Although the primary reason for obesity is energy intake exceeding energy expenditure, it is becoming increasingly clear that overweight can also be associated with individual differences in energy extraction capacity, genetic background and gut microbiota(Reference Bäckhed, Ding and Wang1–Reference Turnbaugh, Ley and Mahowald6). While intestinal microbes contribute to colonisation resistance, nutrition, and the development and tuning of the immune system(Reference McFarland7), studies in both human subjects and animals have also shown a connection between the gut microbiota and obesity(Reference Bäckhed, Ding and Wang1–Reference Cani, Bibiloni and Knauf5).
Antibiotic treatment is known to manipulate the intestinal microbiota. In experimental studies, it has been postulated that antibiotic treatment during pregnancy impairs the physiology and intestinal microbiota of rat pups(Reference Fåk, Ahrné and Molin8), and antibiotic modulation of mouse gut microbiota has been shown to change the metabolic status of the host(Reference Cani, Bibiloni and Knauf5).
Since the sterile fetus is colonised by micro-organisms from the mother and the surrounding environment at birth, it can be hypothesised that the microbial heritage from the mother can be important for offspring development and health. Furthermore, the microbiota of the mother can be affected by dietary factors. For example, a high intake of fat can affect the gut microbiota(Reference Cani, Bibiloni and Knauf5).
Constant exposure to bacteria through diet may well have effects in the long run. In the present study, the two non-pathogenic bacteria Lactobacillus plantarum DSM 15313, designated in the division Firmicutes (family Lactobacillaceae), and Escherichia coli CCUG 29300T (T = type strain), designated in the division Proteobacteria (family Enterobacteriaceae), were given to rat dams and their offspring. L. plantarum frequently occurs spontaneously and in high numbers in most lactic acid-fermented foods, especially those based on plant material, for example, brined olives, capers, sauerkraut, salted gherkins, cassava, and in grape juice and wine(Reference Molin and Mazza9). However, L. plantarum is also present in the human intestinal mucosa(Reference Molin, Jeppsson and Johansson10), and probiotic effects of certain strains of this species have been documented in human subjects, for example, reduction in abdominal bloating and pain in patients with the irritable bowel syndrome(Reference Nobaek, Johansson and Molin11) and reduction in CVD risk factors(Reference Naruszewicz, Johansson and Zapolska-Downar12). Furthermore, administration of L. plantarum to pregnant dams has been shown to stimulate gut growth and development in their suckling offspring(Reference Fåk, Ahrné and Molin13). The particular strain, L. plantarum DSM 15313, used in the present study has been shown to mitigate colitis, translocation and liver injury in rats(Reference Osman, Adawi and Ahrné14, Reference Osman, Adawi and Ahrné15). Refrigerated foods such as milk, meat and vegetables in an early phase of deterioration, before loss of apparent edibility, expose the consumer to different types of bacteria. Those bacteria are typically Gram-negative Proteobacteria such as Pseudomonas and Enterobacteriaceae that have pro-inflammatory lipopolysaccharides (LPS) associated with their cell membranes(Reference Gram, Ravn and Rasch16). E. coli is a Gram-negative commensal in the gastrointestinal tract. Non-pathogenic strains of E. coli can also exert inflammatory stimuli by their LPS, and changed caecal microbiota, as well as increased inflammatory level, have been observed in rat pups with mothers receiving E. coli during pregnancy and lactation(Reference Fåk, Ahrné and Molin8). It has been shown in mice that LPS in the blood (endotoxaemia) is a starting point for insulin resistance and obesity(Reference Cani, Amar and Iglesias17). Moreover, it has been depicted that a high-fat diet increased LPS-containing organisms in the gut and increased transfer of LPS into the blood, which resulted in increased systemic inflammation(Reference Cani, Bibiloni and Knauf5).
High biological diversity normally indicates an ecosystem in healthy balance, while a low bacterial diversity in the gastrointestinal tract has been observed in patients with Crohn's disease, ulcerative colitis and atopic eczema(Reference Dicksved, Halfvarson and Rosenquist18–Reference Wang, Karlsson and Olsson20). Recently, obese twins have been reported to have lower intestinal diversity compared with lean twins(Reference Turnbaugh, Hamady and Yatsunenko4). Interestingly, the probiotic strain L. plantarum DSM 9843 has been shown to improve colonic bacterial diversity in patients with incipient arteriosclerosis(Reference Karlsson, Ahrné and Molin21). However, there is a lack of knowledge of the long-term effect of probiotic administration regarding both gut microbiota and body-weight regulation.
The present study was designed to evaluate the long-term effects on microbiota, weight gain and fattening of regular intake of two fundamentally different non-pathogenic commensal bacteria in combination with a challenging energy-rich diet. To further stress the system, by exposure from fetal life to adulthood, rat dams of the outbreed Sprague–Dawley stock were mated and fed with a high-energy-dense diet (HEDD) and bacterial supplement during pregnancy and lactation, and then their offspring received the same treatment for 6 months.
Experimental methods
Study design and animals
For each study group, three adult female Sprague–Dawley rats (Taconic, Ry, Denmark) of similar weight and age were mated with different male rats. From 2 weeks before parturition, dams were housed individually and were assigned diet and bacterial supplements at that time as described below. At weaning, about 4 weeks of age, nine of the female offspring from each study group were randomly mixed into cages with three animals in each cage. They continued in the experiment for 6 months. Throughout the experiment, animals were housed in polycarbonate cages on chopped aspen wood bedding (Beekay bedding; Scanbur BK AB, Sollentuna, Sweden), and the experiment was run in a controlled environment (21 ± 1°C and 50 ± 10 % relative humidity; 12 h light–12 h dark cycle). The experiment was conducted in compliance with all relevant Swedish laws and institutional guidelines, and the Regional Ethical Review Board, Lund, Sweden, approved the study (approval no. M16-07).
Diets, bacterial supplement and body-weight recordings
The purified HEDD with 41 % of energy as fat, 41 % of energy as carbohydrates, of which half was sucrose, and 18 % of energy as protein was produced by Research Diets (New Brunswick, NJ, USA; see Table S1 of the supplementary material, available online at http://www.journals.cambridge.org/bjn). Feed was administered ad libitum, and the consumption per cage was recorded once a week. In addition to the HEDD, one group received Lactobacillus plantarum DSM 15313 suspended in freezing medium (4·28 mm-K2HPO4, 1·31 mm-KH2PO4, 1·82 mm-sodium citrate, 0·87 mm-MgSO4.7H2O and 1·48 mm of 98 % glycerol) (Lp), while another group was given Escherichia coli CCUG 29300T suspended in the freezing medium (Ec) in their drinking-water. The control (C) group received a corresponding amount of freezing medium in their water. The treatment to dams began during the last two-thirds of pregnancy and continued during lactation. The offspring were then given the same treatment as their mothers until 6 months of age.
A frozen high-density preparation of L. plantarum DSM 15313, originating from the intestine of a healthy human individual, was provided by Probi AB (Lund, Sweden). E. coli CCUG 29300T, a non-pathogenic strain of human origin, was grown in brain heart infusion medium (Oxoid, Basingstoke, Hampshire, UK) in a shaking water-bath at 36°C for 16 h. The cells were stored in freezing medium after harvesting. All bacteria were kept at − 80°C until feeding. The bacterial suspension was poured into the drinking-water on the day of administration. Water was changed, and consumption was recorded daily. Confirmed by culturing, viable counts of L. plantarum and E. coli were stable during 24 h in tap water at room temperature. Each cage was given 109 colony-forming units/d of L. plantarum or E. coli in 200 ml water. Every rat consumed approximately 108 colony-forming units/d of L. plantarum or E. coli. The birth weight of the litter (all pups taken together) was recorded the day after delivery, and a mean birth weight of each animal was assigned. Body weight was measured weekly from weaning until killing.
Procedure at animal killing and blood and organ sampling
At 6 months of age, non-fasted animals were anaesthetised by subcutaneous injection of 0·375 mg fluanisone and 0·012 mg fentanyl citrate/100 g body weight (Hypnorm® VetPharma Limited, Leeds, Yorkshire, UK) and 0·188 mg midazolam/100 g body weight (Dormicum®; Roche, Basel, Switzerland). Under aseptic conditions, a laparotomy was performed through a midline incision. Blood from the aorta was sampled into ice-chilled endotoxin-free polystyrene tubes (Lonza, Copenhagen, Denmark) containing EDTA as an anticoagulant to obtain plasma. Blood was also collected in BD Vacutainer tubes (Becton Dickinson, Plymouth, Devon, UK) to obtain serum. Plasma and serum were stored at − 80°C until further analyses. Plasma for the determination of endotoxin was stored in endotoxin-free tubes (Biopur 1·5 ml tubes; Eppendorf, Hamburg, Germany). Left retroperitoneal and periovarial adipose tissue, spleen and adrenals were carefully dissected and weighed. Caecal and rectal content and liver tissue were frozen, in freezing medium for culturing, in liquid N2, and stored at − 80°C until analyses.
Biochemical analyses
Plasma leptin was analysed using ELISA (Crystal Chem, Inc., Downers Grove, IL, USA), with the detection limit of 0·2 ng/ml. Plasma haptoglobin was analysed colorimetrically, with an assay sensitivity of 0·05 mg/ml (Phase™ Range Haptoglobin Assay; Tridelta Development Limited, Kildare, Republic of Ireland), according to the manufacturer's instructions. Plasma endotoxin was determined by an accredited chromogenic endpoint method based on limulus amebocyte extract (Charles River Laboratories, Charleston, SC, USA). Samples were diluted 1:10 and heated at 75°C for 10 min; the detection limit was 0·03 IU/ml. Each sample was spiked with endotoxin, and 50 % recovery was ensured in order to analyse lack of inhibitors and verify reliability. Serum bilirubin, aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase were analysed colorimetrically, and rectal content of calprotectin was analysed using ELISA (Immundiagnostic AG, Bensheim, Germany).
Liver histology
Liver tissue was fixed in 4 % buffered formaldehyde (Histolab, Göteborg, Sweden), rinsed in water and dehydrated in graded ethanol concentrations, followed by xylene and embedded in paraffin. Sections (5 μm) were mounted on slides, deparaffinised and stained with haematoxylin and eosin, and the prevalence of steatosis was scored as follows: 0, no visible steatosis; 1, steatosis in < 1/3 of the hepatocytes; 2, steatosis in 1/3–2/3 of the hepatocytes; 3, steatosis in >2/3 of the hepatocytes. The histological evaluation was performed by a blinded scientist.
Microbial analyses
In order to analyse the amount of viable lactobacilli and Enterobacteriaceae in the caecum, a conventional dilution series was performed, and samples from appropriate dilutions were plated on Rogosa agar (Oxoid) and violet red bile dextrose agar (Oxoid) and incubated at 37°C for 72 h anaerobically and 24 h aerobically, respectively. Translocation of viable bacteria was evaluated by anaerobic and aerobic cultivation of liver tissue on the brain heart infusion agar (Oxoid) at 37°C for 72 h. Microbial diversity was assessed by the terminal restriction fragment length polymorphism (T-RFLP) method, and cloning and sequencing were used to imply phylogenetic affiliations of the intestinal microbiota. The procedures are described in the supplementary material (available online at http://www.journals.cambridge.org/bjn).
Calculations
The relative abundance of each terminal restriction fragment (T-RF) within a given T-RFLP pattern was calculated as the peak area of the respective T-RF divided by the total peak area of all T-RF, in the given T-RFLP pattern, detected within a fragment length of 20–600 bp. Relative abundance was used for calculations of diversity index. Shannon and Weaner (Shannon, H′) and Simpson (D) indices were calculated for all individuals by using the equations:
where D = Σp i2 and p i is the relative abundance of the ith peak in the community(Reference Magurran22). T-RF incidences were calculated using Fisher's exact test, and two-tailed P values were reported.
Statistical evaluation was performed using the Kruskal–Wallis test followed by the Nemenyi–Damico–Wolfe–Dunn test(Reference Hollander and Wolfe23) using the package coin(Reference Hothorn, Hornik and van de Wiel24) in the statistical software R 2.9.1 (The R Foundation for Statistical Computing, Vienna, Austria). Spearman rank order correlation coefficients were calculated using SigmaStat 3.1 (Systat Software, Point Richmond, CA, USA), and Fisher's exact test was performed with Statistics Online Computational Resource (http://www.socr.ucla.edu/SOCR.html). A P value of < 0·05 was considered significant.
Multivariate data analysis with orthogonal partial least squares to latent structures discriminant analysis was performed using SIMCA-P+12.0.1 (Umetrics, Umeå, Sweden) to reveal differences between the treatment groups(Reference Trygg25, Reference Wiklund, Johansson and Sjöström26).
Results
Animal health and performance
All animals (nine animals per group) appeared healthy throughout the study, with the exception of one rat in group Ec that was killed after 12 weeks after veterinarian consultation and recommendation, due to the finding of a subcutaneous tumour. Data from this rat were excluded. Of the remaining Ec rats, one was perceptibly leaner than the others, as confirmed at dissection at the end of the study. No signs of disease could be diagnosed, and this rat was included in the data evaluation. No difference in feed and water consumption was observed among the groups.
Effects on body and organ weights
Pups in group Lp had the lowest (7·47 g) and group Ec had the highest (9·65 g) mean birth weight, while group C had intermediate birth weight (8·15 g). However, because of the limited number of litters, statistical analysis could not be performed. Group Lp had lower body weight throughout the study compared with groups C and Ec, and at 6 months; the difference approached statistical significance (P = 0·086; Fig. 1 and Table 1). Significantly more retroperitoneal fat was observed in group Ec compared with group Lp (P = 0·03; Table 1). The weight of retroperitoneal fat correlated positively with body weight after 6 months (r 0·454, P = 0·02). Group Ec had a higher median weight of the periovarial fat deposit compared with the other groups, but the difference was not significant (Table 1). The weight of spleen was higher in group Ec than in group Lp (P = 0·006; Table 1). Group Lp had heavier adrenals than group C (P = 0·022; Table 1).

Fig. 1 Body weight development. Values are means (from weaning until 6 months of age), with their standard errors represented by vertical bars. * The body weights were significantly different at 4 weeks; the group exposed to Escherichia coli CCUG 29300T (Ec, ![]() ) had a significantly higher body weight than the control group (C) (P = 0·002). The body weights were significantly different at 5 weeks; group Ec was heavier than groups C (■) and the group exposed to Lactobacillus plantarum DSM 15313 (Lp,
) had a significantly higher body weight than the control group (C) (P = 0·002). The body weights were significantly different at 5 weeks; group Ec was heavier than groups C (■) and the group exposed to Lactobacillus plantarum DSM 15313 (Lp, ![]() ): † P = 0·003; ‡ P = < 0·001, respectively. The body weights were not significantly different after 6 months, but group Lp tended to have a significantly lower body weight (P = 0·086).
): † P = 0·003; ‡ P = < 0·001, respectively. The body weights were not significantly different after 6 months, but group Lp tended to have a significantly lower body weight (P = 0·086).
Table 1 Body weights, weights of fat deposits, spleen weight, adrenal weights and liver steatosis in rats fed a high-energy-dense diet with bacterial supplements for 6 months
(Medians and interquartile ranges)
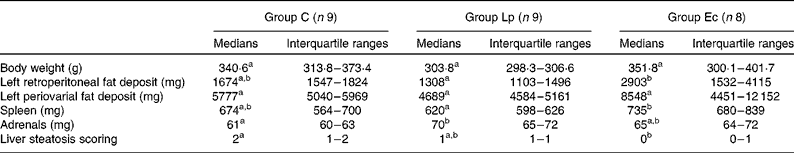
C, control; Lp, Lactobacillus plantarum DSM 15313; Ec, Escherichia coli CCUG 29300T.
a,b Values within a row with unlike superscript letters were significantly different (P ≤ 0·05).
Effects on liver histology
The incidence of liver steatosis was significantly lower in group Ec compared with that in group C (P = 0·008; Table 1).
Effects on biochemical markers
Group Ec showed significantly higher plasma leptin concentrations than group Lp (P = 0·035; Table 2). Haptoglobin was significantly lower in group Ec compared with group C (P = 0·003; Table 2), but no significant differences were observed in the blood levels of endotoxin, bilirubin or the liver enzymes aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase, nor in intestinal calprotectin (Table 2). However, group C had the highest aspartate aminotransferase concentration, which approached statistical significance (P = 0·087).
Table 2 Concentrations of biochemical markers in rats fed a high-energy-dense diet with bacterial supplements for 6 months
(Medians and interquartile ranges)

C, control; Lp, Lactobacillus plantarum DSM 15313; Ec, Escherichia coli CCUG 29300T; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase.
a,b Median values within a row with unlike superscript letters were significantly different (P ≤ 0·05).
* P = 0·087.
Effects on microbiota
Significantly lower bacterial diversity was observed in the caecum of group Ec compared with that in group C when diversity indices were calculated for the T-RFLP profiles (Table 3). When MspI was used for DNA digestion, group Ec had significantly lower diversity than group Lp (Table 3).
Table 3 Microbial diversity in caecal content, calculated with Shannon and Simpson diversity indices, and viable counts of Enterobacteriaceae and lactobacilli
(Medians and interquartile ranges)
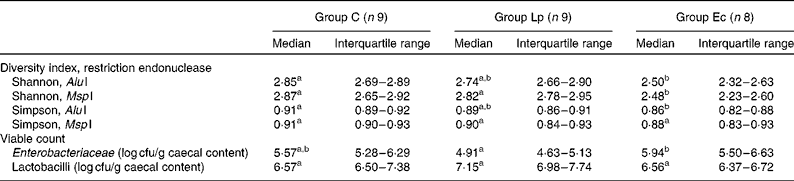
C, control; Lp, Lactobacillus plantarum DSM 15313; Ec, Escherichia coli CCUG 29300T; cfu, colony-forming units.
a,b Median values within a row with unlike superscript letters were significantly different (P ≤ 0·05).
Clone libraries from the different groups showed that divisions Firmicutes, Bacteroidetes and Verrucomicrobia were represented in all three groups at a level ranging 65–77, 4–26 and 5–15 %, respectively. At the hierarchical level of family, the same five taxa dominated the microbiota in animals with the most diverse microbiota (according to T-RFLP profiles) in groups C and Ec, while nine taxa were seen in group Lp (Fig. 2). As regards proportions of the microbiota, significant differences were observed between the treatment groups at division level. Members of the Bacteroidetes were less prevalent in the Ec library than in the C library (LibCompare in Ribosomal Database Project, P = 0·036) and were close to significance for less prevalence in the Lp library compared with the C library (P = 0·056). No significance in proportions of sequences belonging to Firmicutes was observed. All sequences of Verrucomicrobia, independent of the treatment group, corresponded to Akkermansia muciniphila. Incertae sedis XIII included sequences most similar to those of Anaerovorax odorimutans but with a low similarity score (0·94). Incidence of T-RF differed significantly between groups Lp and C, and between groups Ec and C (see Table S2 of the supplementary material, available online at http://www.journals.cambridge.org/bjn). The gut microbiota was also significantly different in groups Lp and Ec, since group Ec, with AluI digestion, had the highest incidence of T-RF at 77 bp and the lowest incidence of 203 bp. With MspI digestion, the T-RF at 257 bp was higher, and T-RF of 80, 258 and 516 bp was found at significantly lower incidence in group Ec than in group Lp.

Fig. 2 Identification of sequences in clone libraries to the level of family. The figure shows the families to which clones in each library are related. Results are given as percentage of total number of clones for each specific library. In the control group (■), forty-one clones were sequenced, and in the Lactobacillus plantarum DSM 15313 (□) and Escherichia coli CCUG 29300T (![]() ) groups, forty-four and eighty-four clones were sequenced, respectively.
) groups, forty-four and eighty-four clones were sequenced, respectively.
The viable count of Enterobacteriaceae in the caecal content was significantly higher in group Ec compared with that in group Lp (P = 0·019; Table 3) and correlated positively with body weight after 6 months (r 0·428, P = 0·029). No significant differences were observed in the lactobacilli count of caecal content or the translocation of viable bacteria to the liver.
Multivariate analysis
Results of multivariate data analysis with orthogonal partial least squares to latent structure discriminant analysis depict a substantial difference between the different groups. Important for the separation of groups Lp and Ec were fat deposition, viable count of Enterobacteriaceae and microbial diversity (Fig. 3). Significant for the separation of groups Ec and C were fat deposition and diversity indices, while separation of groups Lp and C were based on body weight and weight of adrenals, Q2 0·79 and 0·58, respectively.

Fig. 3 Multivariate analysis with orthogonal partial least squares to latent structures (OPLS-DA). OPLS-DA score and loading column plots ((a) and (b), respectively) to discriminate the correlation between the Lactobacillus plantarum DSM 15313 (Lp) and Escherichia coli CCUG 29300T (Ec) groups. Fat deposition, viable count of Enterobacteriaceae and diversity of the intestinal microbiota were important for the separation of groups Lp and Ec. Q2 was 0·55. ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; ●, 0; ▲, 1; t [1], 1st score vector; to [1], orthogonal 1st vector; pq [1], 1st predicted OPLS component (p loading for x and q loading for y); Var ID, variable ID.
Discussion
Feeding with an HEDD and the two bacterial supplements was initiated during pregnancy, because it is known that metabolic malprogramming in the offspring can occur when the dietary intake of the pregnant dam is manipulated(Reference Srinivasan, Katewa and Palaniyappan27, Reference Bayol, Farrington and Stickland28). In the present study, E. coli was chosen as one supplement, since it has been shown that this Gram-negative bacterium, with pro-inflammatory LPS associated with its cell wall, can occasionally dominate the intestinal microbiota in human infants(Reference Wang, Ahrné and Antonsson29). E. coli was also observed to dominate in the sigmoidal mucosa in one out of nine 60-year-old volunteers without clinical symptoms or medication when sequence analysis of cloned 16S rRNA genes, as enriched by PCR from biopsies, was applied (91 % of the clone library were related to E. coli; G Molin, unpublished results). In contrast, L. plantarum was selected since it is a Gram-positive commensal of the gastrointestinal tract and can confer health benefits as probiotics(Reference Molin, Jeppsson and Johansson10, Reference Nobaek, Johansson and Molin11).
In the present 6-month study, long-term bacterial supplementation seemed to affect the weight development of the rats, without having any effect on the feed consumption. Furthermore, when repeated-measures ANOVA was calculated, group Lp had significantly lower body weight compared with group C (data not shown). Importantly, L. plantarum decreased body-weight gain, and E. coli increased body fat, the latter being consistent with the hypothesis of Cani et al. (Reference Cani, Bibiloni and Knauf5), which suggests increased body-weight gain by high-fat feeding and an increased load of Gram-negative bacteria. To our knowledge, effects of probiotics on weight gain have not previously been experimentally evaluated from fetal life to adulthood. However, Lactobacillus gasseri SBT2055 has been shown to significantly reduce abdominal visceral and subcutaneous fat(Reference Kadooka, Sato and Imaizumi30), and lower BMI has been suggested in children after Lactobacillus rhamnosus GG administration(Reference Luoto, Kalliomäki and Laitinen31).
Intestinal diversity has previously been shown to increase in rodents receiving probiotics(Reference Fuentes, Egert and Jimenez-Valera32, Reference Montesi, Garcia-Albiach and Pozuelo33). However, these findings were from short-term studies, in which no physiological parameters were evaluated. Thus, there is a general lack of knowledge regarding the long-term effects of bacterial supplements on intestinal microbial diversity in combination with changed homeostasis. Calculation of diversity index is a common way to estimate microbial diversity(Reference Dicksved, Halfvarson and Rosenquist18, Reference Wang, Karlsson and Olsson20–Reference Magurran22), and low diversity has been associated with various diseases(Reference Dicksved, Halfvarson and Rosenquist18–Reference Wang, Karlsson and Olsson20).
In the present study, reduced bacterial diversity indices were observed in group Ec compared with group C. Group Ec had a significantly increased viable count of Enterobacteriaceae in the caecum compared with group Lp, as well as lower diversity indices, thereby indicating that E. coli consumption might associate with a tendency of overgrowth of unfavourable bacterial groups. Furthermore, the amount of Enterobacteriaceae in the caecal content was positively correlated with body weight. The incidence of specific T-RF varied among the groups, which further supports the observation of different intestinal microbiota according to treatment.
Previous reports have suggested a reduction in the abundance of Bacteroidetes and a proportional increase in Firmicutes in obese individuals(Reference Ley, Bäckhed and Turnbaugh2, Reference Ley, Turnbaugh and Klein3). However, this was not the case in the present study as both the groups of the heavier Ec animals and the leaner Lp animals had lower proportions of Bacteroidetes compared with group C. Furthermore, the proportion of Firmicutes did not vary between the groups. This suggests that the microbiota must be dealt with on lower hierarchical levels than that of division, preferably on the species level but at least at family and genus levels. It also seems important to look into different experimental models with different strains and species of animals, and also different categories of human subjects. Hence, more extensive studies are required to fully explain the connection between gut microbiota and obesity.
In an attempt to evaluate the correlation of intestinal microbiota and changed homeostasis, we measured various biochemical markers. Previously, adult offspring to LPS-exposed dams have been reported to have higher body weight, heavier fat deposits and higher serum leptin(Reference Nilsson, Larsson and Jennische34). The same scenario was true in the present study for the group where the mothers and the offspring received E. coli. Interestingly, the opposite effect was observed in the group receiving L. plantarum: lower leptin levels were observed in combination with lower body weight.
The acute-phase protein haptoglobin, mainly produced by the liver(Reference Taes, De Bacquer and De Backer35), is known to increase during inflammation, but contradictory results have also been reported(Reference Perez-Echarri, Perez-Matute and Marcos-Gomez36, Reference Due, Toubro and Stender37). Since obesity is characterised by low-grade inflammation(Reference Cani, Bibiloni and Knauf5, Reference Fantuzzi38) and group Ec in the present study had more body fat and higher leptin levels, the animals in this group were assumed to have a higher inflammatory tone. In spite of this, group Ec had significantly lower haptoglobin concentrations compared with group C. Interestingly, one of the primary functions of haptoglobin is to restrict Fe availability to pathogenic bacteria by the formation of an irreversible complex with Hb released from damaged erythrocytes(Reference Wejman, Hovsepian and Wall39, Reference Eaton, Brandt and Mahoney40). The decreased haptoglobin concentration found in group Ec might be a result of even non-pathogenic E. coli strains being equipped with efficient means (siderophores) to absorb Fe(Reference Smarda, Smajs and Horynova41). In the present study, significantly heavier spleens were found in group Ec compared with group Lp, indicating systemic inflammation in group Ec(Reference Siegmund, Rieder and Albrich42). Furthermore, intravascular haemolysis is associated with splenomegaly and decreased haptoglobin levels(Reference Tolosano, Fagoonee and Hirsch43).
Esposito et al. (Reference Esposito, Iacono and Bianco44) reported reduced liver damage and inflammation in young rats on a high-fat diet when this was combined with the probiotic mixture VSL#3 containing different strains of Lactobacillus and Bifidobacterium, but no microbial evaluation was performed. In the present study, no statistically significant changes in liver enzymes were observed. However, aspartate aminotransferase and haptoglobin concentrations were highest in group C, which is in accordance with the higher occurrence of liver steatosis in this group. Non-pathogenic strains of E. coli have indicated improvement of liver function, while other Enterobacteriaceae showed the opposite(Reference Li, Wang and Chen45). This highlights the potential to influence the level of steatosis and liver function by modulating the intestinal microbiota. Notably, in the present study, eating behaviour was not controlled before blood sampling at killing, and, for example, plasma LPS is enhanced after a meal(Reference Erridge, Attina and Spickett46), thus some measurements may be on the small side.
It should be stressed that in the present study, calprotectin in the intestinal content did not vary between the groups, suggesting absence of intestinal inflammation. Hence, although systemic effects were observed, neither E. coli nor L. plantarum seemed to have injured the gastrointestinal tract.
The weight of adrenals was significantly higher in group Lp than in group C, but it was not possible to distinguish the weights of the medulla and cortex, making it hard to evaluate the consequences and underlying relevance of the difference in the weight of this organ.
Although not totally conclusive, the present results indicate that it may be possible to affect host homeostasis by bacterial supplementation to rats consuming an HEDD. In future studies, larger study populations should be used, and physiological and microbial characterisation of rat dams should be addressed more extensively. Furthermore, extensive evaluation of the offspring early in life should be performed to distinguish the effect obtained on the fetus in utero, during the neonatal and weaning periods. Litter sizes and social behaviour, such as cage hierarchy, should also be considered.
In summary, the long-term effects of bacterial supplements to HEDD-fed rats were studied from fetal life to adulthood in outbreed rats. Animals receiving L. plantarum had lower body weight, less retroperitoneal adipose tissue and lower plasma leptin compared with those that were given E. coli. The diversity of the intestinal microbiota was reduced in rats consuming E. coli, which highlights the potential of modulating intestinal microbiota and homeostasis. As shown by the multivariate analysis, fat deposition and weight gain were affected by the gut microbiota, and manipulated by the supplementation of bacteria to an HEDD.
Acknowledgements
Mrs Camilla Björklöv is thanked for her assistance with the animal care and Mrs Susanne Eiswold for embedding of the liver tissues. Dr Fredrik Nilsson at the Competence Centre for Clinical Research, Region Skåne (Lund, Sweden), is greatly acknowledged for his statistical recommendations and calculations in R, and Martin Berntsson at Umetrics (Umeå, Sweden), for his guidance in multivariate evaluation of the results. The present study was supported by grant from the Swedish Research Council Formas (grant no. 222-2007-501). G. M., B. J. and S. A. are minority shareholders in Probi AB (Lund, Sweden), and the authors' relationship between the company and their positions at the university has been regulated by an agreement between Lund University (Lund, Sweden) and Probi AB (Lund, Sweden). C. L. J. K., F. F., M.-L. J. H., M. J., Å. H. and B. W. disclose no conflicts of interest. C. L. J. K., G. M., F. F., M. J., Å. H., B. J., B. W. and S. A. designed the study; C. L. J. K., F. F., M. J. and Å. H. collected the data; C. L. J. K., G. M., M.-L. J. H. and S. A. analysed and interpreted the data; C. L. J. K., G. M. and S. A. wrote the manuscript; all authors read and approved the final version of the manuscript.








