To the Editor—Mechanical ventilation is part of the supportive care arsenal used for patients admitted to intensive care units (ICUs). Currently, with the worldwide coronavirus disease 2019 (COVID-19) pandemic, many patients present severe pulmonary symptoms, and the use of mechanical ventilation has increased dramatically. Reference Grasselli, Zangrillo and Zanella1 Although life saving, mechanical ventilation use can lead to ventilator-associated pneumonia (VAP), with high mortality rates, especially when multidrug-resistant bacteria (eg, Acinetobacter baumannii) are involved. Reference Lima, Brito and Nizer2,Reference Sharifipour, Shams and Esmkhani3
Cases of A. baumannii infection were recently reported in COVID-19 patients. Reference Sharifipour, Shams and Esmkhani3,Reference Gottesman, Fedorowsky and Yerushalmi4 In Iran, A. baumannii comprised 90% of coinfections with severe acute respiratory coronavirus virus 2 (SARS-CoV-2), with mortality rates up to 100%. Reference Sharifipour, Shams and Esmkhani3 In Israel, Gottesman et al Reference Gottesman, Fedorowsky and Yerushalmi4 described an outbreak (5 cases) of carbapenem-resistant A. baumannii (CRAb) in 2 wards of a COVID-19 hospital. To the best of our knowledge, ours is the first study to report a monoclonal outbreak of an endemic CRAb strain in a new COVID-19 ICU, presenting a series of 14 cases.
Due to the COVID-19 pandemic, a tertiary teaching hospital in southern Brazil expanded the number of beds from 123 to 173 to treat COVID-19 patients. All new beds were physically isolated from the other hospital wards. Of the new beds, 20 were in an ICU with 2-bed rooms.
The outbreak occurred between September to December 2020 in this new ICU (Fig. 1). Cases of the present study were defined as all patients with positive SARS-CoV-2 RNA by the RT-qPCR method and a positive culture for CRAb. Bacterial identification and antimicrobial susceptibility testing results were obtained with a BD Phoenix automated system (Becton-Dickinson, Franklin Lakes, NJ). All isolates were typed by the enterobacterial repetitive intergenic consensus-PCR (ERIC-PCR) technique. Reference Silbert, Pfaller and Hollis5 BioNumerics version 6.5 software (Applied Maths, Sint-Martens-Latem, Belgium) was used to analyze band patterns. Isolates with a Dice similarity coefficient ≥ 0.93 were classified as belonging to the same cluster.
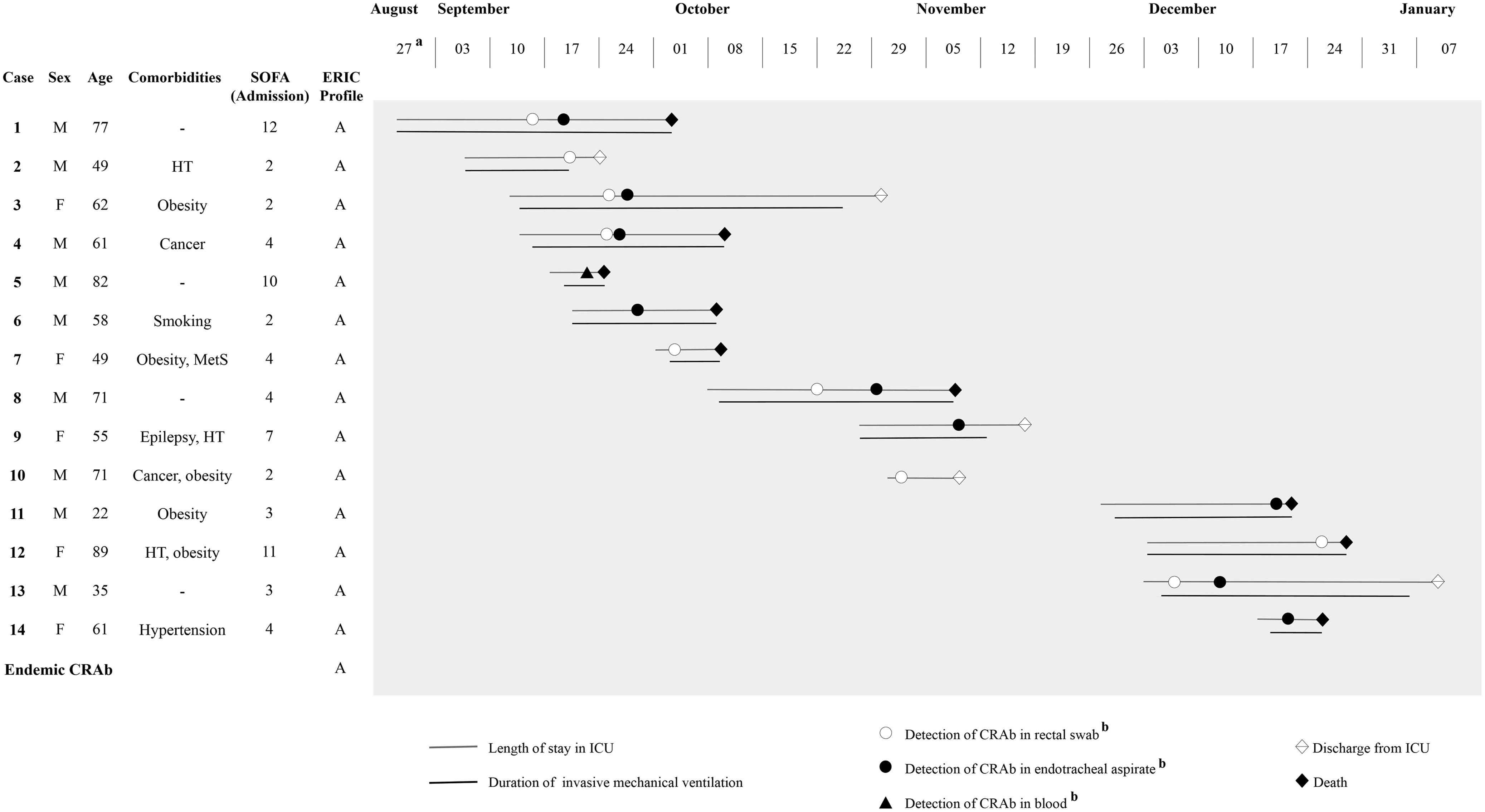
Fig. 1. Schematic description of cases in an outbreak of CRAb in a COVID-19–specific intensive care unit.
aTemporal representation of cases (weekly).
bDate of sample collection for bacterial isolation.
Note. M, male; F, female; SOFA, sequential organ failure assessment score calculated on admission to COVID-19 ICU; ERIC, Enterobacterial Repetitive Intergenic Consensus Polymerase Chain Reaction; HT, hypothyroidism; MetS, metabolic syndrome; CRAb, carbapenem-resistant Acinetobacter baumannii; ICU, COVID-19 intensive care unit.
In total, 14 cases were included in the study (Fig. 1). The mean patient age was 60 years, with male patients predominating (64%). The median duration of the ICU stay was 24 days (interquartile range [IQR], 14–34), duration of invasive mechanical ventilation was 25 days (IQR, 11–32), and Sequential Organ Failure Assessment score on admission to ICU was 4 (IQR, 3–9).
Overall, 19 CRAb isolates were recovered from blood, end otracheal aspirates, and/or rectal swabs of 14 patients. Of these patients, 13 received invasive mechanical ventilation, 9 were diagnosed with VAP and 1 with bacteremia. Among the 9 patients with a CRAb-positive rectal swabs, 5 also had CRAb-positive endotracheal aspirate (>1.0×106 CFU/mL). Of the 4 patients only colonized with CRAb (not infected), 2 survived. A colonized patient can serve as a source or reservoir and thus can increase the spread of CRAb. CRAb colonization may prolong the hospital stay and increase medical costs and the ICU mortality rate. Reference Lee and Lee6 Of the 10 patients with VAP or bacteremia, 7 died. Our findings support a previous report associating CRAb infection in COVID-19 patients with worse outcomes. Reference Sharifipour, Shams and Esmkhani3
All isolates proved to be resistant to penicillins, cephalosporins, aminoglycosides, fluoroquinolones, and carbapenems, greatly limiting options for treatment. All patients with CRAb infection were previously treated with azithromycin, ceftriaxone, and piperacillin/tazobactam. Of these, 1 (patient 5) died before starting appropriate antibiotic treatment, 5 (patients 1, 3, 6, 11 and 14) received polymyxin monotherapy, and 4 received combination therapy. Of the 4 patients treated with combination therapy, 2 (patients 8 and 13) received polymyxin B plus meropenem; 1 (patient 4) received polymyxin B, meropenem, and vancomycin; and 1 (patient 9) received meropenem and vancomycin. Only 1 (20%) of 5 patients treated with polymyxin monotherapy survived; 2 (50%) of 4 patients who received combination therapy recovered.
The best treatment for CRAb infections is a matter of debate. Although polymyxin monotherapy is widely used against CRAb infections, combination therapy has been associated with higher probabilities of therapeutic success. Reference Weinberg, Villedieu and Bagdasarian7 Our results suggest that combination therapy may be more effective in treating COVID-19 patients with CRAb infection, although further studies are needed to evaluate this possibility.
ERIC-PCR results showed a monoclonal spread of CRAb in the COVID-19 ICU within a short period, characterizing an outbreak. The band profile of these isolates showed 100% similarity to representatives of an endemic CRAb clone (previously reported). Reference Saalfeld, Viana and Siqueira8,Reference Moreira, Viana and Moraes9 This CRAb clone has been a persistent problem in our region since 2004, and although the newly opened ICU may have initially been contamination free, the clone spread rapidly in this unit. A. baumannii can survive for long periods on surfaces, including dry surfaces and human skin, which could facilitate its persistence and spread in ICUs. Reference Weinberg, Villedieu and Bagdasarian7 CRAb cross transmission between equipment (eg, ventilators, infusion pumps, and hemodialysis machines) and COVID-19 patients may also partly explain the onset of this outbreak. Furthermore, in several countries, including Brazil, health personnel were hired on an emergency basis to respond to the COVID-19 pandemic, impeding adequate training in infection prevention and control.
In our hospital, stricter barrier measures were implemented, increasing the effectiveness of screening and surveillance for CRAb. The active surveillance culture and efficient performance of a multidisciplinary team were highly important in detecting and controlling the CRAb outbreak in the COVID-19 ICU.
In conclusion, constant infection-control measures are necessary to stop the spread of CRAb in the hospital environment, prevent outbreaks, and lower mortality rates, especially in this time of the SARS-CoV-2 pandemic. With overloaded health systems and shortages of health workers trained in infection management, as well as medical consumables and equipment, the best preventive measure remains changing gloves and hand washing.
Acknowledgments
The authors thank Dr Janet W. Reid for her English text review.
Financial support
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil (CAPES), Finance Code 001. These government funds covered only the cost of laboratory materials and had no role in the study design or the decision to submit the work for publication.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.




