Introduction
Leishmaniasis is one of the neglected vector-borne tropical diseases endemic in almost 100 countries worldwide caused by Leishmania spp. (Euglenozoa: Trypanosomatidae) (Bruschi and Gradoni, Reference Bruschi and Gradoni2018; Kostygov et al., Reference Kostygov, Karnkowska, Votýpka, Tashyreva, Maciszewski, Yurchenko and Lukeš2021b). Between 10 and 15 million people in the world are infected, and the annual rate of new infections is over 2 million cases (WHO, 2022). The mortality per year from leishmaniasis is second only to malaria among all parasitic diseases (Pace, Reference Pace2014). Its clinical manifestations range from cutaneous ulcers to systemic multiorgan diseases in the cases of cutaneous leishmaniasis (CL) and visceral leishmaniasis (VL), respectively (Bruschi and Gradoni, Reference Bruschi and Gradoni2018).
In the Old World, the main areas of CL circulation are northern Africa, central Asia (hereafter, Kazakhstan, Kyrgyzstan, Mongolia, Turkmenistan and Uzbekistan) and the Middle East (Alvar et al., Reference Alvar, Velez, Bern, Herrero, Desjeux, Cano, Jannin and den Boer2012; Torres-Guerrero et al., Reference Torres-Guerrero, Quintanilla-Cedillo, Ruiz-Esmenjaud and Arenas2017). The most common Leishmania spp. documented in the Old World are Leishmania aethiopica, Leishmania major, Leishmania tropica and species of the Leishmania donovani complex (L. donovani and Leishmania infantum) (Lukeš et al., Reference Lukeš, Mauricio, Schönian, Dujardin, Soteriadou, Dedet, Kuhls, Tintaya, Jirků, Chocholová, Haralambous, Pratlong, Oborník, Horák, Ayala and Miles2007; Bruschi and Gradoni, Reference Bruschi and Gradoni2018). The VL in humans is mainly caused by members of the L. donovani complex and may manifest in damages to the liver, spleen, lymph nodes and bone marrow often resulting in death of a patient, if not diagnosed and treated in a timely manner (Strelkova et al., Reference Strelkova, Ponirovsky, Morozov, Zhirenkina, Razakov, Kovalenko, Schnur and Schönian2015; Mann et al., Reference Mann, Frasca, Scherrer, Henao-Martinez, Newman, Ramanan and Suarez2021). Infection with these species may also present skin manifestations in the cases of post-kala-azar dermal leishmaniasis or atypical leishmaniases (Guan et al., Reference Guan, Yang, Qu, Ren and Chai2013; Zhang et al., Reference Zhang, Ramasamy, McCall, Haydock, Ranasinghe, Abeygunasekara, Sirimanna, Wickremasinghe, Myler and Matlashewski2014; Ben-Shimol et al., Reference Ben-Shimol, Sagi, Horev, Avni, Ziv and Riesenberg2016; Zijlstra, Reference Zijlstra2016). Leishmania aethiopica, L. major and L. tropica mostly cause CL, although some isolates of L. major and L. tropica were occasionally identified from patients with VL (Alborzi et al., Reference Alborzi, Pouladfar, Fakhar, Motazedian, Hatam and Kadivar2008; Bruschi and Gradoni, Reference Bruschi and Gradoni2018; Charyyeva et al., Reference Charyyeva, Çetinkaya, Özkan, Şahin, Yaprak, Şahin, Yurchenko and Kostygov2021). The CL can be further subdivided into anthroponotic (ACL) and zoonotic (ZCL) forms, which are predominantly caused by L. tropica and L. major, respectively (Akilov et al., Reference Akilov, Khachemoune and Hasan2007; Ghatee et al., Reference Ghatee, Taylor and Karamian2020). Great gerbils (Rhombomys opimus) and fat sand rats (Psammomys obesus) serve as the main animal reservoirs for ZCL in central Asia and the Middle East, correspondingly (Elfari et al., Reference Elfari, Schnur, Strelkova, Eisenberger, Jacobson, Greenblatt, Presber and Schönian2005; Akhavan et al., Reference Akhavan, Yaghoobi-Ershadi, Khamesipour, Mirhendi, Alimohammadian, Rassi, Arandian, Jafari, Abdoli, Shareghi, Ghanei and Jalali-zand2010c), although other animal species – for example, Libyan jird (Meriones libycus), Shaw's jird (Meriones shawi), Indian gerbil (Tatera indica) or Indian desert gerbil (Meriones hurrianae) – may play this role in particular geographic regions (Yaghoobi-Ershadi et al., Reference Yaghoobi-Ershadi, Akhavan and Mohebali1996; Rassi et al., Reference Rassi, Jalati, Javadian and Moatazadian2001; Mohebali et al., Reference Mohebali, Javadian, Yaghoobi-Ershadi, Akhavan, Hajjaran and Abaei2004; Parvizi et al., Reference Parvizi, Moradi, Akbari, Farahmand, Ready, Piazak, Assmar and Amirkhani2008; Ghawar et al., Reference Ghawar, Toumi, Snoussi, Chlif, Zaatour, Boukthir, Hamida, Chemkhi, Diouani and Ben-Salah2011; Akhoundi et al., Reference Akhoundi, Mohebali, Asadi, Mahmodi, Amraei and Mirzaei2013).
The great gerbils may simultaneously host several species of Leishmania. In addition to pathogenic to humans L. major, they may also be infected by gerbil-restricted Leishmania turanica and Leishmania gerbilli (Strelkova et al., Reference Strelkova, Shurkhal, Kellina, Eliseev, Evans, Peters, Chapman, Le Blancq and van Eys1990b, Reference Strelkova, Eliseev, Ponirovsky, Dergacheva, Annacharyeva, Erokhin and Evans2001; Akhavan et al., Reference Akhavan, Mirhendi, Khamesipour, Alimohammadian, Rassi, Bates, Kamhawi, Valenzuela, Arandian, Abdoli, Jalali-zand, Jafari, Shareghi, Ghanei and Yaghoobi-Ershadi2010b). Here, we reviewed the literature on the mixed infections of L. major, L. turanica and L. gerbilli in central Asia and neighbouring countries with a focus on their natural animal reservoirs. As a second aim, we wanted to highlight some important papers on this topic published in Russian, and, as such, not well-known to the researchers in other countries.
Historical notes
Parasites of the genus Leishmania were first formally described by Leishman and Donovan in 1903 in patients infected with kala-azar in India (Donovan, Reference Donovan1903; Leishman, Reference Leishman1903). The parasite causing tropical ulcer was described as Helcosoma tropicum by Wright in the same year (Wright, Reference Wright1903) and renamed as Leishmania tropica in 1906 by Lühe (Reference Lühe and Mense1906). Yet, the first scientist documented the presence of a parasite now known as L. tropica was Cunningham in 1885 (Cunningham, Reference Cunningham1885). Its protistan nature (‘class of protozoa’) was discovered by Borovsky (Reference Borovsky1898), but remained unrecognized until much later (Hoare, Reference Hoare1938). In 1914, Yakimov identified 2 variants of Leishmania sp., based on the size of amastigotes in the macrophages of patients and named them L. tropica minor and L. tropica major (Yakimov and Schokhor, Reference Yakimov and Schokhor1914). Later studies revealed that L. t. minor causes dry ulcers usually lasting for over a year and it is more commonly spread in the cities. In contrast, L. t. major manifests in wet ulcers, the course of the disease is shorter and it is more commonly spread in rural areas (Latyshev and Krukova, Reference Latyshev and Krukova1941; Kozevnikov, Reference Kozevnikov1963; Schnur, Reference Schnur and Hart1987). In 1973, summarizing the accumulated data, Bray proposed to reclassify parasites as L. tropica for the causative agent of ACL and L. major for the causative agent of ZCL (Bray et al., Reference Bray, Ashford and Bray1973).
It is important to note that for about 50 years all isolates of Leishmania coming from animals and people in central Asia and neighbouring countries with a characteristic clinical picture were classified as L. major, with the only exception being a description of another Leishmania sp., L. gerbilli, from R. opimus in 1964 in China (Wang et al., Reference Wang, Qu and Guan1964). In line with that, in vitro experiments in animals demonstrated that different isolates have different levels of virulence. The highly virulent (HV) strains were invariably isolated from humans. They caused a progressive disease with obligatory ulceration in golden hamsters and domestic mice. Conversely, the strains with virulence ranging from low (low virulent strains, LV) to high could be isolated from gerbils. The LV strains caused a slow course of the disease that was limited to infiltrates and never led to ulceration. The strains with intermediate virulence caused a prolonged disease manifesting in small abortive ulcers in the later stages (Kellina, Reference Kellina1965; Lavrova et al., Reference Lavrova, Kellina, Passova and Shuikina1973; Kellina et al., Reference Kellina, Passova and Alekseev1981). Experimental infection of different animal species (in which Leishmania presence was documented in nature) with clonal cultures or strains of Leishmania with different virulence (HV and LV) revealed that HV parasites infected all the tested animals – great gerbils, Libyan jirds, Severtzov's jerboa (Allactaga severtzovi), long-eared hedgehogs (Hemiechinus auritus), domestic mice (Mus musculus) and golden hamsters (Mesocricetus auratus). In contrast, only the great gerbils, some Libyan jirds and golden hamsters could be infected by the LV clones or strains (Eliseev et al., Reference Eliseev, Strelkova and Passova1980; Strelkova et al., Reference Strelkova, Eliseev, Passova and Valevich1980).
The mystery of strains with different virulence was solved only with an advent of molecular techniques in the late 1980s. The isoenzyme analysis revealed that the strains previously identified as L. major include 3 independent species – L. major sensu stricto, L. turanica and L. gerbilli (Strelkova, Reference Strelkova1990; Strelkova et al., Reference Strelkova, Shurkhal, Kellina, Eliseev, Evans, Peters, Chapman, Le Blancq and van Eys1990b). These experiments also confirmed that HV and LV strains belonged to L. major and L. turanica/L. gerbilli, respectively. Notably, all strains isolated from humans were L. major, implying that L. turanica and L. gerbilli are restricted to gerbils. To sum up, all 3 abovementioned species can infect great gerbils, golden hamsters and Libyan jirds, but only L. major (neither L. turanica nor L. gerbilli) can infect Severtzov's jerboa, long-eared hedgehogs and domestic outbred mice.
Communal epidemiology and ecology of L. gerbilli, L. major and L. turanica
Central Asia
Ecology
In the natural foci of the ZCL on the territory of Turkmenistan, Uzbekistan, Kazakhstan, Kyrgyzstan and Mongolia great gerbils are the main natural hosts of Leishmania spp. discussed above (Eliseev and Kellina, Reference Eliseev and Kellina1963; Dubrovsky, Reference Dubrovsky1978; Eliseev and Neronov, Reference Eliseev and Neronov1997; Bruschi and Gradoni, Reference Bruschi and Gradoni2018) (Fig. 1, Table 1). Parasites’ life cycle not involving R. opimus was shown in some rare cases (e.g. in the lower reaches of the Surkhandarya river it involves M. libycus) (Strelkova et al., Reference Strelkova, Eliseev, Passova and Valevich1980). Great gerbils form topical and, as a result, trophic relationships with sand flies (Abai et al., Reference Abai, Oshaghi, Tajedin, Rassi and Akhavan2010; Akhavan et al., Reference Akhavan, Yaghoobi-Ershadi, Khamesipour, Mirhendi, Alimohammadian, Rassi, Arandian, Jafari, Abdoli, Shareghi, Ghanei and Jalali-zand2010c). They dig large and complex burrows (colonies), ideal for hatching and feeding of sand flies. Other animals [such as M. libycus, midday jird Meriones meridianus, H. auritus or M. musculus (Dubrovsky, Reference Dubrovsky1978)] may cohabitate with the greater gerbils for some time usually occupying the outer parts of the colony. This significantly reduces the likelihood and intensity of sand flies’ feeding on them.
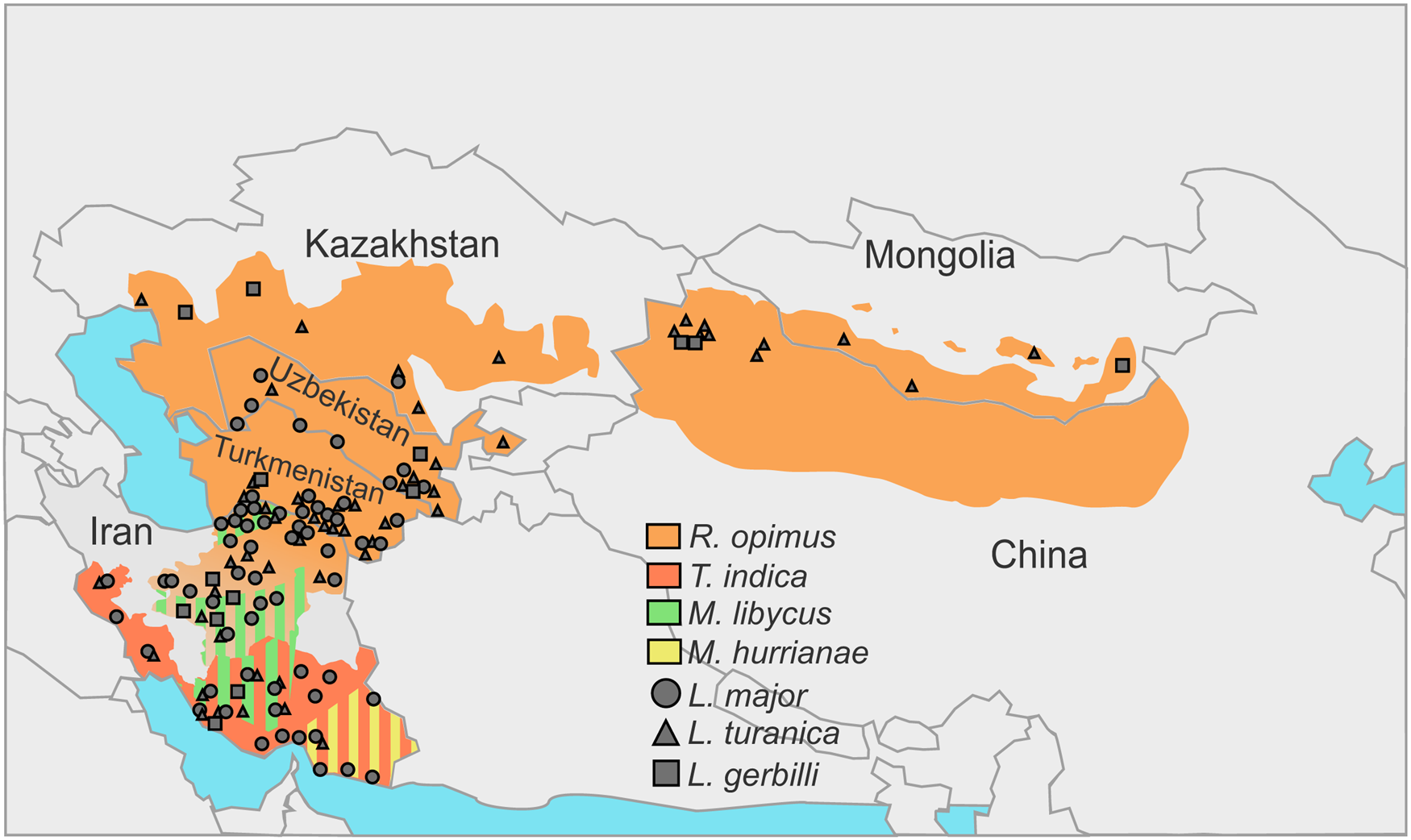
Fig. 1. Incidences of Leishmania spp. in the central Asia and Middle East over the distribution areas of their predominant animal reservoirs. Stripes indicate the presence of 2 species serving as animal reservoirs in the same territory.
Table 1. ZCL in central Asia, Iran and China (data summarized for field studies)

a Suspected vector.
Note: Leishmania infantum may also manifest skin symptoms, main reservoir hosts are dogs, vectors are Phlebotomus wui, Phlebotomus longiductus, Phlebotomus alexandri, Phlebotomus chinensis, Phlebotomus sichuanensis and Phlebotomus smirnovi in China; P. alexandri, Phlebotomus transcaucasicus, Phlebotomus kandelakii, P. chinensis and P. major s. l. in Iran; P. longiductus and P. smirnovi in Kazakhstan and Uzbekistan; Phlebotomus turanicus in Turkmenistan.
Hosts and vectors
In central Asian countries, the spectra of documented hosts and vectors vary for different Leishmania spp.: L. major has been isolated from humans, great gerbils, Libyan jirds, Phlebotomus papatasi and Phlebotomus andrejevi, while L. turanica and L. gerbilli have been found in great gerbils, P. papatasi, P. andrejevi, Phlebotomus caucasicus, Phlebotomus mongolensis, Phlebotomus alexandri, Sergentomyia clydei, and great gerbils and P. mongolensis, respectively (Strelkova, Reference Strelkova1996). Out of all the identified sand fly species, only P. papatasi and P. mongolensis are anthropophilic (Killick-Kendrick, Reference Killick-Kendrick1990; Guan et al., Reference Guan, Yang, Qu and Shen1995). Notably, experimental coinfections of L. major and L. turanica in P. papatasi revealed that 2 parasite species do not outcompete each other and develop in parallel (Chajbullinova et al., Reference Chajbullinova, Votýpka, Sádlová, Kvapilová, Seblová, Kreisinger, Jirků, Sanjoba, Gantuya, Matsumoto and Volf2012). In addition to (albeit, loose) vector specificity and host specificity, different Leishmania spp. inhabit somewhat different geographic areas. While L. turanica was found infecting R. opimus throughout its distribution areas (see Fig. 1 in Strelkova, Reference Strelkova1996), L. major parasitizes great gerbils and Libyan jirds in more southern parts of their distribution areas predominantly in river valleys, oases, areas adjacent to the oases and foothill plains (Neronov et al., Reference Neronov, Strelkova, Shurkhal, Luschekina and Artemyev1987; Strelkova et al., Reference Strelkova, Shurkhal, Eliseev, Kellina, Rakitskaia, Zviagintseva, Peters and Evans1990a). Conversely, in the southern and south-western areas of Iran not populated by great gerbils, the circulation of CL is unstable confirming the role of these animals as the main reservoirs (Neronov and Farang-Azad, Reference Neronov and Farang-Azad1973) (Table 1, see below). Leishmania gerbilli is not as widespread as the other 2 species discussed above and epizootics caused by this parasite are limited to the local populations of R. opimus in several regions of China, Iran, Kazakhstan, Turkmenistan and Uzbekistan (Strelkova, Reference Strelkova1996; Strelkova et al., Reference Strelkova, Shendrik, El Fari and Schönian2003).
Infection prevalence and seasonal dynamics
The prevalence of L. turanica infection in R. opimus is high (over 50%, sometimes achieving 100% in certain populations) and fairly stable over the years. The transmission season lasts from late April to mid-October and from late May to early September for southern and northern parts of the area, respectively; it usually peaks around late June–early July and remains stable until the end of transmission period. The prevalence of L. major in R. opimus is lower than that of L. turanica; it peaks in July–August achieving 10–50% in different years and places. The prevalence of this parasite in M. libycus (usually, within the R. opimus distribution range) does not exceed 3–4%. The prevalence of L. gerbilli infection in R. opimus is even lower than that of L. major (Strelkova, Reference Strelkova1996).
Coinfections, sympatric and allopatric populations
A remarkably high proportion of great gerbils in central Asia is infected by more than 1 Leishmania spp.; occasionally, all 3 species have been documented in the same animal (Strelkova, Reference Strelkova1990; Strelkova et al., Reference Strelkova, Shurkhal, Eliseev, Kellina, Rakitskaia, Zviagintseva, Peters and Evans1990a) (Fig. 1, Table 1). Populations of L. gerbilli, L. major and L. turanica are sympatric in some areas of R. opimus distribution (Strelkova et al., Reference Strelkova, Shendrik, El Fari and Schönian2003). The human ZCL caused by L. major in these areas always occurs on the background of L. turanica or, more rarely, L. gerbilli infection. It has been demonstrated that coinfection with L. turanica facilitates L. major perseverance in rodents during the 6–10 months break in the sand flies-mediated transmission of these parasites in central Asia (Strelkova et al., Reference Strelkova, Eliseev, Ponirovsky, Dergacheva, Annacharyeva, Erokhin and Evans2001). In contrast, the populations of L. turanica in northern Kazakhstan and Mongolia are allopatric, as the Iran–Turanian part of the ZCL range is geographically isolated from the central Asian one (Shurkhal et al., Reference Shurkhal, Strelkova, Passova, Rakitskaia and Podogas1985; Neronov et al., Reference Neronov, Strelkova, Shurkhal, Luschekina and Artemyev1987).
The dynamics of parasite presence in sympatric populations was investigated in the cases of predominant L. turanica and subsidiary L. major in great gerbils in central Asia (Eliseev et al., Reference Eliseev, Strelkova and Zherikhina1991; Strelkova et al., Reference Strelkova, Eliseev, Ponirovskii, Erokhin, Rakitskaia, Valevich, Sysoev, Allenov, Adamishina and Dergacheva1993, Reference Strelkova, Shendrik, El Fari and Schönian2003). Three main types of natural foci are described in R. opimus (Lysenko and Beljaev, Reference Lysenko, Beljaev, Peters and Killick-Kendrick1987). The hypoendemic foci are characterized by the very low-density circulation of L. major; here L. turanica predominates throughout the year, often for several years in a row. As a consequence, human ZCL in these foci is rare. In the hyperendemic foci, the proportion of L. major goes up during the transmission period (May–September), in some cases achieving 50% by the end of the season. This results in a substantially higher incidence of human ZCL. Leishmania major in the mesoendemic foci may accumulate up to 50% prevalence in R. opimus for several years, while being virtually absent in certain years in-between (Eliseev et al., Reference Eliseev, Strelkova and Zherikhina1991; Strelkova, Reference Strelkova1996).
Coinfections with L. turanica and L. major in great gerbils appear to be evolutionarily beneficial over the single-species infections. Indeed, while individual experimental infections with L. major, L. turanica and L. gerbilli lasted for 7, 15 and 18 months, respectively, the coinfection of L. major and L. turanica lasted for over 25 months (Strelkova, Reference Strelkova1991), indirectly confirming an early observation that duration of Leishmania spp. infection may be comparable with the life time of a host, R. opimus (Shishliaeva-Matova et al., Reference Shishliaeva-Matova, Ni and Zviagintseva1966). While infections with L. major alone were mostly self-healing, those with L. gerbilli, L. turanica, or coinfections with L. major and L. turanica led to chronic diseases in vast majority of cases (Strelkova, Reference Strelkova1991).
The situation in neighbouring countries (particularly, Iran and China) is somewhat different and deserves special discussion.
Iran
Hosts and vectors
The animal hosts of L. major are R. opimus in the north-eastern and central Iran, M. libycus in the south-western and central regions, T. indica in the south-west, west and south of the country and M. hurrianae in the south-east (Mohebali et al., Reference Mohebali, Javadian, Yaghoobi-Ershadi, Akhavan, Hajjaran and Abaei2004; Akhoundi et al., Reference Akhoundi, Mohebali, Asadi, Mahmodi, Amraei and Mirzaei2013). The presence of L. turanica was documented in great gerbils from the central and north-eastern Iran (Mohebali et al., Reference Mohebali, Javadian, Yaghoobi-Ershadi, Akhavan, Hajjaran and Abaei2004; Mirzaei et al., Reference Mirzaei, Rouhani, Taherkhani, Farahmand, Kazemi, Hedayati, Baghaei, Davari and Parvizi2011), M. libycus from Fars and Esfahan provinces (Akhoundi et al., Reference Akhoundi, Mohebali, Asadi, Mahmodi, Amraei and Mirzaei2013; Asl et al., Reference Asl, Mohebali, Jafari, Akhavan, Shirzadi, Zarei, Fadaei, Ramezanpour, Hassanpour, Izadi, Hajjaran and Elikaee2022), Rattus norvegicus and T. indica in Bushehr province (Yaghoobi-Ershadi et al., Reference Yaghoobi-Ershadi, Shahbazi, Darvishi, Akhavan, Jafari, Khajeian, Rassi, Soleimani, Shirzadi, Hanafi-Bojd, Darabi, Arandian, Sanei-Dehkordi and Heidari2013), and short-tailed bandicoot rats (Nesokia indica) in the western province of Kermanshah (Hajjaran et al., Reference Hajjaran, Mohebali, Alimoradi, Abaei and Edrissian2009) (Fig. 1, Table 1).
Infection prevalence, seasonal dynamics and coinfections
The reported prevalence of single infections and coinfections with L. major and L. turanica in great gerbils varied greatly between different studies (Akhavan et al., Reference Akhavan, Mirhendi, Khamesipour, Alimohammadian, Rassi, Bates, Kamhawi, Valenzuela, Arandian, Abdoli, Jalali-zand, Jafari, Shareghi, Ghanei and Yaghoobi-Ershadi2010b, Reference Akhavan, Yaghoobi-Ershadi, Khamesipour, Mirhendi, Alimohammadian, Rassi, Arandian, Jafari, Abdoli, Shareghi, Ghanei and Jalali-zand2010c; Akhoundi et al., Reference Akhoundi, Mohebali, Asadi, Mahmodi, Amraei and Mirzaei2013; Hajjaran et al., Reference Hajjaran, Mohebali, Abaei, Oshaghi, Zarei, Charehdar, Mirjalali, Sharifdini and Teimouri2013; Mirzaei et al., Reference Mirzaei, Schweynoch, Rouhani, Parvizi and Schönian2014; Asl et al., Reference Asl, Mohebali, Jafari, Akhavan, Shirzadi, Zarei, Fadaei, Ramezanpour, Hassanpour, Izadi, Hajjaran and Elikaee2022), indicating that more studies are needed to address this question. Leishmania gerbilli was previously detected only in the Esfahan and Bushehr provinces in single infections and coinfections with L. major, L. turanica or both, but usually in a small fraction of animals (Mirzaei et al., Reference Mirzaei, Rouhani, Taherkhani, Farahmand, Kazemi, Hedayati, Baghaei, Davari and Parvizi2011; Yaghoobi-Ershadi et al., Reference Yaghoobi-Ershadi, Shahbazi, Darvishi, Akhavan, Jafari, Khajeian, Rassi, Soleimani, Shirzadi, Hanafi-Bojd, Darabi, Arandian, Sanei-Dehkordi and Heidari2013). A recent study reported a higher frequency of L. gerbilli in the central Iran: out of 162 R. opimus and M. libycus analysed, 28 and 43 were infected by L. gerbilli alone and L. gerbilli with L. turanica, respectively (Asl et al., Reference Asl, Mohebali, Jafari, Akhavan, Shirzadi, Zarei, Fadaei, Ramezanpour, Hassanpour, Izadi, Hajjaran and Elikaee2022). The seasonal dynamics of R. opimus infection in Iran also differs from that described for the former USSR: while single L. major infections were observed in autumn and winter, and coinfections with L. major and L. turanica in all seasons except for summer, the single L. turanica infections were present throughout the year (Akhavan et al., Reference Akhavan, Yaghoobi-Ershadi, Mirhendi, Alimohammadian, Rassi, Shareghi, Jafari, Arandian, Abdoli, Ghanei, Jalali-Zand and Khamesipour2010a, Reference Akhavan, Yaghoobi-Ershadi, Khamesipour, Mirhendi, Alimohammadian, Rassi, Arandian, Jafari, Abdoli, Shareghi, Ghanei and Jalali-zand2010c). The proven vectors of L. major in this country include P. papatasi, Phlebotomus salehi, P. caucasicus and (suspected) Phlebotomus ansari (Azizi et al., Reference Azizi, Fakoorziba, Jalali and Moemenbellah-Fard2012; Yaghoobi-Ershadi, Reference Yaghoobi-Ershadi2012; Maroli et al., Reference Maroli, Feliciangeli, Bichaud, Charrel and Gradoni2013; Rafizadeh et al., Reference Rafizadeh, Saraei, Abaei, Oshaghi, Mohebali, Peymani, Naserpour-Farivar, Bakhshi and Rassi2016). The presence of L. major, L. turanica, and L. gerbilli in Iranian sand flies (P. papatasi, P. caucasicus, P. mongolensis, P. salehi, Phlebotomus sergenti, P. ansari, P. alexandri and Sergentomyia sintoni) was reported mostly from north-eastern and central regions (Parvizi and Ready, Reference Parvizi and Ready2008; Bakhshi et al., Reference Bakhshi, Oshaghi, Abai, Rassi, Akhavan, Sheikh, Mohtarami, Saidi, Mirzajani and Anjomruz2013; Bordbar and Parvizi, Reference Bordbar and Parvizi2014a; Sharbatkhori et al., Reference Sharbatkhori, Spotin, Taherkhani, Roshanghalb and Parvizi2014; Rafizadeh et al., Reference Rafizadeh, Saraei, Abaei, Oshaghi, Mohebali, Peymani, Naserpour-Farivar, Bakhshi and Rassi2016). Coinfections with L. major and L. turanica were rare and documented only in P. papatasi (Parvizi and Ready, Reference Parvizi and Ready2008; Darvishi et al., Reference Darvishi, Yaghoobi-Ershadi, Shahbazi, Akhavan, Jafari, Soleimani, Yaghoobi-Ershadi, Khajeian, Darabi and Arandian2015; Rafizadeh et al., Reference Rafizadeh, Saraei, Abaei, Oshaghi, Mohebali, Peymani, Naserpour-Farivar, Bakhshi and Rassi2016). Coinfections with L. turanica and L. gerbilli were seen in P. papatasi and P. caucasicus (Bakhshi et al., Reference Bakhshi, Oshaghi, Abai, Rassi, Akhavan, Sheikh, Mohtarami, Saidi, Mirzajani and Anjomruz2013; Darvishi et al., Reference Darvishi, Yaghoobi-Ershadi, Shahbazi, Akhavan, Jafari, Soleimani, Yaghoobi-Ershadi, Khajeian, Darabi and Arandian2015). Overall, P. papatasi is the species, in which L. turanica has been found most frequently in Iran, both in single and coinfections with L. major or L. gerbilli. As its competence to support the development of L. turanica has also been demonstrated in the laboratory (Chajbullinova et al., Reference Chajbullinova, Votýpka, Sádlová, Kvapilová, Seblová, Kreisinger, Jirků, Sanjoba, Gantuya, Matsumoto and Volf2012), P. papatasi is probably the main vector of L. turanica in Iran. Its vector competence for L. gerbilli has yet to be established.
China
In China, both VL and CL leishmaniasis are endemic in the western and north-western areas, with a predominance of VL and rather rare cases of CL (Wang et al., Reference Wang, Gao, Yang, Chen, Zhu, Lv, Chen, Tong, Steinmann, Ziegelbauer and Zhou2010). Leishmania major in these regions has not been documented in rodents or humans, and human CL is caused by L. infantum transmitted by Phlebotomus major wui (Guan et al., Reference Guan, Yang, Qu, Ren and Chai2013). Leishmania gerbilli has been described from desert areas inhabited by R. opimus since the 1960s (Wang et al., Reference Wang, Qu and Guan1964), and L. turanica was first identified in R. opimus in mid-1990s in Xinjiang (Guan et al., Reference Guan, Yang, Qu and Shen1995). Phlebotomus mongolensis and P. andrejevi are considered the main vectors; both are ecologically connected to R. opimus (Table 1). While P. andrejevi is scarcely captured in residential quarters, P. mongolensis is anthropophilic and dominates in human baits. In theory, reptilian reservoirs could also be involved in the circulation of L. turanica in the arid desert areas of north-western China, as this parasite has been found in 4 and 2 species of the genera Phrynocephalus and Eremias, respectively (Zhang et al., Reference Zhang, Guo, Chen, Liu, Gong, Chen and Chen2019).
In summary, the division of previously recognized as a single-species L. major into 3 Leishmania spp., of which only 1 is pathogenic for humans, facilitated revision of the earlier concepts about the epidemiology and endemicity of L. major in the territory that was considered well-studied.
Pathogenicity of L. turanica infections for humans
In Iran, asymptomatic Leishmania infections prevail in the natural rodent hosts: approximately 90% of the infected R. opimus showed no cutaneous lesions on their earlobes (Akhavan et al., Reference Akhavan, Mirhendi, Khamesipour, Alimohammadian, Rassi, Bates, Kamhawi, Valenzuela, Arandian, Abdoli, Jalali-zand, Jafari, Shareghi, Ghanei and Yaghoobi-Ershadi2010b, Reference Akhavan, Yaghoobi-Ershadi, Khamesipour, Mirhendi, Alimohammadian, Rassi, Arandian, Jafari, Abdoli, Shareghi, Ghanei and Jalali-zand2010c; Akhoundi et al., Reference Akhoundi, Mohebali, Asadi, Mahmodi, Amraei and Mirzaei2013). Notably, among symptomatic R. opimus, both L. major and L. turanica were detected but not scrutinized further (Hajjaran et al., Reference Hajjaran, Mohebali, Abaei, Oshaghi, Zarei, Charehdar, Mirjalali, Sharifdini and Teimouri2013). In line with these, single infections with L. turanica can cause lesions in humans too, as was demonstrated in a single study in northern Iran (Bordbar and Parvizi, Reference Bordbar and Parvizi2014b). Subcutaneous inoculation of human volunteers with 2 strains of L. turanica (1 from a Mongolian great gerbil and 1 from a sand fly from Uzbekistan) resulted in mild cutaneous manifestations. The Mongolian isolate infection manifested in a small nodule, which persisted for 2 weeks and resolved with no ulceration. Infection with a strain originated from a sand fly led to a prolonged incubation period and ulceration. The lesions persisted for about 2.5 months and healed spontaneously leaving small scars (Strelkova et al., Reference Strelkova, Shurkhal, Kellina, Eliseev, Evans, Peters, Chapman, Le Blancq and van Eys1990b). In China, 2 healthy volunteers injected with L. turanica also had mild cutaneous manifestations (Guan et al., Reference Guan, Yang, Qu and Shen1995). Collectively, L. turanica, a species of Leishmania might be categorized better as being low in pathogenicity, rather than being non-pathogenic in humans. Further studies are warranted to clarify this issue, as the number of volunteers involved in experimental infections was too small to generalize.
Conclusions and perspectives
Genomics
Genomes of all Leishmania spp. discussed above have been sequenced. The iconic one, L. major MHOM/IL/81/Friedlin had been sequenced earlier in 2005 (Ivens et al., Reference Ivens, Peacock, Worthey, Murphy, Aggarwal, Berriman, Sisk, Rajandream, Adlem, Aert, Anupama, Apostolou, Attipoe, Bason, Bauser, Beck, Beverley, Bianchettin, Borzym, Bothe, Bruschi, Collins, Cadag, Ciarloni, Clayton, Coulson, Cronin, Cruz, Davies, De Gaudenzi, Dobson, Duesterhoeft, Fazelina, Fosker, Frasch, Fraser, Fuchs, Gabel, Goble, Goffeau, Harris, Hertz-Fowler, Hilbert, Horn, Huang, Klages, Knights, Kube, Larke, Litvin, Lord, Louie, Marra, Masuy, Matthews, Michaeli, Mottram, Muller-Auer, Munden, Nelson, Norbertczak, Oliver, O'Neil, Pentony, Pohl, Price, Purnelle, Quail, Rabbinowitsch, Reinhardt, Rieger, Rinta, Robben, Robertson, Ruiz, Rutter, Saunders, Schafer, Schein, Schwartz, Seeger, Seyler, Sharp, Shin, Sivam, Squares, Squares, Tosato, Vogt, Volckaert, Wambutt, Warren, Wedler, Woodward, Zhou, Zimmermann, Smith, Blackwell, Stuart, Barrell and Myler2005) and since then was used as a reference in numerous comparative studies (El-Sayed et al., Reference El-Sayed, Myler, Blandin, Berriman, Crabtree, Aggarwal, Caler, Renauld, Worthey, Hertz-Fowler, Ghedin, Peacock, Bartholomeu, Haas, Tran, Wortman, Alsmark, Angiuoli, Anupama, Badger, Bringaud, Cadag, Carlton, Cerqueira, Creasy, Delcher, Djikeng, Embley, Hauser, Ivens, Kummerfeld, Pereira-Leal, Nilsson, Peterson, Salzberg, Shallom, Silva, Sundaram, Westenberger, White, Melville, Donelson, Andersson, Stuart and Hall2005; Peacock et al., Reference Peacock, Seeger, Harris, Murphy, Ruiz, Quail, Peters, Adlem, Tivey, Aslett, Kerhornou, Ivens, Fraser, Rajandream, Carver, Norbertczak, Chillingworth, Hance, Jagels, Moule, Ormond, Rutter, Squares, Whitehead, Rabbinowitsch, Arrowsmith, White, Thurston, Bringaud, Baldauf, Faulconbridge, Jeffares, Depledge, Oyola, Hilley, Brito, Tosi, Barrell, Cruz, Mottram, Smith and Berriman2007; Lukeš et al., Reference Lukeš, Butenko, Hashimi, Maslov, Votýpka and Yurchenko2018; Zakharova et al., Reference Zakharova, Albanaz, Opperdoes, Škodová-Sveráková, Zagirova, Saura, Chmelová, Gerasimov, Leštinová, Bečvář, Sádlová, Volf, Lukeš, Horváth, Butenko and Yurchenko2022). The genomes of L. turanica MRHO/SU/65/VL (LEM423) and L. gerbilli MRHO/CN/60/GERBILLI (LEM452) are also available, but they have not been scrupulously analysed yet (Warren et al., Reference Warren, Akopyants, Dobson, Hertz-Fowler, Lye, Myler, Ramasamy, Shanmugasundram, Silva-Franco, Steinbiss, Tomlinson, Wilson and Beverley2021). It goes without saying that more strains of these Leishmania spp. need to be sequenced and analysed in order to shed light on molecular mechanisms driving speciation in allopatric and sympatric populations of Leishmania spp., as well as the details defining hypoendemic, mesoendemic and hyperendemic foci of this parasite in central Asia.
Presence of leishmaniaviruses
Another interesting and very important topic of investigation is interaction between Leishmania spp. and their endosymbiotic viruses. The fact that viruses can infect Leishmania was established half-a-century ago (Molyneux, Reference Molyneux1974), but the real breakthrough came only after about 40 years of active investigation (Tarr et al., Reference Tarr, Aline, Smiley, Scholler, Keithly and Stuart1988; Stuart et al., Reference Stuart, Weeks, Guilbride and Myler1992; Widmer and Dooley, Reference Widmer and Dooley1995; Grybchuk et al., Reference Grybchuk, Kostygov, Macedo, d'Avila-Levy and Yurchenko2018a), when it was demonstrated that the presence of Leishmania RNA virus 1 (Leishmaniavirus 1, LRV-1) in Leishmania guyanensis controls the severity of mucocutaneous leishmaniasis (Ives et al., Reference Ives, Ronet, Prevel, Ruzzante, Fuertes-Marraco, Schutz, Zangger, Revaz-Breton, Lye, Hickerson, Beverley, Acha-Orbea, Launois, Fasel and Masina2011). The LRV-1 and LRV-2 are restricted to the New and Old World Leishmania spp., respectively and generally co-evolve with their hosts (Scheffter et al., Reference Scheffter, Ro, Chung and Patterson1995; Widmer and Dooley, Reference Widmer and Dooley1995; Cantanhêde et al., Reference Cantanhêde, Mata-Somarribas, Chourabi, Pereira da Silva, Dias das Chagas, de Oliveira, Cortes Boite and Cupolillo2021; Kostygov et al., Reference Kostygov, Grybchuk, Kleschenko, Chistyakov, Lukashev, Gerasimov and Yurchenko2021a). Interestingly, their elimination from L. major and L. guyanensis in vitro prompts different cellular responses (Saura et al., Reference Saura, Zakharova, Klocek, Gerasimov, Butenko, Macedo, Servienė, Zagirova, Meshcheryakova, Rogozin, Serva, Kostygov and Yurchenko2022). Our previous analyses of the available central Asian strains of L. gerbilli (n = 2), L. major (n = 14) and L. turanica (n = 12) revealed the presence of LRV-2 only in L. major with the prevalence of about 65% (Kleschenko et al., Reference Kleschenko, Grybchuk, Matveeva, Macedo, Ponirovsky, Lukashev and Yurchenko2019; Kostygov et al., Reference Kostygov, Grybchuk, Kleschenko, Chistyakov, Lukashev, Gerasimov and Yurchenko2021a) corroborating findings of LRV-2 in the same parasite species in Iran and Turkey (Hajjaran et al., Reference Hajjaran, Mahdi, Mohebali, Samimi-Rad, Ataei-Pirkooh, Kazemi-Rad, Naddaf and Raoofian2016; Kurt et al., Reference Kurt, Mansur, Çavuş, Özcan, Batir, Gündüz, Sezerman and Özbilgın2019; Nalçacı et al., Reference Nalçacı, Karakuş, Yilmaz, Demir, Özbilgin, Özbel and Töz2019; Saberi et al., Reference Saberi, Fakhar, Hajjaran, Ataei-Pirkooh, Mohebali, Taghipour, Ziaei Hezarjaribi, Moghadam and Bagheri2020, Reference Saberi, Fakhar, Hajjaran, Abbaszadeh Afshar, Mohebali, Hezarjaribi, Moghadam and Sharbatkhori2022; Farrokhi-Karibozorg et al., Reference Farrokhi-Karibozorg, Ghayour-Najafabadi, Hejazi, Ataei-Pirkooh, Mohebali, Teimouri and Hajjaran2022; Moin-Vaziri et al., Reference Moin-Vaziri, Zare, Seyyed Tabaei, Saberi and Hajjaran2022). The prevalence of L. major infection by LRV-2 in these studies varied between 2 and 68% and the presence of LRV-2 was confirmed in 87 out of 221 isolates of L. major analysed (only 1 isolate of L. turanica was included and reported as negative and no isolates of L. gerbilli were analysed).
These findings also pose an intriguing question – why L. turanica and L. gerbilli (note that conclusion about L. gerbilli is based on analysis of a very few isolates) are not infected with LRV-2? This is unlikely to be a virus-related feature, as LRVs were shown to be able to cross species barriers and even infect trypanosomatids of other genera (Grybchuk et al., Reference Grybchuk, Kostygov, Macedo, Votýpka, Lukeš and Yurchenko2018b). This is also unlikely to be determined by the vector, because all 3 Leishmania spp. are transmitted by the same or closely related species of sand flies (Strelkova, Reference Strelkova1996). The most parsimonious explanation is that L. gerbilli and L. turanica are naturally resistant against LRV-2. If this is really the case, analysis of additional strains of L. gerbilli and L. turanica will help us to discover genetic elements making them resilient to viral infections possibly providing new tools for treatment of leishmaniases. It is also important to consider how LRVs are transmitted. While to the best of our knowledge no experimental studies were performed on LRV-2s, in the cases of LRV-1 and New World Leishmania spp. it has been shown that viruses are transmitted horizontally via extracellular vesicles (Atayde et al., Reference Atayde, da Silva Lira Filho, Chaparro, Zimmermann, Martel, Jaramillo and and Olivier2019; Olivier and Zamboni, Reference Olivier and Zamboni2020).
In conclusion, we are now on the verge of new and exciting findings fuelled by recent development in sequencing technologies that may dramatically expand our knowledge about parasites causing leishmaniasis and change the way this disease is diagnosed and treated.
Author's contributions
Writing and revision – all authors.
Financial support
This work was supported by the Russian Science Foundation (no. 19-15-00054) to D. S. C. (section Historical notes), L. V. A. (section Introduction) and M. V. S. (section Communal epidemiology and ecology of L. gerbilli, L. major and L. turanica) and the Grant Agency of Czech Republic (no. 20-22689) to V. Y. (section Presence of leishmaniaviruses).
Conflict of interest
None.