Introduction
Tuberculosis (TB) continues to present a considerable public health concern, particularly in developing countries where high rates of human immunodeficiency virus (HIV) infection and adverse socio-economic factors contribute to high rates of TB disease and TB-related mortality [Reference Abdool Karim1, 2]. In 2016, South Africa had the highest estimated incidence of TB in the world at 781 cases per 100 000 population [2]. This overall estimate includes both notified and estimated undiagnosed cases, whereas only 244 053 of the estimated 438 000 cases (56%) were notified [2]. Within South Africa, the Western Cape Province is known to carry a particularly high burden of TB, with an incidence of 681 notified cases per 100 000 population and HIV co-infection rate of 38.5% in 2015, according to the electronic TB register [Reference Massyn3]. In this instance, the estimated number of undiagnosed/unreported cases has not been accounted for and the true incidence of TB in the province is almost certainly higher. Within such a high-burden setting, it is not surprising that sites of TB disease that constitute only a small percentage of all cases amount to a relatively large absolute number of individuals affected. For example, although spinal TB accounts for only 1–5% of all TB cases [Reference De la Garza Ramos4–Reference Jutte6], previous reports suggest that spinal TB is a common pathology at tertiary orthopaedic units within the province [Reference Held7–Reference Held9].
TB of the spine typically occurs following haematogenous spread of Mycobacterium tuberculosis from a primary infection site and is characterised by gradual destruction of one or more spinal vertebrae [Reference Rajasekaran, Kanna and Shetty10–Reference Turgut13]. Although progression of the disease is relatively slow and insidious, diagnosis is often delayed and advanced cases may present with kyphotic deformity, spinal instability and neurological deficits due to compression of the spinal cord [Reference Rajasekaran, Kanna and Shetty10–Reference Turgut13]. These sequelae may be particularly prevalent in children as the disease progresses more rapidly in the high cartilage content of the immature spine and there is a greater likelihood of buckling and collapse [Reference Rajasekaran, Kanna and Shetty10, Reference Jain14]. As a result, many children with spinal TB sustain significant kyphotic deformity during the active phase of the disease and all children remain at risk of secondary complications until skeletal maturity [Reference Jain14, Reference Rajasekaran15].
Within the Western Cape, the recommended diagnostic procedure for patients with suspected spinal TB involves a magnetic resonance imaging (MRI) scan and a biopsy of the spinal lesion or paraspinal abscess [Reference Watt and Davis8, Reference Dunn and Zondagh16]. While TB disease is treated with antituberculosis medication, many patients may also require corrective orthopaedic surgery [Reference Rajasekaran, Kanna and Shetty10, Reference Mak and Cheung17]. Anecdotal evidence suggests that in-patient diagnosis and surgical intervention for patients with spinal TB constitutes a significant burden on specialist orthopaedic services in this TB endemic setting. However, the true burden of spinal TB in the province has not been investigated. Furthermore, it is unclear whether the recent decline in the incidence of all reported TB cases within the province, from 777 per 100 000 population in 2012 to 681 per 100 000 population in 2015 [Reference Massyn3], has had any impact on the number of new spinal TB cases over recent years.
Increased understanding of the burden of spinal TB in this setting may provide key insights for health system adjustments and the planning of future spinal TB research, a field in which Africa is currently poorly represented despite its high TB burden [Reference Held18]. Therefore, the aim of the current study was to assess the burden of spinal TB at tertiary hospitals in the Western Cape, including the overall number of adult and child cases, the clinical and demographic characteristics of patients affected and the change in case numbers by year.
Methods
Study design and setting
This retrospective, descriptive study identified cases of spinal TB treated at tertiary hospitals in the Western Cape Province of South Africa between 1 January 2012 and 31 December 2015. The study was conducted in collaboration with orthopaedic services at each of the province's three tertiary hospitals (Tygerberg Hospital, Groote Schuur Hospital and Red Cross War Memorial Children's Hospital) and was approved by the Health Research Ethics Committee of Stellenbosch University and by the management of the hospitals involved. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Case identification and data collection
Cases of spinal TB in children and adults were identified using clinicians’ databases, theatre log books and a search of the National Health Laboratory Service (NHLS) TB investigations. Cases identified were cross-referenced and all duplicates removed.
Clinical and demographic data including age, gender, HIV status and the results of samples submitted for bacteriological and histological testing were captured from NHLS laboratory results systems and available medical records. Additional information was captured for HIV-positive patients, including CD4 count, viral load (VL), timing of HIV diagnosis and antiretroviral therapy (ART) status. CD4 count and/or VL are typically measured only once or twice a year according to the standard ART monitoring in South Africa and results closest to hospital admission, from 12 months prior to admission to 1 month after admission, were included as available. Diagnosis with HIV at approximately the same time as presenting to the health system with spinal TB was considered a new HIV diagnosis as opposed to a prior HIV diagnosis. HIV-positive patients initiated on ART only during or after presentation with spinal TB were categorised as not on ART within the descriptive analysis.
Radiological outcomes for all patients including the number of vertebrae affected and diagnosis were captured from MRI reports. Furthermore, the presence or absence of kyphotic deformity was assessed by a spine surgeon based on available imaging. If applicable, a description of the spine surgery performed was obtained from the afore-mentioned clinicians’ databases, theatre log books or medical records. Corrective surgery included procedures such as instrumented fusion whereas needle biopsies, abscess drainage and costotransversectomy were considered minor surgical procedures. All data collected for the study was de-identified and entered into custom-designed forms using REDCap electronic data capture tools hosted at Stellenbosch University.
Case classification
Cases were classified as ‘bacteriologically confirmed’ or ‘clinically diagnosed’, according to the 2013 revised World Health Organization (WHO) case definitions [19]. In the current study, confirmed cases were those patients for whom the presence of M. tuberculosis in a spine biopsy was demonstrated by a positive Xpert MTB/RIF test (Cepheid, Sunnyvale, California, USA; available for routine testing from approximately 2014 onwards), positive culture for M. tuberculosis or positive smear microscopy for acid-fast bacilli. Clinically diagnosed cases were those patients for whom spine imaging, clinical presentation and, in some instances, confirmed TB from other sites indicated spinal TB, but there was no bacteriological confirmation from a spine biopsy. Patients 0–14 years of age and patients 15 years or older at the time of diagnosis were classified as child cases and adult cases, respectively.
Data analysis
Upon completion of the study, data were exported from REDCap to Microsoft Excel and then transferred to Graphpad Prism (GraphPad Prism version 6.00 for Windows, GraphPad Software, La Jolla, California, USA, http://www.graphpad.com) for analysis. The overall burden of spinal TB cases was presented as counts. Case counts per year and annual, age-specific Western Cape population estimates [20–23] were used to estimate the incidence of spinal TB in the Western Cape during the study period. No report of population estimates was available for 2012 and this estimate was taken as the average of 2011 [20] and 2013 [21] values. Categorical descriptive data were presented as counts and percentages, as appropriate. Odds ratios (ORs) were used to investigate the association between female gender and HIV infection among adults with known HIV status and the association between CD4 count <200 cells/μl and ⩾3 vertebrae affected and CD4 count <200 cells/μl and receiving corrective surgery among HIV-positive adults. The severity of spinal TB presentation in children vs. adults was compared using the OR for ⩾3 vertebrae affected, presence of kyphotic deformity and having received corrective spine surgery. All ORs were calculated with 95% confidence intervals (CIs) and significance was accepted at P < 0.05. Finally, a two-sided Jonckheere–Terpstra test was used to assess the trends in counts and TB incidence over the study period. This trend analysis was conducted using STATA version 15 (Stata Corporation, College Station, Texas, USA) and significance was accepted at P < 0.05.
Results
A total of 393 cases of spinal TB were diagnosed at tertiary hospitals in the Western Cape within the 4-year study period, including 319 adults and 74 children. The majority of cases presented from the Cape Town Metro (n = 313, 80%) with much smaller representation from the surrounding rural districts (Cape Winelands, n = 39 (10%), West Coast, n = 13 (3%), Overberg, n = 10 (3%), Eden, n = 9 (2%)) and outside of the province (n = 8, 2%). Demographic data, HIV status and the results of diagnostic tests are presented in Table 1.
Table 1. Demographics, investigation of spine lesions and case classification of children and adults with spinal tuberculosis at tertiary hospitals in the Western Cape, 2012–2015
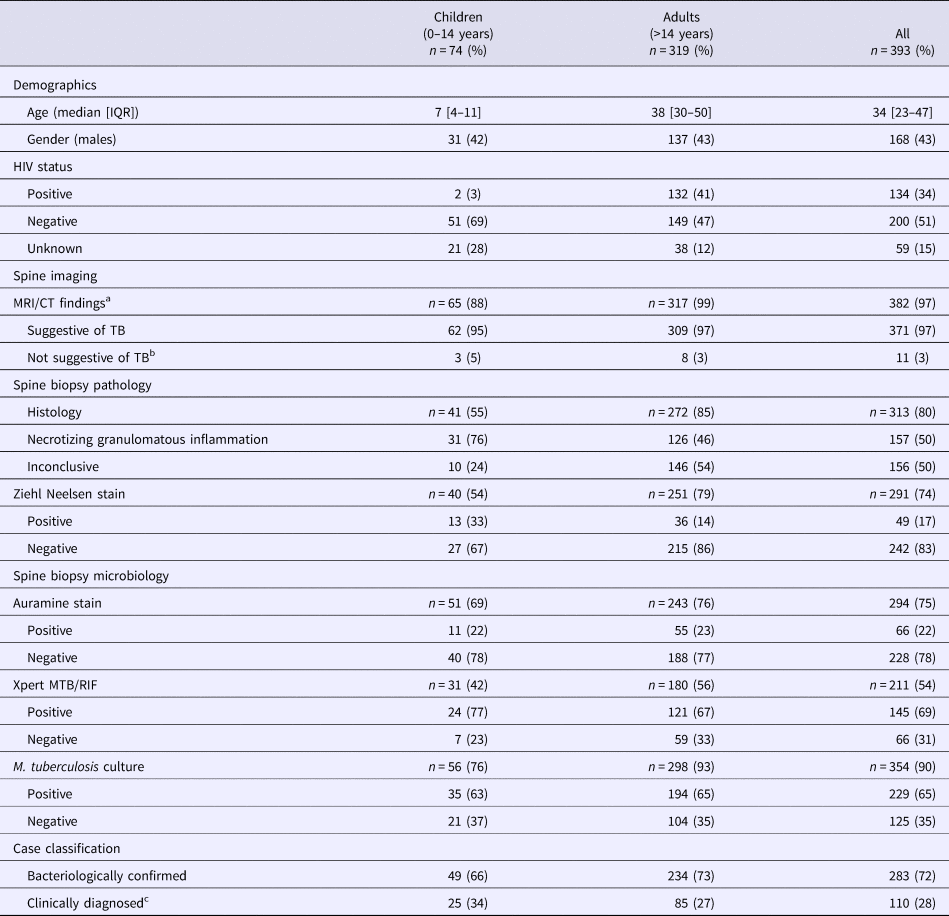
Data are presented as n (%) for non-missing data, unless otherwise indicated, with % values rounded to the nearest whole number.
a MRI, magnetic resonance imaging (n = 378); CT, computed tomography (n = 4).
b Although MRI findings did not suggest TB, spinal TB was diagnosed following bacteriological (n = 10) or histological (n = 1) spine biopsy findings.
c Among the clinically diagnosed cases (no bacteriological confirmation from a spine biopsy), seven adults and seven children had bacteriological confirmation from another site (lungs, n = 8; lymph node, n = 2; pleura, n = 1; knee, n = 1; groin, n = 1; liver, n = 1).
Age and gender
The median age of adults was 38 years with a range of 16–82 years, while the median age of children was 7 years with a range from 1 year 7 months to 14 years 9 months. Approximately 57% of patients were female in both adult and child groups. The percentage of spinal TB cases within each 5-year age bracket, in total and by gender, is shown in Figure 1.
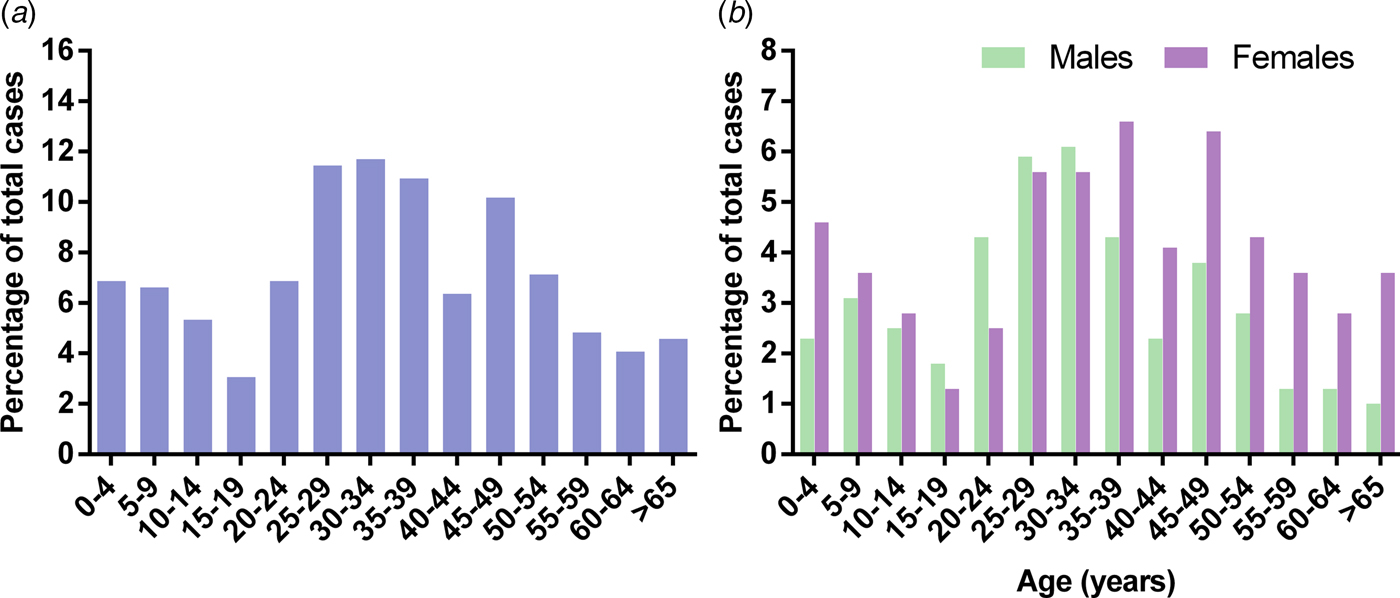
Fig. 1. Cases of spinal tuberculosis presented as (A) total cases and (B) male and female cases, per 5-year age bracket.
HIV status
Based on available HIV test results, 41% of adults with spinal TB were HIV-positive vs. only 3% of children. However, HIV status was unknown or could not be determined from the available data in 12% of adults and 28% of children. When examining HIV status by 5-year age bracket, the highest proportion of HIV-positive cases were observed among 25–54 years age groups whereas those ⩽14 or ⩾55 years of age were predominantly HIV-negative or of unknown HIV status (Fig. 2). When comparing HIV status between genders, 89 of 160 (56%) adult females and 43 of 121 (36%) adult males with known HIV status were HIV-positive. Based on these proportions, females were significantly more likely to be HIV-positive than males (OR 2.3, 95% CI 1.4–3.7, P = 0.001).
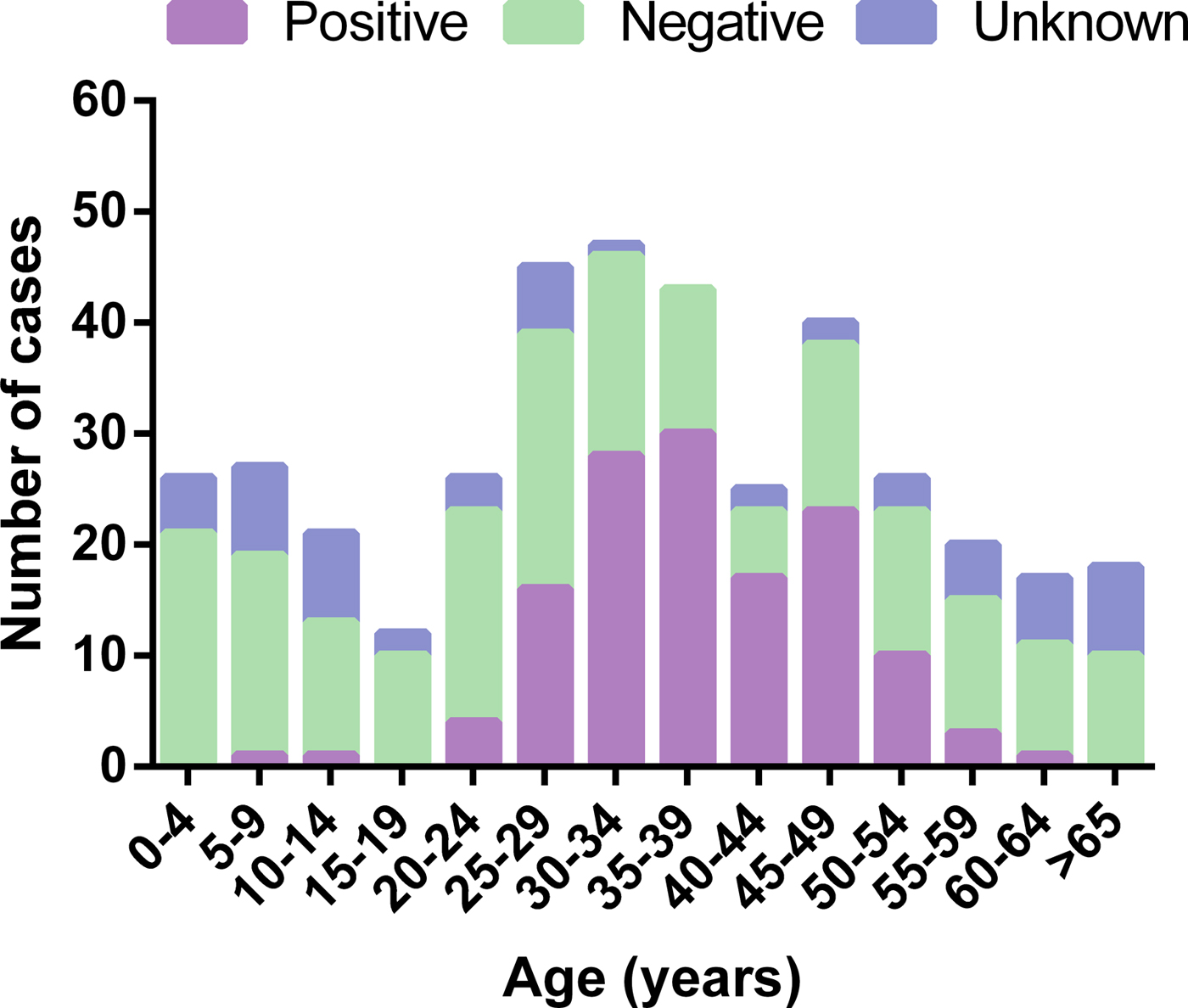
Fig. 2. HIV status of spinal tuberculosis cases by 5-year age bracket.
CD4 counts were available in 117/132 (89%) HIV-positive adults; the median CD4 count was 325 cells/μl (IQR 193–520 cells/μl) with a median CD4% of 21.38% (IQR 14.95–28.12%). Of 74 (56%) adults with available VL, 54 had lower than detectable VL (<20 copies/ml) and 20 had a detectable VL with a median of 3.25 log10 copies/mL (IQR 2.24–5.02 log10 copies/ml). The median absolute time difference between CD4 test results and admission and VL test results and admission was 2 weeks (IQR 0–10 weeks) and 10 weeks (IQR 4–18 weeks), respectively. Most adults had prior HIV diagnosis and were on ART upon presentation with spinal TB. Further HIV-specific details are shown in Table 2.
Table 2. Clinical characteristics at spinal tuberculosis diagnosis among HIV-infected cases
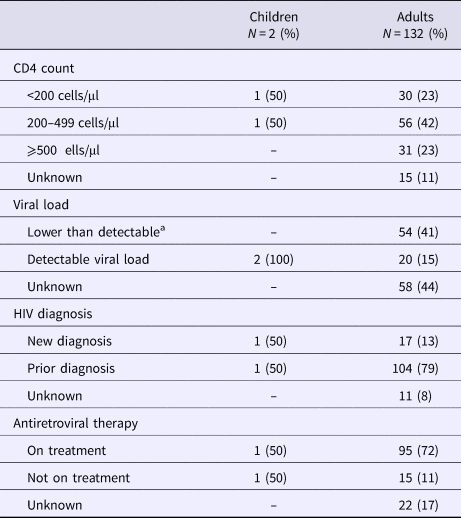
a <20 viral RNA copies/ml.
Only two children were confirmed HIV-positive; an 8-year-old boy with a prior HIV diagnosis on ART (CD4 count: 105 cells/μl, CD4%: 6.86% and VL: 3.13 log10 copies/ml) and an 11-year-old girl newly diagnosed with HIV not yet on ART (CD4 count: 283, CD4%: 13.73 and VL: 4.04 log10 copies/ml).
Diagnosis
Imaging was available for 318/319 adults (n = 314 MRI scans, n = 3 computed tomography (CT) scans and n = 1 radiograph only) and 72/74 children (n = 64 MRI scans, n = 1 CT scan, n = 7 radiograph only) with MRI or CT findings specifically suggestive of TB in 309 (97%) adults and in 62 (95%) children. Of patients who received an MRI or CT scan, 53/382 (14%) had non-contiguous involvement of two or more vertebrae. Of 211 patients with spine biopsies submitted for Xpert MTB/RIF testing, 145 (69%) were positive. Similarly, of 354 patients with spine biopsies submitted for mycobacterial culture, 229 (65%) were positive. Further details of how MRI and spine biopsy results related to one another are included as online Supplementary Material (Fig. S1).
Among 263 (70%) patients with known drug susceptibility test (DST) results, 239 (91%) had susceptibility to isoniazid and rifampicin or rifampicin based on DST on mycobacterial culture or Xpert MTB/RIF, respectively. However, 24 patients (9%) had drug-resistant TB: eight patients (3%) had mono-resistant TB (n = 4 rifampicin, n = 4 isoniazid), 14 patients (5%) had multidrug-resistant (MDR)-TB and two patients (<1%) had extensively drug-resistant-TB. Although two cases of MDR were based on Xpert only, all other cases of drug resistance were identified from culture DST.
When considering all diagnostic evidence together, 283 (72%) of all spinal TB cases were bacteriologically confirmed from a spine biopsy. The proportion of confirmed cases was slightly higher in adults than in children (73% vs. 66%). However, 315 of 319 (99%) adults and only 57 of 74 (77%) children had spine biopsy specimens sent for bacteriology, affording greater opportunity for bacteriological confirmation among the adults (data not shown).
Severity of presentation
A large number of adults and children presented with severe spinal disease, based on the number of vertebrae affected, the presence of kyphotic deformity and corrective surgery received (Table 3). While children were no more likely than adults to have ⩾3 vertebrae affected or to present with kyphotic deformity, children were 2.7 (95% CI 1.6–4.5, P = 0.0003) times more likely to have received corrective surgery, compared with adults (Table 3). Overall, 115 (29%) spinal TB patients received corrective surgery. Among HIV-positive adults with an available CD4 count, there was no association between CD4 count <200 cells/μl and having ⩾3 vertebrae affected or receiving corrective surgery.
Table 3. Number of affected vertebrae, spine deformity and spine surgery among children and adults with spinal tuberculosis
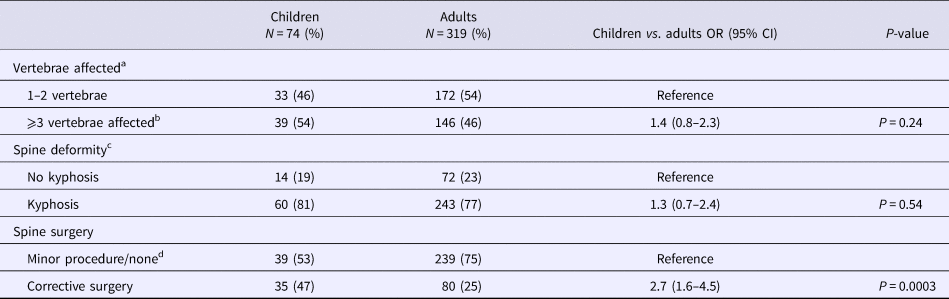
a n = 72 children and 318 adults due to missing data.
b Three to four vertebrae affected (n = 26 (36%) children, n = 94 (30%) adults), ⩾5 vertebrae affected (n = 13 (18%) children, n = 52 (16%) adults).
c n = 315 adults due to missing data.
d No spine surgery (n = 13 (18%) children, n = 3 (1%) adults), biopsy or other minor procedure (n = 26 (35%) children, n = 236 (74%) adults).
Cases by year
Adult cases of spinal TB decreased year-on-year during the study period with 105 cases in 2012 and 56 cases in 2015 (Fig. 3). This decrease was accompanied by a decrease in the incidence of spinal TB among adults in the Western Cape from 2.57 cases per 1 00 000 in 2012 to 1.22 cases per 1 00 000 in 2015. Both case counts and incidence of spinal TB among adults showed a significant downward trend over the 4-year study period (z = −2.04, P = 0.04 for both counts and incidence). Conversely, the number of children diagnosed with spinal TB each year showed no significant trend with 16 cases in both 2012 and 2015 (Fig. 3). The incidence of spinal TB among children also remained similar at 1.03 cases per 1 00 000 and 0.99 cases per 1 00 000 in 2012 and 2015, respectively, with no significant trend.
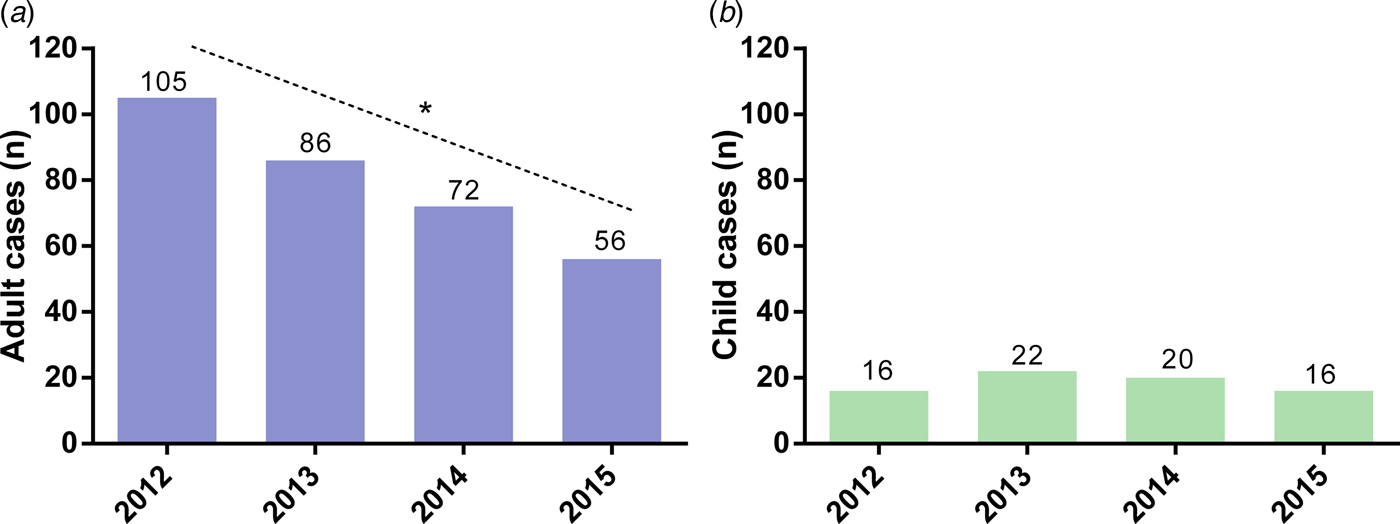
Fig. 3. Number of (A) adults and (B) children diagnosed with spinal tuberculosis at tertiary hospitals in the Western Cape between January 2012 and December 2015 *Significant trend for decrease in cases over the study period (P = 0.04).
Discussion
The main finding of the study was that 393 new cases of spinal TB were treated at tertiary hospitals in the Western Cape between 2012 and 2015 with 72% of all cases bacteriologically confirmed from a spine biopsy. The majority of patients presented with kyphotic deformity and 115 (29%) of all patients received corrective spinal surgery. However, indications for surgical intervention were particularly prevalent among children with approximately one in two children vs. one in four adults receiving corrective surgery (Table 3). These findings highlight the considerable burden of spinal TB in the current setting, including the impact on affected individuals and on the public health system.
Spinal TB among adults in the Western Cape
In the current study, 50% of all spinal TB cases were between 23 and 47 years old with a median age of 38 years among adult cases (Table 1). While previous studies from the USA [Reference De la Garza Ramos4] and Eastern Mediterranean [Reference Batirel11] reported a somewhat older median age of approximately 50 years among spinal TB cases, the current age distribution is similar to findings from Kenya [Reference Mwachaka24] and China [Reference Shi12] and suggests that spinal TB primarily affects adults from a relatively young, economically active population in regions with a high TB burden.
Fifty-seven per cent of adults in the current study were female, a finding in keeping with previous spinal TB studies from South Africa [Reference Held7, Reference Watt and Davis8, Reference Godlwana25, Reference Daniel and Dunn26] but in contrast to other large studies in which 51–61% of spinal TB cases were male [Reference De la Garza Ramos4, Reference Batirel11, Reference Shi12, Reference Mwachaka24]. One possible explanation for this difference is HIV infection and its oft-cited association with extra-pulmonary TB disease [Reference Naing27, Reference Shivakoti28]. For example, whereas HIV co-infection was ⩽4% [Reference De la Garza Ramos4, Reference Mwachaka24] or not reported [Reference Batirel11, Reference Shi12] in the afore-mentioned studies from other parts of the world, 56% of adult females and 36% of adult males with known HIV status in the current study were HIV-positive, indicating a significantly higher HIV-positive prevalence among adult females in this study.
Overall, 41% of adults in the current study were HIV-positive, an HIV prevalence notably higher than the 20% HIV prevalence reported among patients with spinal TB treated at Groote Schuur Hospital in 2013–2014 [Reference Held9]. This difference is most likely explained by the inclusion of children ⩽14 years (none of whom were confirmed HIV-positive) in the HIV prevalence from this previous study along with the inclusion of high HIV prevalence areas in the Tygerberg Hospital catchment area for the current study [29]. Unknown HIV status among 15% of all cases in the current study is in keeping with the 16% unknown HIV status in the afore-mentioned study at Groote Schuur [Reference Held9]. The apparent absence of HIV testing among these cases is a notable concern. However, it is acknowledged that some patients may have received HIV testing without this being reflected in the records available for the current study, e.g. a rapid test conducted at a primary care clinic.
The overall HIV prevalence in South Africa and in the Western Cape has been estimated at 12% and 5% [Reference Shisana30], respectively, suggesting that HIV prevalence may be considerably higher among individuals with spinal TB than in the general population. Most adults with confirmed HIV were diagnosed prior to presentation with spinal TB, were on ART and had lower than detectable VLs (Table 2). Although the majority had CD4 counts <500 cells/μl, only 23% had severe immunosuppression. Those with CD4 count <200 cells/μl did not show increased odds of having ⩾3 vertebrae affected or receiving corrective surgery. However, variation in the timing of CD4 tests and little sensitivity in the measure of disease severity limit the interpretation of these findings.
In addition to the findings of a large burden of cases, relatively young median age and high HIV prevalence, the current study suggests that many adults present with relatively advanced spinal TB disease, given that 46% of adult cases had three or more vertebrae affected and 77% had kyphotic deformity (Table 3). Furthermore, 25% of adults required corrective surgery, on the basis of indications such as spinal instability or progressive neurological deficit [Reference Mak and Cheung17]. Notably, those who received corrective surgery comprised only 30% of all adults with kyphosis and it is assumed that surgery could not be justified in the remainder of the patients with spinal deformity. While treatment outcomes fell beyond the scope of the current study, it has previously been reported that at least 23% of patients managed non-surgically have kyphosis of ⩾10 degrees at the affected level post-treatment [Reference Nene and Bhojraj31]. This permanent deformity may or may not impact on the individual's physical function in the long term. Nevertheless, it is likely to have significant cosmetic and psychological implications for the relatively young, economically active population concerned.
Spinal TB among children in the Western Cape
Approximately 19% of spinal TB cases in the current study were in children (⩽14 years old) with 14% of all cases involving children younger than 10 years old. In contrast to previous findings of a large peak in all TB disease among 0–4 years old in our setting [Reference Snow32, Reference Wood33], the current study found an equal distribution of spinal TB cases in 0–4 and 5–9 years old. This is likely due to a time delay in the onset of spinal TB after initial TB infection; Perez-Velez and Marais suggested that osteoarticular TB manifests 1–3 years after primary infection in children [Reference Perez-Velez and Marais34]. However, it is possible that delayed presentation to the health system and delayed diagnosis may also contribute to the observed distribution of cases. In comparison to young children, there were relatively fewer cases of spinal TB among adolescents (defined as 10–19 years), in keeping with previous findings of an increase in pulmonary rather than extra-pulmonary TB in this age group [Reference Wood33, Reference Marais35].
Gender distribution among children was almost identical to that of adults with 58% of spinal TB cases in children involving females (Table 1). However, the smaller overall number of child cases translated into a smaller absolute difference in male vs. female cases and limits the extent to which this gender distribution can be interpreted. In a similar way, only two of the children in the current study were known to be HIV-positive and the relationship between HIV status and gender in children could not be assessed. The low prevalence of HIV among children in the current study is in keeping with the previous reports of a 2% HIV prevalence among South African children ⩽14 years of age [Reference Shisana30]. However, it is unclear whether this is a true reflection of HIV prevalence within this group given that HIV status was unknown in 28% of children. The relatively large proportion of children with unknown HIV status is of major concern, as young children remain at risk for late diagnosis of perinatal HIV infection [Reference Byamungu36]. While it is possible that evidence of HIV testing may have existed beyond the records available for the current study, previous studies in our setting support a comparatively high proportion of unknown HIV status among children under 5 years old [Reference Held9, Reference Wood33].
The majority of children with spinal TB could be described as having severe disease with 54% having ⩾3 vertebrae affected, 81% presenting with kyphosis and 47% receiving corrective surgery (Table 3).
While children were no more likely than adults to have ⩾3 vertebrae affected or to have kyphotic deformity, they were almost three times more likely to have received corrective surgery (Table 3). The most probable explanation for this is that active spinal TB disease progresses more rapidly in children than in adults with a particularly high risk of deformity among children younger than 10 years old [Reference Rajasekaran15]. The current study recorded kyphotic deformity as a binary outcome rather than quantifying it and one would expect that deformity would have been more severe among children, increasing the risk of spinal instability or progressive neurological deficit. Furthermore, in contrast to adults, children remain at risk of progressive deformity through disproportional growth of vertebral remnants after the active stage of the spinal TB disease [Reference Rajasekaran15]. It follows that increased corrective surgery among children may also partly be explained by surgery to prevent severe deformity in the future. Risk factors for severe deformity may be identified very early in the course of the disease and include age below 10 years, cervical thoracic or thoracolumbar junctional lesions and the well-established ‘spine at risk’ signs described by Rajasekaran [Reference Rajasekaran15].
Trends in the burden of spinal TB
The current study found that adult cases of spinal TB showed a significant decline between 2012 and 2015, whereas cases of spinal TB among children showed no clear trend over the study period (Fig. 3). These patterns were similarly reflected in incidence measures with the incidence of spinal TB among adults decreasing from 2.57 per 100 000 in 2012 to 1.22 per 100 000 in 2015. While these values are broadly similar to the 1.32 spinal cases per 100 000 reported among adults in KwaZulu-Natal in 2005–2006 [Reference Godlwana25], factors such as changes in TB control programmes and the provision of ART in the intervening years limit the scope for further incidence comparison.
A decline in spinal TB cases is consistent with a decrease in the incidence of all TB cases at both national level [37, 38] and in the Western Cape [Reference Massyn39] during the period of study – progress that may be largely attributed to the National Strategic Plan for HIV, STIs and TB (2012–2016) [40]. This plan was developed as the next phase of response to the HIV and TB epidemics within the country and some key applications have included the introduction of the Xpert test for TB diagnosis from 2011, increased TB screening and prophylaxis in high-risk populations and increased ART coverage [Reference Churchyard41]. While these factors are likely to contribute to a decrease in the incidence of all TB cases, it could be speculated that increased ART coverage may be particularly relevant to decrease the incidence of extra-pulmonary TB; earlier initiation of ART would be expected to protect against severely impaired immunity and increased risk of disseminated TB disease among HIV-positive individuals [Reference Jones42].
Unfortunately there is little data available on other types of extra-pulmonary TB in South Africa with which to compare the current trends in child and adult cases of spinal TB. A retrospective review of TB registers at hospitals in the rural Mopani District reported an increase in bone and joint TB from five to 13 cases between 2009 and 2013 [Reference Hoogendoorn43]. However, the study had a number of limitations and it is unclear whether the change in bone and joint TB should be regarded as meaningful. Data from the Electronic Treatment Register (ETR) in the Western Cape suggest a decrease in all new TB cases among children ⩽14 years (6 350 cases in 2012 vs. 5 095 cases in 2015) (unpublished data, Western Cape Department of Health). However, in principle, it is likely to be more difficult to observe trends among child cases of spinal TB given the small absolute numbers involved. A further explanation could be that although the incidence of TB has decreased in recent years, it remains exceptionally high and much larger decreases may be necessary before the benefit can be seen among the vulnerable paediatric population.
The current study focused on trends in absolute case numbers and it fell beyond the scope of the study to estimate trends in the proportion of spinal TB among all TB cases. Some studies from developed countries have reported an increase in extra-pulmonary TB as a proportion of all TB cases [Reference Kruijshaar and Abubakar44–Reference Sandgren, Hollo and van der Werf46]. However, this trend has been attributed to an increase in immigrant populations from Africa and elsewhere and is therefore not necessarily applicable in the developing world. WHO estimates suggest that the proportion of exclusively extra-pulmonary TB cases in South Africa decreased from 14% in 2012 to 12.7% in 2013 and 10% in 2015 [37, 38, 47].
In the case of spinal TB, previous studies from developed countries are inconclusive with some suggesting an increase [Reference Jutte6, Reference Kruijshaar and Abubakar44] and others a decrease [Reference De la Garza Ramos4] in spinal TB cases. At present, trends in the proportion of spinal TB among all TB cases in South Africa and other developing countries remain unclear, partly due to reporting methods that tend to group all types of extra-pulmonary TB together and to record only pulmonary TB when patients have concomitant pulmonary and extra-pulmonary infection. In the future, increased differentiation for reporting severe forms of TB would provide greater insight into the true impact of TB and allow for trends over time to be established.
Limitations
Limitations of the current study include the retrospective nature of the study and the fact that it relied on possible spinal TB patients being referred to tertiary orthopaedic units for diagnostic confirmation. It is possible that some patients may have been diagnosed presumptively without tertiary referral, although these numbers should be low. The current study also does not account for any cases of spinal TB diagnosed in the private health sector. However, these numbers would be expected to be very low, given that only 16% of South Africans belong to private medical schemes [48] and that these individuals are likely to be better resourced with less of the socio-economic adversity associated with TB. Nevertheless, the current findings should be taken to represent the minimum burden for spinal TB in the province rather than the total burden.
Other limitations of the study include the fact that it did not include information about previous episodes of TB or symptoms at presentation, may have overestimated the number of cases with unknown HIV status and had incomplete information regarding the state of HIV illness among HIV-positive individuals. Finally, it is noted that the number of bacteriologically confirmed cases may have been higher had the Xpert test been in use for the whole study period rather than primarily 2014 and 2015.
Conclusion
The current study demonstrated a high absolute burden of spinal TB at tertiary hospitals in the Western Cape in recent years with 19% of cases involving children. Adult cases appear to have declined between 2012 and 2015, although the trend among child cases is less obvious. While this apparent progress is encouraging, the number of cases remains considerable and severe presentation requiring corrective surgery was common, particularly in children. It follows that spinal TB remains a public health concern in the Western Cape, with increased vigilance required for earlier diagnosis, especially among children.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268818002649
Acknowledgements
Many thanks to Mr W. Kleinhans and Mr J. Goodway (National Health Laboratory Service, Tygerberg Hospital) for their assistance with NHLS records and to Dr V. Gezengana (Division of Orthopaedic Surgery, Tygerberg Hospital) for his assistance with clinical imaging.
Financial support
This work was supported by the South African National Research Foundation (NRF). However, it is noted that the NRF was not involved in any aspect of designing or conducting the study. Furthermore, the views expressed in the current report are those of the authors and cannot necessarily be attributed to the NRF. KDP is supported by a South African NRF SARchi Chair in Paediatric Tuberculosis grant to A. C. Hesseling.
Conflict of interest
None.









