Vegetarianism, defined as the practice of abstaining from meat, meat products, poultry, seafood and the flesh from any other animal is becoming increasingly popular( Reference Leitzmann 1 ). According to the Academy of Nutrition and Dietetics, appropriately planned vegetarian diets are healthful, nutritionally adequate, and may provide protection against chronic diseases( Reference Melina, Craig and Levin 2 ). The total or partial restriction of animal products, however, may result in inadequate intake of vitamin B12, which is an essential vitamin found in substantial quantities only in animal foods( Reference Melina, Craig and Levin 2 ).
Vitamin B12 has an important role in cellular metabolism: its physiological functions include erythropoiesis, DNA synthesis and the synthesis and maintenance of the myelin sheath. Furthermore, vitamin B12 acts as an anti-inflammatory agent through the inhibition of nitric oxide synthase, the down-regulation of the transcription factor NF-κB, and the promotion of oxidative phosphorylation( Reference Wheatley 3 ). Inadequate intake of vitamin B12 leads to low serum concentrations and elevated homocysteine, an amino acid linked independently to the risk of CVD( 4 ).
Epidemiological studies have shown a high prevalence of vitamin B12 deficiency among vegetarians, regardless of other factors such as age, demographic characteristics or geographical region( Reference Gilsing, Crowe and Lloyd-Wright 5 ). In a systematic literature review, a deficiency in up to 87 % of adults and the elderly, 17–39 % of pregnant women, and up to 33 % of children and adolescents on a vegetarian diet was documented( Reference Pawlak, Lester and Babatunde 6 ). In addition, a 5-year prospective study reported a significant decrease in circulating vitamin B12 in omnivores that switched to a vegan diet( Reference Madry, Lisowska and Grebowiec 7 ).
It has long been thought that only vegans, who avoid all animal-derived products, are at risk for vitamin B12 deficiency. Some studies recently have reported that also individuals whose diet includes low intake of animal foods, such as lacto-ovo-vegetarians, may incur in vitamin B12 deficiency( Reference Rizzo, Laganà and Rapisarda 8 ). All these findings, however, come from observational studies. Few randomised dietary intervention studies investigated the effects of a lacto-ovo-vegetarian diet (VD) in clinically healthy omnivorous participants( Reference Kestin, Rouse and Correll 9 – Reference Gardner, Coulston and Chatterjee 13 ). The aim of the present analysis was to evaluate the effects of a 3-month dietary intervention with a VD on the levels of circulating vitamin B12 in a group of omnivorous subjects.
Methods
Study population and dietary intervention
Data presented were obtained from the CARDIVEG (Cardiovascular Prevention with Vegetarian Diet) study, a randomised, open, crossover trial that compared the effects of a VD with a Mediterranean diet over a 3-month period on several CVD risk factors( Reference Sofi, Dinu and Pagliai 14 ). The study design and the characteristics of the participants are described elsewhere( Reference Sofi, Dinu and Pagliai 15 ) and are briefly reported here. A total of 118 clinically healthy subjects with low to moderate cardiovascular risk profile (<5 % at 10 years, according to the European Society of Cardiology) were recruited from the Clinical Nutrition Unit of Careggi University Hospital, Florence, Italy. After a 2-week run-in period, subjects were randomly assigned to a VD or a Mediterranean diet. Following the first phase of intervention, they crossed over to the opposite treatment. The analysis presented in this paper is based on data obtained from fifty-four participants (forty-three females; eleven males) who were randomised to a VD at the beginning of the study and completed the 3-month dietary intervention period.
The VD was characterised by abstinence from meat, meat products, poultry, fish and seafood consumption but included dairy products, eggs and all the other food groups. It was a low-energy diet with respect to the energy requirements of participants and consisted of approximately 50–55 % of energy from carbohydrate, 15–20 % from proteins and 25–30 % from fat. All the participants received a detailed 1-week menu plan and recipes for preparing meals.
The study was approved by the Ethic Committee (SPE 15.054) of the Tuscany Region, Careggi University Hospital, was registered at clinicaltrials.gov (identifier: NCT02641834) and adhered to the principles of the Declaration of Helsinki and the Data Protection Act.
Data collection
Data collection was performed at the Clinical Nutrition Unit of Careggi University Hospital, Florence, Italy. Participants were interviewed, according to standardised methods, to obtain information about demographics, risk factors, comorbidities, use of drugs, dietary supplements or fortified foods and lifestyle habits. Before starting the study, each participant completed a 3-d dietary record that was analysed by a dietitian using a nutrition-specific database. Body weight, body composition and blood samples were obtained both at the beginning and at the end of the VD intervention period. Measurements were made between 06.30 and 09.30 hours after an overnight fast. BMI was calculated as weight (kg)/height (m2). Body composition was determined by a bioelectrical impedance analysis device (model TBF-410; Tanita).
Compliance to the VD was assessed through a modified version of the National Health and Nutrition Examination Survey Food Questionnaire( 16 ) and through a 24-h dietary recall interview. Adherence to the VD was defined as the absence of meat, meat products, poultry, fish and seafood in the participants’ diet.
Laboratory measurements
Venous blood samples were collected into evacuated plastic tubes (vacutainer; Beckton Dickinson). Samples were centrifuged at 3000 rpm for 15 min (4°C) and stored in aliquots at –80°C until further analyses. Vitamin B12, as well as all the other biochemical parameters, was measured according to conventional laboratory standard methods. Pro- and anti-inflammatory cytokines were determined using a Bio-Plex cytokine assay (Bio-Rad Laboratories Inc.), according to the manufacturer’s instructions.
Statistical analysis
The statistical package PASW 20.0 for Macintosh (SPSS Inc.) was used. Data were presented as means and standard deviations or medians and ranges, as appropriate. Categorical variables were presented in terms of frequencies and percentages. The χ 2 test was used for dichotomous variables. The Mann–Whitney test was used to test for differences between males and females. The Spearman (R) test was used to estimate correlations between the changes in circulating vitamin B12 levels and changes in dietary profiles, anthropometric, biochemical and inflammatory parameters.
Subgroup analyses were performed to analyse the differences in the changes of circulating vitamin B12 levels, according to the median age of the study population (≤50·5, >50·5 years), to the obesity status (BMI <30, ≥30 kg/m2), to the smoking status (smokers; non-smokers) and to the cut-off values for determining high levels of total cholesterol levels (≤4·9, >4·9 mmol/l) and TAG (≤1·7, >1·7 mmol/l). A logistic regression analysis was performed to test for the probability of experiencing a reduction in circulating vitamin B12 after 3 months of VD. A reduction in circulating vitamin B12 was used as independent variable, while age, sex, BMI, smoking status, cholesterol levels, TAG and a reduction in vitamin B12 intake >28·5 % (i.e. the value of the delta changes corresponding to the first quartile of distribution in the study population) were used as covariates. Results were reported as OR and 95 % CI. A P value <0·05 was considered statistically significant.
Results
Baseline characteristics
Demographic characteristics, anthropometric parameters, prevalence of traditional risk factors for CVD and nutritional characteristics of the study population are shown in Table 1. Significant differences between males and females were found for body weight, percentage of fat mass and hypercholesterolaemia (total cholesterol >4·9 mmol/l). Intake of vitamin B12 was not significantly different between males and females at baseline. Participants reported no use of vitamin of B12 supplements or fortified foods.
Table 1 Characteristics of the study population (Mean values and standard deviations; medians and ranges; numbers and percentages)
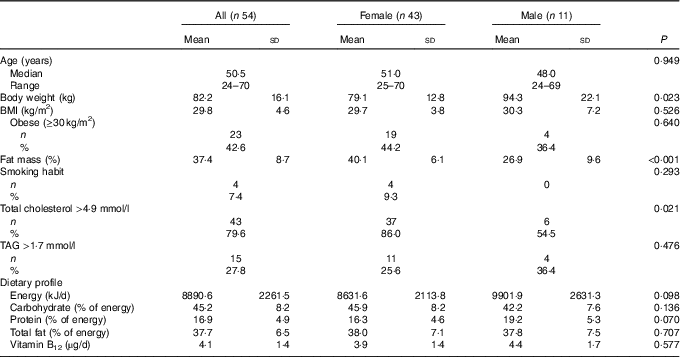
Regarding the circulating vitamin B12, mean levels in the study population were 392·3 (sd 131·3) pg/ml, with a non-significant difference (P=0·723) between females (395·5 (sd 135·9) pg/ml) and males (380·1 (sd 116·3) pg/ml). Five (9·3 %) subjects reported a vitamin B12 deficiency (<250 pg/ml), and a significant and positive correlation was found between vitamin B12 intake and circulating levels at baseline (R 0·47; P<0·001). As expected, a significantly lower dietary intake of vitamin B12 was found in subjects deficient in vitamin B12 compared with those with normal levels (2·4 (sd 0·6) v. 4·2 (sd 1·4) µg/d; P=0·005).
Effect of the dietary intervention with a lacto-ovo-vegetarian diet
The dietary intervention with a low-energy VD resulted in significant changes in dietary profiles compared with baseline (Table 2). All subjects experienced a reduction in total energy content, carbohydrates and total fat intake. The reduction in protein intake was significant only in males. Regarding daily vitamin B12 intake, a significant decrease of 2·1 (sd 1·4) µg/d was observed, with an average decrease of 51·2 % compared with baseline in both females and males.
Table 2 Changes in dietary profile during the lacto-ovo-vegetarian diet intervention period (Mean values and standard deviations)
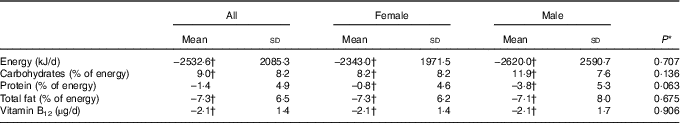
* P for differences between changes in females and males.
† P<0·05 for changes from pre- to post intervention.
Along with the significant reduction in vitamin B12 intake, thirty-five participants (64·8 %) reported a significant reduction in circulating vitamin B12 levels. In the whole study population, circulating vitamin B12 levels significantly decreased by 6·2 %, from 392·3 (sd 131·3) to 367·8 (sd 122·5) pg/ml, with a greater reduction in males (pre: 380·1 (sd 116·3) pg/ml v. post: 338·5 (sd 95·7) pg/ml; –10·9 %) compared with females (pre: 395·5 (sd 135·9) pg/ml v. post: 375·4 (sd 128·3) pg/ml; –5·1 %) (Fig. 1). The difference between males and females, however, was not statistically significant.
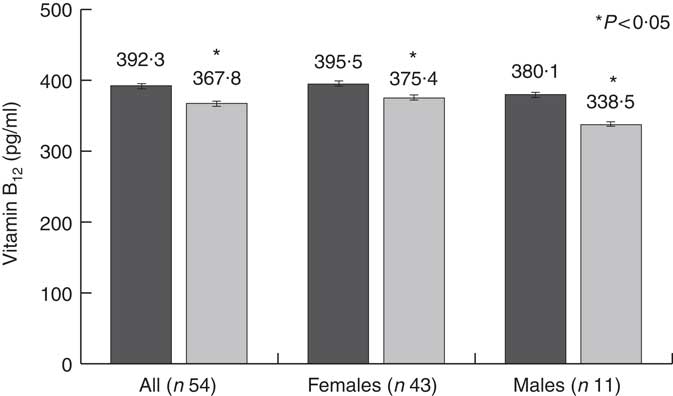
Fig. 1 Changes in circulating vitamin B12 levels. ![]() , Pre-dietary intervention;
, Pre-dietary intervention; ![]() , post-dietary intervention.
, post-dietary intervention.
Subgroup analyses showed that the reduction in circulating vitamin B12 levels was statistically significant in (a) younger subjects than in older subjects (<50·5 years, i.e. the median age of the study population), (b) overweight subjects compared with obese, (c) non-smokers subjects compared with smokers, (d) subjects with high levels of serum cholesterol compared with subjects with normal serum cholesterol levels and (e) subjects with normal levels of TAG compared with subjects with elevated levels of TAG (Table 3).
Table 3 Subgroup analyses of changes in circulating vitamin B12 levels (pg/ml) (Mean values and standard deviations; medians and ranges)
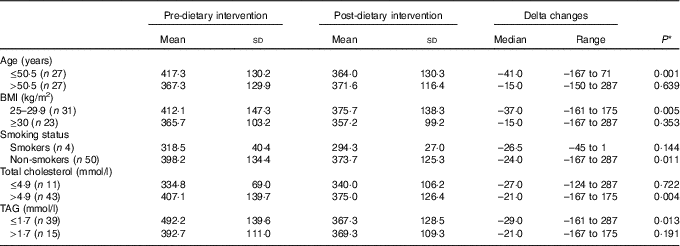
* P for changes from pre- to post intervention.
Correlation analyses conducted between changes in circulating vitamin B12 levels and changes in other variables after the dietary intervention period showed a significant positive correlation of circulating vitamin B12 levels with vitamin B12 intake (R 0·61; P<0·001). No significant correlations were found for all the other anthropometric, biochemical and inflammatory parameters evaluated.
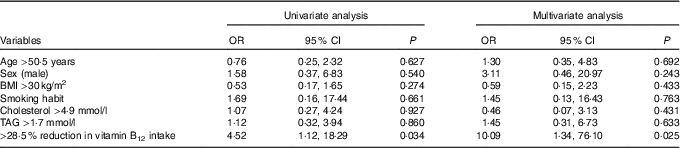
Finally, a logistic regression analysis was performed with the aim of assessing the probability of experiencing a decrease in circulating vitamin B12 levels after a VD intervention period (Table 4). In univariate analysis, only a reduction in vitamin B12 intake greater than the first quartile of the distribution of the delta changes obtained in the study population (28·5 %) conferred a significantly higher risk of experiencing a decrease in circulating vitamin B12. At multivariate analysis, after controlling for confounding variables, this association remained significant (OR 10·1; 95 % CI 1·3, 76·1).
Table 4 Logistic regression analysis of the probability of experiencing a reduction in circulating vitamin B12 levels after a 3-month dietary intervention with a lacto-ovo-vegetarian diet (Odds ratios and 95 % confidence intervals)
Discussion
The present study is the first clinical trial that evaluated the effect of a dietary intervention with VD on vitamin B12 levels in a group of omnivorous individuals. After 3 months of VD, a significant reduction in circulating levels of vitamin B12 was observed, positively correlated with the observed decrease in dietary vitamin B12 intake. Furthermore, a reduction in vitamin B12 intake above the first quartile of the delta changes in vitamin B12 intake in the study population increased the risk of experiencing a reduction in circulating levels of vitamin B12, regardless of age, sex and other demographic and clinical characteristics.
Vitamin B12 is a B-group vitamin that has important function in metabolism and prevention of neurological, haematological and cardiovascular disorders. It is present exclusively in animal and animal-derived foods, thus suggesting that subjects who adopt a diet that exclude animal products may have an increased risk of vitamin B12 deficiency due to the reduced amount of vitamin B12 in their diet. A meta-analysis including studies that analysed subjects consuming VD reported a compromised vitamin B12 status in vegetarians compared with non-vegetarians( Reference Obersby, Chappell and Dunnett 17 ). In fact, food sources of vitamin B12 in VD are milk, dairy products and eggs and their content ranges from 0·4 to 4·2 µg/100 g, with the highest values for dairy products( 18 , 19 ). However, considering the specific absorption rate and losses during cooking, these quantities seem not to be sufficient to ensure an adequate daily intake in subjects following a VD( Reference Watanabe 20 ).
In our dietary intervention study, despite baseline dietary intake of vitamin B12 similar to that observed in the general adult population( Reference Partearroyo, Samaniego-Vaesken and Ruiz 21 , Reference Sukumar, Adaikalakoteswari and Venkataraman 22 ), the exclusion of meat and fish during the VD period resulted in a reduction in vitamin B12 intake and a consequent decrease of circulating levels. Although clinically irrelevant and within the normal range, probably due to the limited duration of the intervention, this reduction confirms that switching from an omnivorous to a VD may result in a reduction in the levels of circulating vitamin B12.
Apart from diet, vitamin B12 is known to be generally affected by some variables such as age, sex and traditional cardiovascular risk factor. Its absorption capacity generally declines with age, mainly due to hypochlorhydria and lack of intrinsic factor( Reference Hunt, Harrington and Robinson 23 ). As a result, low levels of circulating vitamin B12 are common in the elderly who are considered a population at risk( Reference Hunt, Harrington and Robinson 23 ). In our study, the reduction in circulating vitamin B12 levels was more evident in the young people than in older participants. Such a result may seem surprising and in contrast with available evidence, but it can be explained by the changes imposed by the intervention diet with respect to participants’ usual diet. In fact, older participants have experienced a lower reduction in vitamin B12 intake compared with younger participants, due to the higher baseline intake of vitamin B12 in the diet of younger participants. Logistic regression analysis conducted in our intervention study allowed us to demonstrate that a reduction >28·5 % in vitamin B12 intake was the only factor that significantly determined an increased risk of reducing circulating vitamin B12 levels after 3 months of intervention with VD. It is therefore possible that younger participants, starting with a higher intake of vitamin B12, are also at higher risk of reporting a reduction of more than 28·5 %. In light of these data, more attention is needed to this particular cohort of the population when starting to adopt a VD.
Sex is another factor that could play a role in vitamin B12 metabolism. Although epidemiological studies evaluating the prevalence of vitamin B12 in vegetarians did not report significant differences between sexes( Reference Pawlak, Lester and Babatunde 6 ), a recent analysis of a large cohort of healthy adults found that males had a risk of deficiency twice as high as females( Reference Margalit, Cohen and Goldberg 24 ). The causal mechanism of this phenomenon is yet to be determined, but previous studies have shown that genetic variations related to sex and oestrogen status could be involved in the absorption of vitamin B12 and the resulting circulating levels( Reference Tanaka, Scheet and Giusti 25 ). In our study, the reduction in circulating vitamin B12 was significant in both females and males, but was greater in males. It is important to note that changes in diet cannot explain this result, as the reduction in vitamin B12 intake was similar in the two groups. The possible relationship between sex and vitamin B12 warrants further investigation since a better understanding of this aspect could contribute to the early identification of individuals at risk.
With regard to traditional cardiovascular risk factors, subgroup analyses revealed that the reduction in circulating vitamin B12 levels was more evident in overweight participants compared with obese, in participants with higher cholesterol and in non-smokers. The effect of body weight, lipid profile and smoking on vitamin B12 has not been studied in detail as of yet. Obesity and overweight have been associated with lower B12 levels in several populations, but a pooled analysis of nineteen studies found no evidence of an inverse association between BMI and circulating vitamin B12 Reference Wiebe, Field and Tonelli (26 ). With regard to the lipid profile, higher cholesterol levels have been found in individuals with vitamin B12 deficiency, and several mechanisms for the role of this vitamin in lipid metabolism have been proposed( Reference Adaikalakoteswari, Jayashri and Sukumar 27 , Reference Adaikalakoteswari, Finer and Voyias 28 ). There are no studies, though, that evaluated the effects of high cholesterol levels on vitamin B12. Lastly, harmful effects of cigarette smoking on vitamin B12 status in vegetarians have been reported( Reference Dastur, Quadros and Wadia 29 ). Our results, which seem to be contrasting these findings, may however be explained by the fact that most participants were non-smokers, and the division into subgroups has reduced the statistical significance in smokers.
Few limitations deserve discussion. A notable weakness is the short duration of the study that limited the possibility to study long-term effects. Since the body stores vitamin B12 in the liver and has a recycling process for this vitamin, it may take from several months to years to develop a deficiency once the intake of this nutrient has ceased. Another limitation is the absence of specific markers of vitamin B12 status such as holotranscobalamin II and methylmalonic acid. Their measurement is a valuable support for the assessment of vitamin B12 status( Reference Del Bo’, Riso and Gardana 30 ), but it is not common because of methodological and economic reasons. Finally, the lack of data on homocysteine levels and the relatively small number of participants limited the opportunity to explore the relationship between homocysteine and vitamin B12 levels and permits only to suggest possible interpretations of the results. However, despite these limitations, the present study includes the largest cohort of omnivorous participants who underwent a period on a VD.
In conclusion, the transition from an omnivorous to a VD is associated with a reduction in circulating vitamin B12, mainly due to a reduction in vitamin intake. This result strengthens the importance of an adequate intake of vitamin B12 to maintain normal circulating levels. Many individuals still think that B12 deficiency is rare and occurs only in a small percentage of vegans( Reference Rizzo, Laganà and Rapisarda 8 ). Findings from the EPIC-Oxford cohort showed that <40 % of the lacto-ovo-vegetarians use vitamin B12 supplements( Reference Sobiecki, Appleby and Bradbury 31 ), and even lower percentages have been reported in other studies( Reference Rizzo, Laganà and Rapisarda 8 ). Supplementation meets opposition due to misconceptions or aversion to products considered artificial( Reference Hokin and Butler 32 ), but underestimating the risk of developing a deficiency may nullify the health benefits of VD( Reference Pawlak 33 ). A compromised vitamin B12 status might therefore explain why the improved CVD risk factors among vegetarians do not always translate into a lower risk of incidence and mortality from CVD( Reference Appleby, Crowe and Bradbury 34 , Reference Chang-Claude, Hermann and Eilber 35 ).
Acknowledgements
The authors thank all the staff of the Department of Geriatric Medicine, Section of Diabetes and Nutrition of the Careggi University Hospital for their invaluable contribution and the participants for their consistent cooperation.
The research received no specific grant from any funding agency, commercial or not-for-profit sectors.
M. D. participated in the design of the study, participated in the clinical evaluations, conducted the statistical analyses, wrote the paper and revised the manuscript. G. P. participated in the clinical evaluations, conducted the statistical analyses and revised the manuscript. F. C. was responsible for the evaluation of all the laboratory parameters and participated in the design of the study. B. G., A. M. G. and R. M. participated in the writing of the study protocol and in the critical revision of the manuscript. A. C. participated in the design of the study and critical revision of the manuscript for important intellectual content. F. S. conceived the study, participated in the design of the study, wrote the study protocol and revised the final version of the manuscript. He has been responsible for recruitment, clinical evaluations and statistical analyses. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.








