comments that improved the manuscript.
Author contribution
S.F.J. led the conceptualization of the manuscript with substantial input from co-authors. S.F.J. led the writing of the original drafts with contribution from co-authors. All authors contributed to revision and review.
Financial support
A.A.-O. acknowledges support from MCIN/AEI RYC2021-034455-I. D.B. acknowledges support by the Delta Science Stewardship Council and the US Department of Energy Ameriflux project. C.L.S. and K.T. acknowledge support by the U.S. Geological Survey Ecosystem Mission Area. E.J.W. acknowledges support from the NASA Terrestrial Ecology Program and Carbon Monitoring System that support the BlueFlux field campaign and related efforts.
Competing interest
The authors declare none.







Comments
Dr. Tom Spencer
Editor-in-Chief
Cambridge Prisms: Coastal Futures
Dr. Spencer,
I am pleased to submit, “When and where can coastal wetland restoration increase carbon sequestration as a natural climate solution” as an invited review in Cambridge Prisms: Coastal Futures. In this manuscript we briefly introduce the concept of coastal wetlands as natural climate solutions (NCS), synthesize the literature into three fundamental requirements for effective coastal wetland restoration as NCS, and provide paths forward on uncertainties that are currently impeding deployment of restoration as one tool in the climate mitigation toolbox. I believe this will be a timely article with a future-facing vision that readers will find compelling.
Thank you for your patience as we developed this manuscript, and to you and your team (especially Laetitia Beck and Jess Jones) for insight and communication during the process. Please let me know if there is any additional information required or missing in our submission, and thank you again for the invitation and for your consideration.
Best,
Scott F. Jones, Ph.D.
Assistant Professor, Department of Biology
University of North Florida
Jacksonville, FL, USA
[email protected]; +1-904-620-3029