Introduction
Parasites that we encounter in nature may be species-specific or may have a wide range of hosts. The latter are strenuous to control because they can lie dormant in their reservoir hosts for long periods of time before infecting other hosts. Similarly, zoonotic parasites are difficult to control and pose a concern to human health due to their proclivity for residing in diverse hosts (Allen et al., Reference Allen, Murray, Zambrana-Torrelio, Morse, Rondinini, Di Marco, Breit, Olival and Daszak2017). Livestock helminths, among other zoonotic viruses, bacteria and other infections, are a cause of health concern for humans (Libera et al., Reference Libera, Konieczny, Grabska, Szopka, Augustyniak and Pomorska-Mól2022). Helminth parasites that infect the livestock, significantly affect their health and reproduction (Rehman & Abidi, Reference Rehman and Abidi2022). Although Haemonchus contortus is considered as the notorious parasite of livestock because of its reproductive potential and blood sucking ability, Trichostrongylus remains one of the most frequent and extremely pathogenic parasites in cattle, and because of its zoonotic potential, Trichostrongylus is dangerous to human health (Getachew et al., Reference Getachew, Dorchies and Jacquiet2007). Human infection by Trichostrongylus is more prevalent in the pastoral communities who raise livestock or eat vegetables that are fecundated with faecal ordure. Humans become infected after consuming food or water contaminated with faeces of the definitive host (ruminant and humans). Gastrointestinal symptoms with disparate morbidity may develop in some patients; however, most patients are asymptomatic but have eosinophilia as the only symptom (Buonfrate et al., Reference Buonfrate, Angheben, Gobbi, Mistretta, Degani and Bisoffi2017). Till date, Trichostrongylus infections in humans have been reported only in a few parts of the world. The reason for such rarity in cases of human trichostrongylosis could most probably be the occurrence of asymptomatic infections and comparably less sensitivity of microscopic detection due to low egg-output (Buonfrate et al., Reference Buonfrate, Angheben, Gobbi, Mistretta, Degani and Bisoffi2017). As a result, there could be many undiagnosed cases of Trichostrongylus infection in the human populations worldwide (Wolfe, Reference Wolfe1978). Trichostrongylus is similar to hook worms, particularly Ancylostoma duodenale and Necator americanus concerning its transmission, pathophysiology and morphology at certain life-cycle stages; as a result, very little is known about the population biology and epidemiology of Trichostrongylus spp. (Yong et al., Reference Yong, Lee and Sim2007). There is lack of information regarding the global status of trichostrongylosis among humans. It is necessary to compile the pertinent scientific material and examine pastoral populations for the incidence of this parasite. Little to no work has been done on the pathogenicity and immune response to this worm in relation to humans. This review aims to compile literature about the: (a) pathogenesis of trichostrongylosis in humans; (b) immune response to Trichostrongylus invasion and its mechanism of action; and (c) epidemiology of this zoonotic parasite in relation to humans.
Life cycle and transmission
Using no intermediate host and exhibiting a direct life cycle, Trichostrongylus adults live in the gastrointestinal tract (GIT) at particular micro-niches depending on the species. Females lay eggs in the host's GIT which are then excreted with the faeces of the host. As soon as the eggs are in a favourable environment, they embryonate into the L1-larvae which moults two times and develops into the infective L3-larvae in about five days and may remain viable for about six months (Levine & Anderson, Reference Levine and Anderson1973). Throughout the summer months of June to August, most trichostrongylid larvae occur on the grass blades representing greater chances of infection in grazing animals (Crofton, Reference Crofton1948). Cattle become infected after consuming the L3-larvae while feeding on contaminated grasses. Once ingested, L3-larvae of Trichostrongylus spp. reach the predilection site, for example, the L3-larvae stage of Trichostrongylus axei inhabits the abomasum of ruminants where they complete their development and the adult worms then penetrate the lining of the abomasum. In some other species, the L3-larvae reach the small intestine and invade the crypts to complete their development into L4 and L5 larval stages. Depending on the species and the host, the prepatent period is usually three to four weeks (Janquera, Reference Janquera2017) but can extend up to two years (Ralph et al., Reference Ralph, O'Sullivan, Sangster and Walker2006). Trichostrongylus colubriformis lives in mucoid passages on the surface of duodenal and intestinal villi (Shaw et al., Reference Shaw, McNeill, Maass, Hein, Barber, Wheeler and Shoemaker2003). Humans acquire trichostrongylosis when L3-larvae of Trichostrongylus spp. are ingested orally while consuming contaminated food. Application of night-soil (human excreta) or livestock faecal matter as manure and the resistant nature of the eggs gives rise to the propagation of this parasite in human populations (Sharma & Anand, Reference Sharma and Anand1997). Shady areas with high humidity and an abundance of grass are more favourable for the spread of Trichostrongylus (Watson, Reference Watson1953).
Methodology
Databases such as Google Scholar, PubMed, Scopus, Web of Science and ScienceDirect were searched to collect and review the published scientific research articles related to trichostrongylosis among humans. Terms such as Trichostrongylus, trichostrongylosis/liasis, zoonosis/es/tic, human, transmission, case/s, report, diagnosis, pathogenicity/sis, immunological, immune response, etc. were used in multiple combinations to search for relevant research articles. Further, the bibliographic section of research articles was also searched to extract relevant references. In addition to these, any other relevant research articles and/or case reports from other sources were also reviewed during the study. Research articles with information regarding number of cases in humans, method of diagnosis, symptoms, country name and/or pathogenesis and immune response were included in this study. Research articles related to other hosts were excluded. We used Endnote to compile the articles and then thoroughly read the papers to extract information such as year, country/region, number of cases, method of examination, mode of transmission, symptoms and any other important findings (table 1). Statistics from these articles are compiled in figs 3–5.
Table 1. Reported cases of human trichostrongylosis globally in scientific publications from 1938 to 2022.
Pathogenicity
Human trichostrongylosis is typically a minor, subclinical condition as indicated by the fact that diagnosed cases have only been identified through screening. Nevertheless, heavier infection may result in emaciation, pain in the abdomen and diarrhoea along with slight anaemia and eosinophilia in adults while causing retardation of development in children (Hollo et al., Reference Hollo, Rovo and Hidvegi1970). Persons with infection intensity of 24–300 eggs per gram (EPG) of faeces are symptomatic while an infection intensity below 24 EPG shows no symptoms (Ghadirian & Arfaa, Reference Ghadirian and Arfaa1975; Wolfe, Reference Wolfe1978). In small ruminants the symptoms of trichostrongylosis are more severe causing ‘black scour disease’ which is characterized by dark green to black diarrhoea, that covers the crutch, hocks and legs. There is extreme emaciation in heavily infected sheep with wasting of musculature and negligible amount of renal and omental fat (Edgar, Reference Edgar1933). Craig (Reference Craig, Anderson and Rings2009) reported that trichostrongylosis may lead to moderate anaemia as the worms may feed on the host's blood from mucosa of the gut. Histopathological studies carried out by Barker (Reference Barker1975) showed that T. colubriformis causes severe villus atrophy and plasma loss in the sheep gut. Acutely infected mucosa becomes flat, has stunted epithelium, with projecting crypts often leaking eosinophilic material, exhibiting hyperplasia with highly inflammated lamina propria due to cell infiltration. The intestinal epithelial surface has erosions and necrosis which may be most probably caused by operational movement of adult nematodes. In the case of Trichostrongylus vitrinus, exsheathed L3-larvae burrow through intestinal villi and form submucosal tunnels and when adults emerge out of these tunnels, they cause considerable damage to the mucosal layer (Beveridge et al., Reference Beveridge, Pullman, Phillips, Martin, Barelds and Grimson1989). Alterations in the intestinal morphology due to Trichostrongylus infection cause a decrease in the activity of enzymes such as alkaline phosphatase and leucine amino peptidase (Shayo & Benz, Reference Shayo and Benz1979). Though mildly pathogenic, Trichostrongylus may cause severe complications among young and weak livestock, sometimes proving fatal (fig. 1).
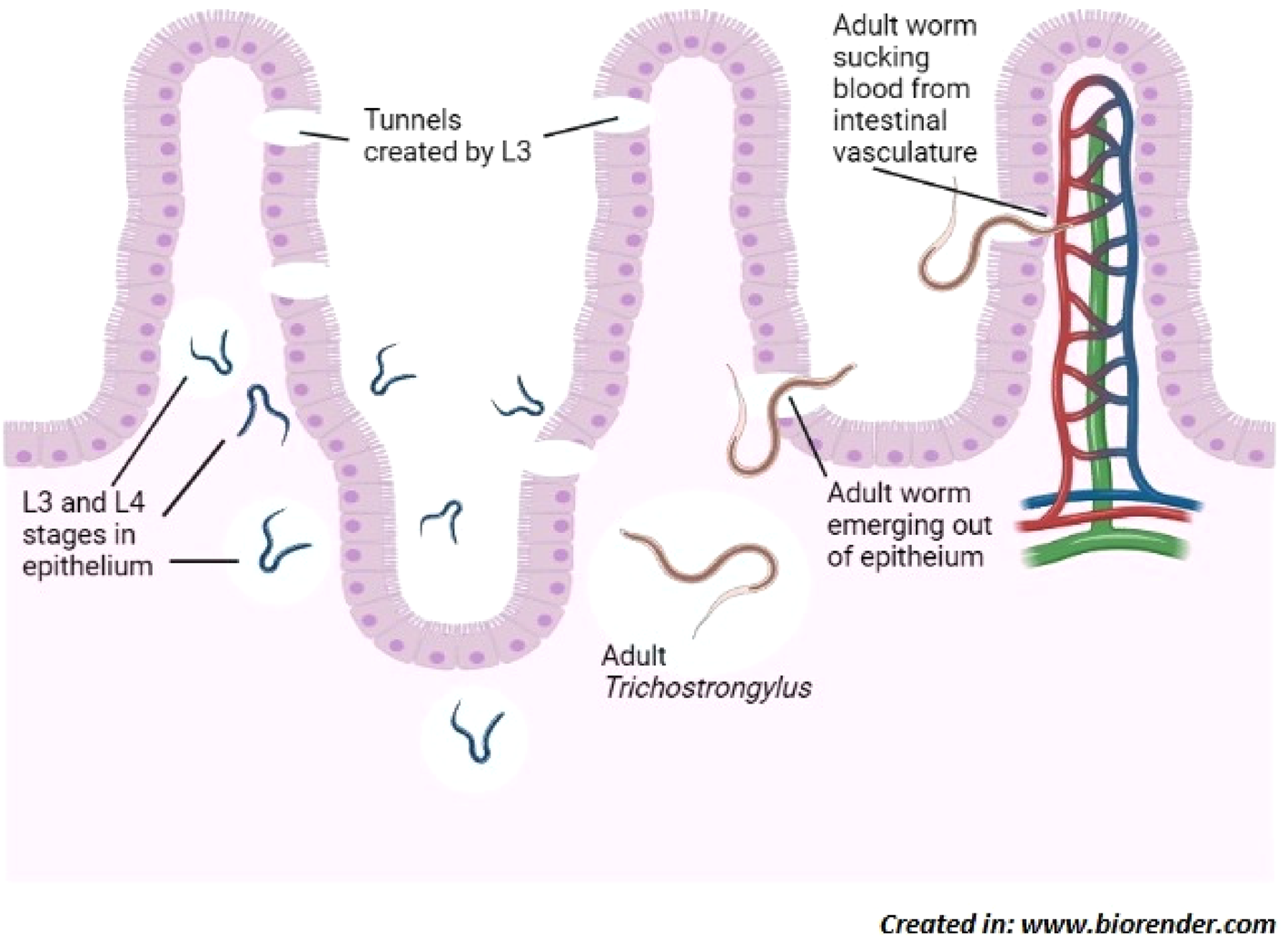
Fig. 1. Activity and development of Trichostrongylus sp. in intestine of host; L3-larvae stages burrow through epithelium resulting in damage to intestinal villi and enteritis. Then adults emerge out through tunnels into the lumen of intestine and occasionally suck blood from the intestinal vasculature which leads to anaemia.
Immune response
The aim of the parasite is to establish itself successfully in/on the host without killing it. To do so, the parasite manipulates the immune response of the host, and either the parasite evades the host immune system by different mechanisms or it alters the immune response of host to make it ineffective. To counter this, the host tries to mount an effective immune response against the parasite to kill and expel it. Usually in helminth infection, T helper 2 or Type 2 response is initiated by the host. It includes expression of interleukin-4, interleukin-5, interleukin-9, interleukin-13, interleukin-21, interleukin-33 (IL-33) and proliferation and activation of plasma cells to secrete immunoglobulin E (IgE), ocytess and mast cells to secrete vasoactive amines. With respect to trichostrongylosis in humans, limited research has been conducted on immunological response. An in vitro study using a co-culture system of T. colubriformis and epithelial cell from humans showed that movement of T. colubriformis at the site of infection creates necrosis of intestinal epithelial cells. The necrosis in turn induces the release of intracellular contents, including IL33 which is elemental in the commencement of appropriate host response to gastrointestinal nematodes (Andronicos et al., Reference Andronicos, McNally, Kotze, Hunt and Ingham2012). It has been found that after recurrent infections in natural conditions, sheep can develop immunity against T. colubriformis. In a natural foraging environment, the ability to withstand nematode establishment occurs after seven weeks of incessant infection (Dobson et al., Reference Dobson, Waller and Donald1990). When a sheep is fed with Trichostrongylus larvae, most of them are expelled in less than 24 h under a hypersensitivity response known as rapid rejection (Miller et al., Reference Miller, Jackson, Newlands and Huntley1985). The sheep shows resistance to Trichostrongylus by mounting an inflammatory response in gastro-intestinal mucosa which is evident by increase in mucosal mast cells and globule leucocytes (Miller et al., Reference Miller, Jackson, Newlands and Huntley1985; Douch et al., Reference Douch, Harrison, Elliott, Buchanan and Green1986). This inflammatory response seems to be genetically controlled and mast cells play a major role in resistance against Trichostrongylus (Gill, Reference Gill1991). When treated with parasite antigen, mast cells from resistant sheep release around 39% of cellular mast cell protease (CMCP) as compared to less than 8% CMCP release by mast cells from sheep with primary infection of Trichostrongylus (Bendixsen et al., Reference Bendixsen, Emery and Jones1995). Jones & Emery (Reference Jones and Emery1991) demonstrated that sheep immunized with T. colubriformis release a number of inflammatory mediators such as histamine, leukotriene C4, 6-keto prostaglandin F1α and thromboxane B2 on secondary infection with leukotriene C4 being the most predominant inflammatory mediator in expulsion of nematodes from intestines. In guinea pigs immunized with irradiated T. colubriformis larvae, release of biological amines (histamine and 5-hydroxytryptamine) and enteric plasma was found to be involved in resistance to secondary infections of this parasite (Steel et al., Reference Steel, Jones and Wagland1990).
Serum IgE levels have been found to escalate following nematode infections (Shaw et al., Reference Shaw, Morris, Green, Wheeler, Bisset, Vlassoff and Douch1999). Shaw et al. (Reference Shaw, McNeill, Maass, Hein, Barber, Wheeler and Shoemaker2003) found T. colubriformis aspartyl inhibitor (Tco-API-1) as a strong allergen that produces an overwhelming IgE response in sheep, when produced endogenously by nematode; however, when administered separately, Tco-API-1 does not evoke IgE response. In response against T. colubriformis, serum immunoglobulin G1 (IgG1) and immunoglobulin M (IgM) titres elevate significantly by 35 days of infection with IgG1 being more persistent than IgM (Douch et al., Reference Douch, Risdon and Green1994). IgG1 and immunoglobulin G2 (IgG2) levels in gut associated lymphoid tissue were observed to be greater in Merino sheep during T. colubriformis larval rejection (McClure et al., Reference McClure, Emery, Wagaland and Jones1992). Treatment with the corticosteroid dexamethasone has been found to inhibit the progress of nematode resistance and reversibly reduce the expression of existing resistance in sheep, as indicated by higher faecal egg count and persistent weight loss. Dexamethasone functions by decreasing the production of leukotrienes and preventing the advent of mast cells in intestines (Douch et al., Reference Douch, Harrison, Elliott, Buchanan and Green1986, Reference Douch, Risdon and Green1994) and eosinophils in serum (Buddle et al., Reference Buddle, Jowett, Green, Douch and Risdon1992). Sheep also counter the nematodes by increased numbers of circulating antibodies and increased number of antibodies in the mucus of the intestine (Dawkins et al., Reference Dawkins, Windon, Outteridge and Dineen1988; Adams et al., Reference Adams, Anderson and Windon1989; McClure et al., Reference McClure, Emery, Wagaland and Jones1992). Rabbit develops resistance against Trichostrongylus retortaeformis through three ways viz: self-cure; inhibition of larval development; and prevention of establishment of infective larval stages (Michel, Reference Michel1952). All of these findings reveal that inflammatory response involving an interplay of different immune mediators including IL33, IgE, IgG1, IgG2, IgM, histamine, leukotriene C4, 6-keto prostaglandin F1α, and thromboxane B2 is vital in the fight against Trichostrongylus infection with mast cells playing a key role (fig. 2).
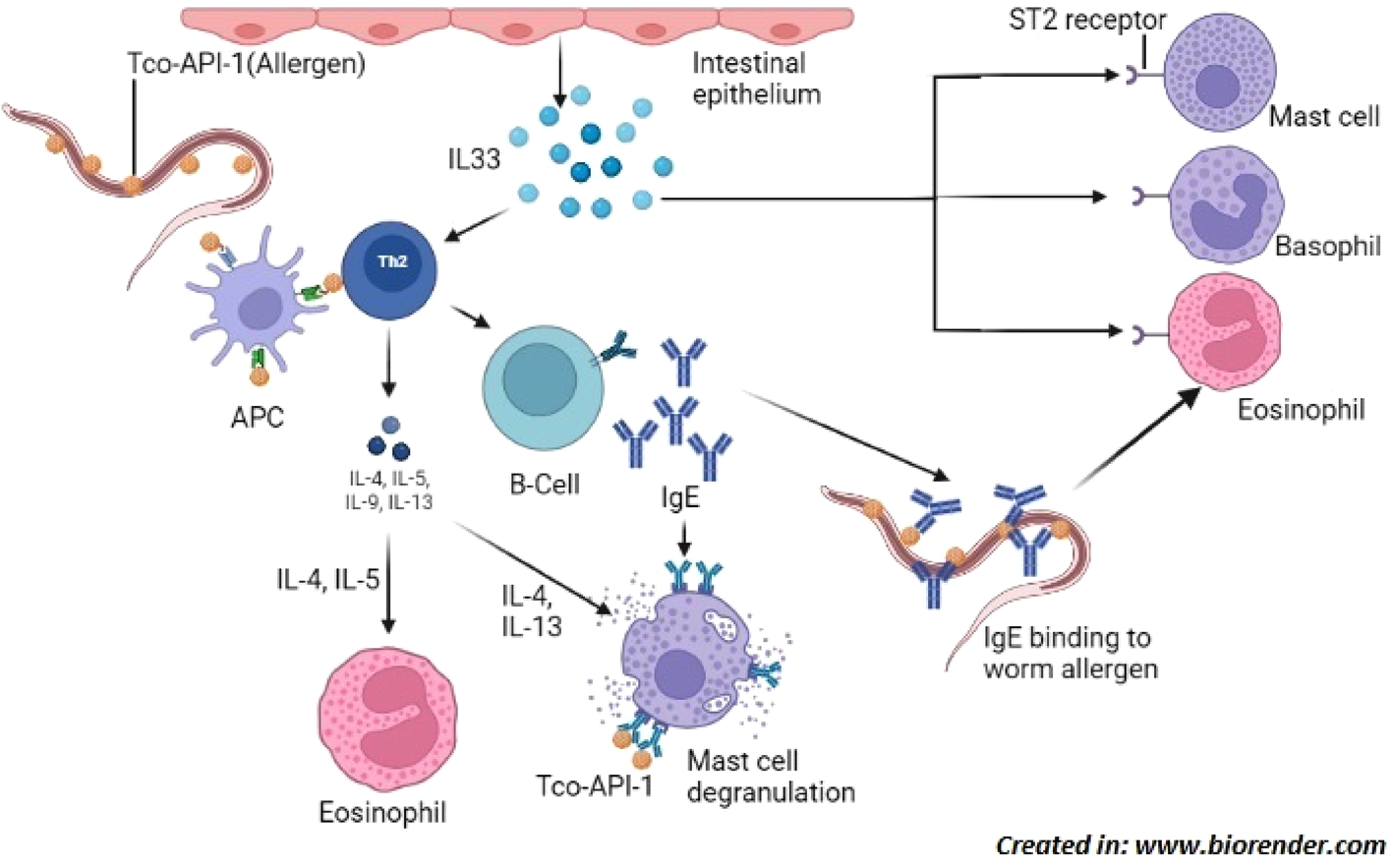
Fig. 2. Induction of Th2 immune response; Trichostrongylus colubriformis expressing allergen Tco-API-1, that is presented to Th2 cells by APC which releases cytokines (IL-4, IL-5, IL-9 and IL-13) that activate other cells including mast cell, eosinophil and B-cells. B-cells produce allergen specific IgE which bind to worm surface by Fab region and eosinophil by Fc region to induce their degranulation in order to kill the parasite. Allergen can also bind to mast cell bound IgE to cause their degranulation. In addition, damaged intestinal epithelial cells release IL-33 which binds to ST-2 receptor expressed by cells such as mast cell, basophil and eosinophil to cause their degranulation. Abbreviations: Tco-API-1, Trichostrongylus colubriformis sspartyl inhibitor; Th2 cell, T-helper 2 cell; APC, antigen presenting cell; IL, interleukin; Fab, fragment antigen-binding; Fc, fragment crystallizable; ST-2 is an IL-33 receptor belonging to the IL-1 family.
Prevalence of Trichostrongylus in humans
Few investigations on Trichostrongylus have revealed its widespread occurrence in human communities around the world. Watson (Reference Watson1953) reported that 48 million people were infected with Trichostrongylus spp. globally. However, researches on the epidemiology of Trichostrongylus proclaim a global distribution but low prevalence of infection in humans (Ghadirian & Arfaa, Reference Ghadirian and Arfaa1975; Cancrini et al., Reference Cancrini, Boemi, Iori and Corselli1982; Millington et al., Reference Millington, Costa, Tavares, Dourado, Reid and Macedo1989; Boreham et al., Reference Boreham, McCowan, Ryan, Allworth and Robson1995; John & Petri, Reference John and Petri2006; Ralph et al., Reference Ralph, O'Sullivan, Sangster and Walker2006; Yong et al., Reference Yong, Lee and Sim2007). Ghadirian & Arfaa (Reference Ghadirian and Arfaa1975) estimated 67%, 86% and 71% prevalence of trichostrongylosis among humans in Isfahan, Bakhtiari and Khuzestan regions of Iran, respectively, with primary species being Trichostrongylus orientalis and T. colubriformis. Watthanakulpanich et al. (Reference Watthanakulpanich, Pongvongsa and Sanguankiat2013) found 36.9% prevalence in Thakamrien Savannakhet, Laos. Trichostrongylus infections often become misreported due to the resemblance of their eggs with those of hookworms, for example, in Lahanam Laos, 2011, 93.5% of positive hookworm cases were of Trichostrongylus (Sato et al., Reference Sato, Yoonuan, Sanguankiat, Nuamtanong and Pongvongsa2011). Joe (Reference Joe1947) reported 36.42% prevalence in Java, Indonesia. Very low prevalence of 0.5% and 1.2% was reported in Chile and Brazil, respectively (Torres et al., Reference Torres, Figueroa and Navarrete1972; Souza et al., Reference Souza, Souza, Menezes, Alcântara, Soares and Teixeira2013). Heydon & Green (Reference Heydon and Green1931) also reported a very low prevalence (0.3–0.4%) of trichostrongylosis in Queensland, Australia. Infection of T. orientalis has been reported in Armenia, China, Japan and Korea (John & Petri, Reference John and Petri2006). Species such as T. axei, Trichostrongylus capricola, T. colubriformis, T. orientalis, Trichostrongylus probolurus, Trichostrongylus skrjabin and T. vitrinus have been found associated with infections in humans with T. axei, T. colubriformis and T. orientalis being the most common species which infect humans, mostly obtained via close contact with livestock (Ghadirian & Arfaa, Reference Ghadirian and Arfaa1975; Millington et al., Reference Millington, Costa, Tavares, Dourado, Reid and Macedo1989; John & Petri, Reference John and Petri2006; Ralph et al., Reference Ralph, O'Sullivan, Sangster and Walker2006; Yong et al., Reference Yong, Lee and Sim2007). In Japan, the most predominant species among humans is T. orientalis which is also found in China and Korea (Miyazaki, Reference Miyazaki1991). Buonfrate et al. (Reference Buonfrate, Angheben, Gobbi, Mistretta, Degani and Bisoffi2017) reported four clusters of Trichostrongylus infection in Italy. Multiple cases were reported from different regions of Hungary from time to time as mentioned by Hollo et al. (Reference Hollo, Rovo and Hidvegi1970). El-Shazy et al. (Reference El Shazly, Awad, Sultan, Sadek, Khalil and Morsy2006) reported 2.6% prevalence of trichostrongylsis in Dakahlia, Egypt. Females of age group 41–50 have been found to be more susceptible to trichostrongylosis than males (Watthanakulpanich et al., Reference Watthanakulpanich, Pongvongsa and Sanguankiat2013) (figs 3–5).
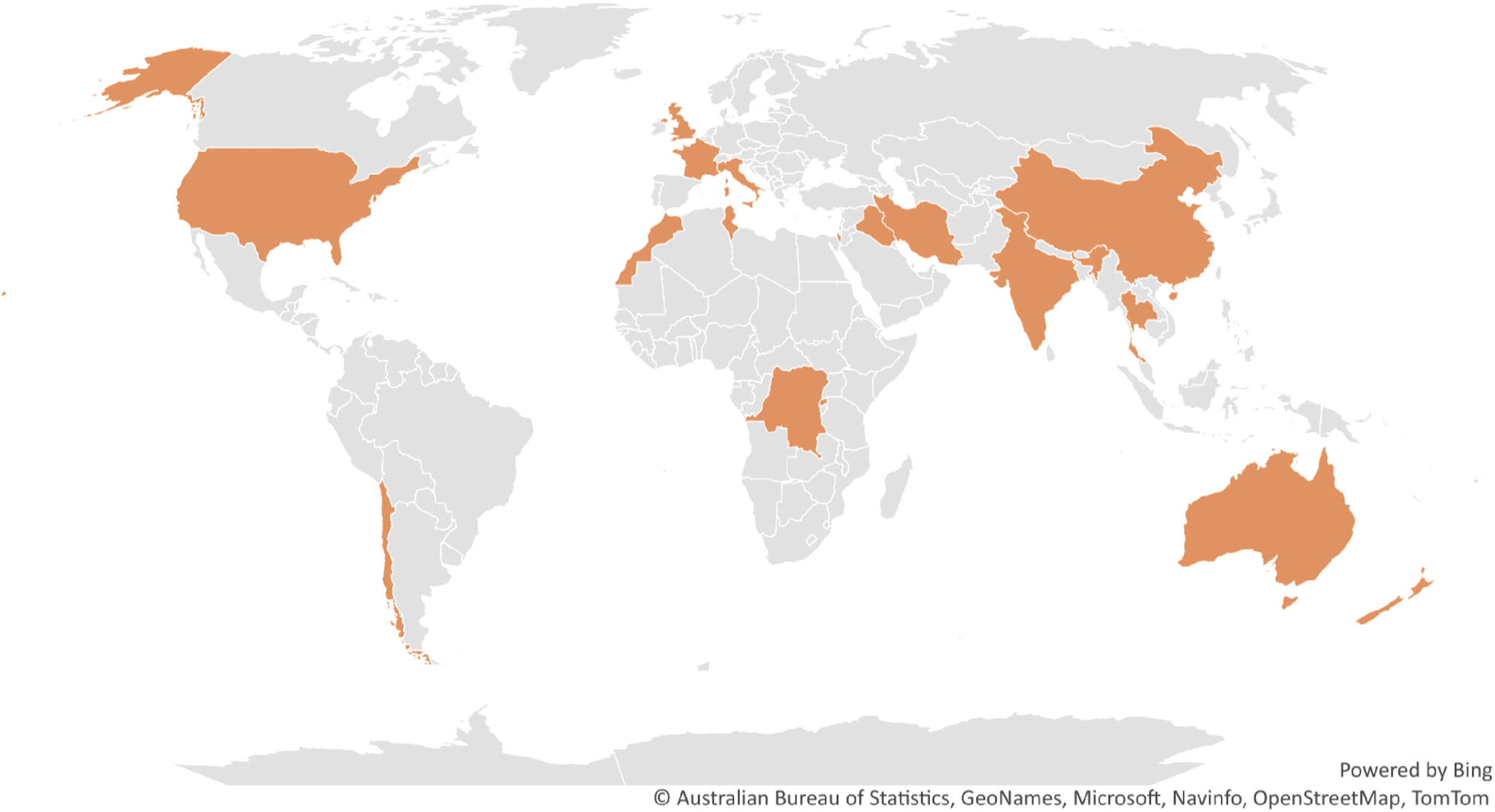
Fig. 3. Countries (coloured) with reported cases of Trichostrongylus infection in humans; Human infection by Trichostrongylus spp. is documented in several countries and is not limited to any one geographical area. Comprehensive inspection of hookworm patients may indicate otherwise in nations where Trichostrongylus has not yet been reported, as trichostrongyle eggs are frequently mistaken for hookworm eggs.
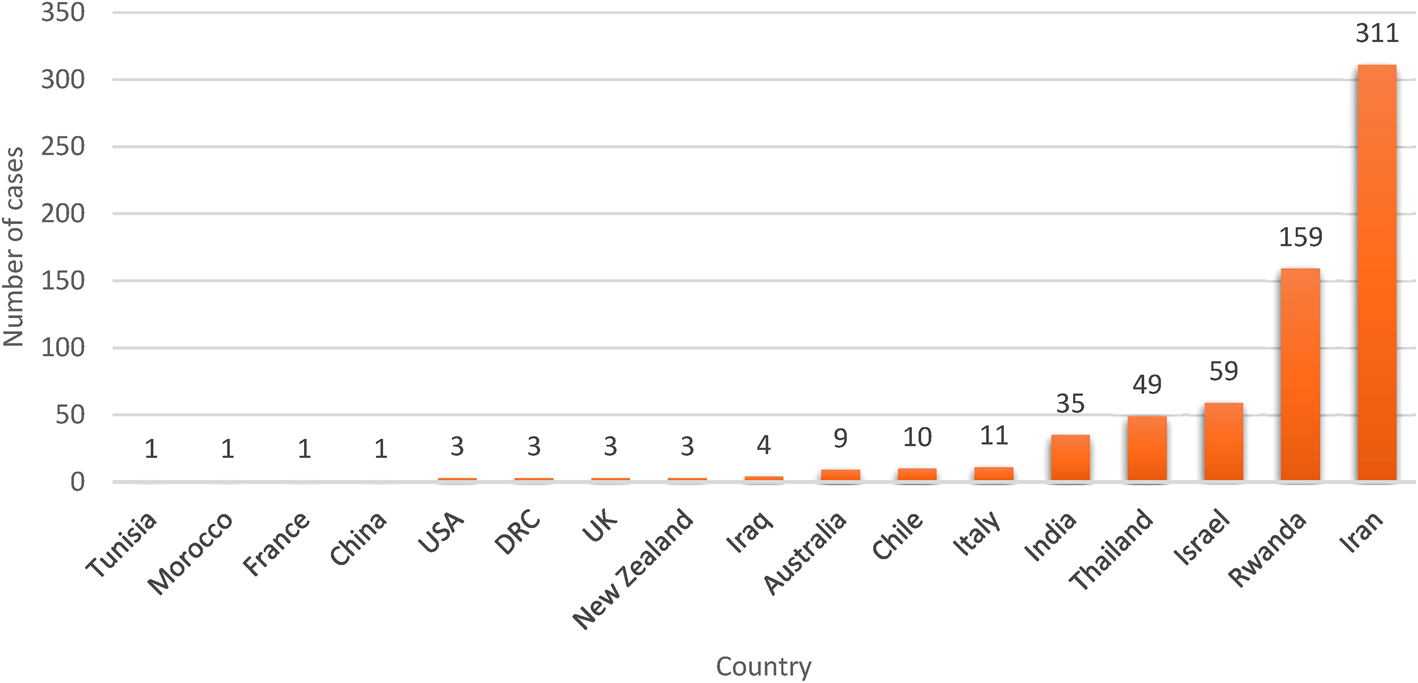
Fig. 4. Human trichostrongylosis case count by country. It is evident that Iran has reported the highest number of cases of Trichostrongylus among humans, which can be attributed to higher screening among human populations in part and the rest for pastoral livelihood of people in rural areas of Iran.
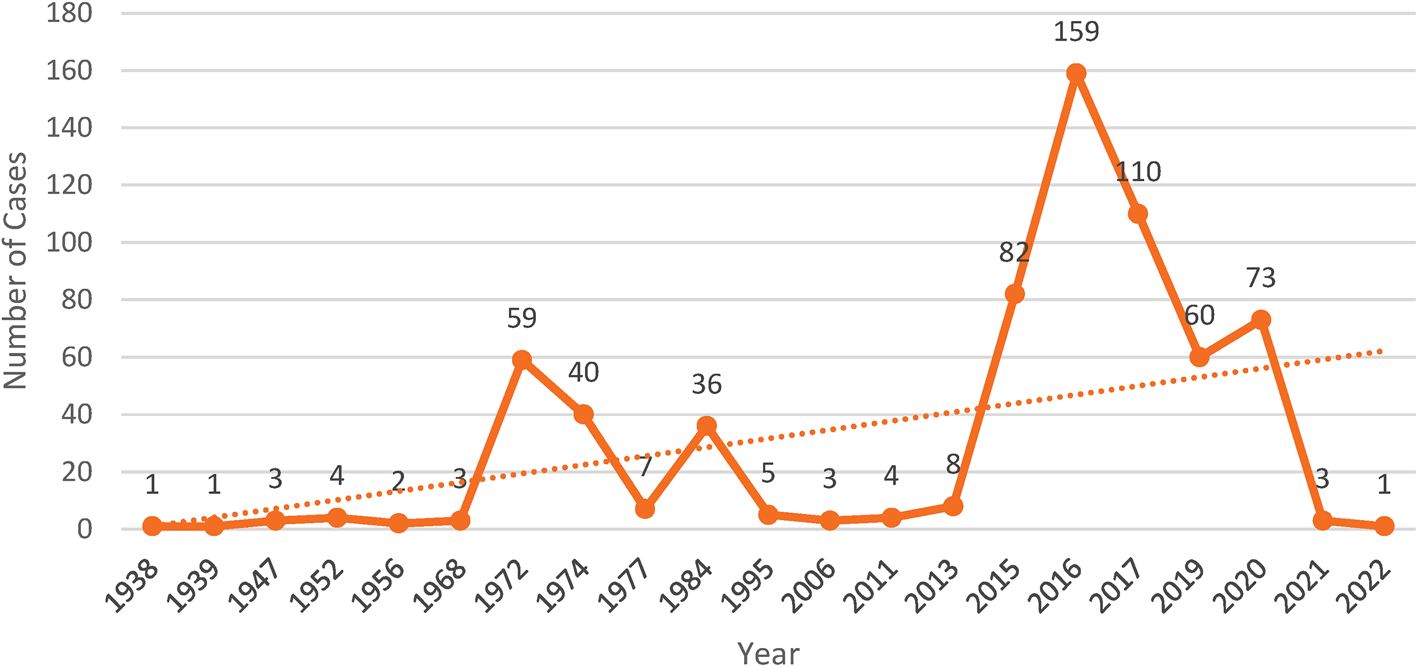
Fig. 5. Year wise human trichostrongylosis cases reported in the scientific literature from 1938 to 2022. Between the years 2015 and 2020, the number of cases is comparatively higher because of increased testing in rural human communities. Investigations in other pastoral communities of the world may detect more infections of trichostrongylosis.
Discussion
Different studies on the incidence of Trichostrongylus prove that this parasite is prevalent among small ruminants worldwide and sporadically occurs in humans that live in close contact with these ruminants. This review, which included almost all cases of Trichostrongylus infection that had been reported and documented in the literature worldwide, showed that trichostrongylosis occur in people occasionally. A systematic review of Trichostrongylus infection in Iran by Rahimi-Esboei et al. (Reference Rahimi-Esboei, Pourhajibagher and Bahador2022) showed its prevalence to be 0.01%. However, in rural communities the prevalence is found to be higher (3.13%) (Sharifdini et al., Reference Sharifdini, Ghanbarzadeh, Barikani and Saraei2020). With ten species of Trichostrongylus reported in human beings, Iran has been a hotspot of human trichostrongylosis; globally, 11 species of Trichostrongylus have been linked to humans (Sharifdini et al., Reference Sharifdini, Derakhshani, Alizadeh, Ghanbarzadeh, Mirjalali, Mobedi and Saraei2017b). The metrics indicate that T. colubriformis, followed by other species, is the predominant species infecting humans around the world (Lattes et al., Reference Lattes, Ferte, Delaunay, Depaquit, Vassallo, Vittier, Kokcha, Coulibaly and Marty2011; Phosuk et al., Reference Phosuk, Intapan, Sanpool, Janwan, Thanchomnang, Sawanyawisuth, Morakote and Maleewong2013; Watthanakulpanich et al., Reference Watthanakulpanich, Pongvongsa and Sanguankiat2013; Gholami et al., Reference Gholami, Babamehmoodi, Abedian, Sharif, Shahbazi, Pagheh and Mehdi2015; Hidalgo et al., Reference Hidalgo, Gacitúa, Melo, Oberg, Herrera and Fonseca-Salamanca2020; Torres et al., Reference Torres, Arcos, Villa and Cerna2021; Du et al., Reference Du, Zhang and Dang2022) with Iran reporting the highest number of cases.
Most cases of human trichostrongylosis around the world have been revealed following a parasitological stool examination particularly using the FEAC technique on persons with gastrointestinal complications (diarrhoea, abdominal pain, weakness and loss of appetite) and eosinophilia. In family outbreaks of trichostrongylosis, some family members with hypereosinophilia prove negative for Trichostrongylus eggs on stool examination which can be attributed to a long prepatent period of the parasite, that is, four months to two years (Wolfe, Reference Wolfe1978; Boreham et al., Reference Boreham, McCowan, Ryan, Allworth and Robson1995; Ralph et al., Reference Ralph, O'Sullivan, Sangster and Walker2006). Saraei et al. (Reference Saraei, Ghanbarzadeh, Hajialilo, Barghandan, Amini and Sharifdini2019) showed a higher sensitivity of FEAC (95.8%) than the agar plate culture method (90.1%) in diagnosis of trichostrongylosis. However, a recent study showed that among conventional parasitological stool examination techniques, Willi's method is more sensitive (91.7%) followed by the agar plate culture method (52.8%), Harada–Mori culture (40.3%), FEAC (37.5%) and 5.6% for the wet mount technique (Pandi et al., Reference Pandi, Sharifdini, Ashrafi, Atrkar Roushan, Rahmati and Hajipour2021). The same study showed the polymerase chain reaction (PCR) assay to be highly sensitive (97.2%) and specific in diagnosing human trichostrongylosis. Recently, Mizani et al. (Reference Mizani, Gill, Daryani, Sarvi, Amouei, Katrimi and Sharif2017) the devised restriction enzyme-PCR assay for the detection of Trichostrongylus species, which has higher specificity to detect even 10 pg/ml of DNA from the sample. Arbabi et al. (Reference Arbabi, Hooshyar, Lotfinia and Bakhshi2020) developed the PCR-high resolution melting assay for the diagnosis of Trichostrongylus species with higher specificity in differentiating T. colubriformis, T. capricula, T. vitrinus and T. probblorous. Most research to date has used the FEAC approach to diagnose human trichostrongylosis, which has lower sensitivity than Willi's method in conventional methods. Therefore, many more cases of trichostrongylosis can be discovered if human populations are screened using highly specific PCR along with the conventional Willi's and FEAC approaches.
Even though helminth zoonoses are declining globally, research has shown that warming caused by climate change, in particular, may eventually lead to a rise in helminth infections. The cause of this is that warming would lessen these helminths’ larval arrest including Trichostrongylus, and so lengthen the time of year when the free-living larval stages would be active. This would result in a rise in the rates of inadvertent human and livestock ingestion of larval stages, as well as the rates of infection (Dobson & Hudson, Reference Dobson and Hudson1992; Mas-Coma et al., Reference Mas-Coma, Valero and Bargues2008). This review indicates that contamination of food (vegetables) by livestock faeces is the main source of trichostrongylosis among humans which is further enhanced by rainfall due to dispersal of faecal material (Ghadirian & Arfaa, Reference Ghadirian and Arfaa1973; Ashrafi et al., Reference Ashrafi, Sharifdini, Heidari, Rahmati and Kia2020; Hidalgo et al., Reference Hidalgo, Gacitúa, Melo, Oberg, Herrera and Fonseca-Salamanca2020). Along with close proximity to livestock, consumption of untreated wild aromatic plants, use of animal dung as fuel (common among rural residents), unhygienic eating practises, use of livestock manure as fertilizer, weakened immune system and advanced age are some other risk factors linked to the rising incidence of human trichostrongylosis (Ashrafi et al., Reference Ashrafi, Sharifdini, Heidari, Rahmati and Kia2020; Rahimi-Esboei et al., Reference Rahimi-Esboei, Pourhajibagher and Bahador2022). In light of many proven ill effects of chemical fertilizers, organic farming with use of animal dung as fertilizer is becoming common among rural areas which could further increase the incidence of human trichostrongylosis and other zoonotic diseases in the near future (Ashrafi et al., Reference Ashrafi, Sharifdini, Heidari, Rahmati and Kia2020). Livestock being our most important source of meat and dairy must be monitored for helminth infections regularly through parasitological stool examination and be given periodic anthelmintic doses in order to prevent spillover of helminthiasis to humans. Proper hygiene decreases the rate of helminth infection greatly (Vaz Nery et al., Reference Vaz Nery, Pickering, Abate, Asmare, Barrett, Benjamin-Chung and Brooker2019). Even though developed countries with advanced health care and proper hygiene have managed to curb the helminth infections, the ‘hygiene hypothesis’ and related studies have correlated the decrease in helminth infections with the increase of inflammatory and autoimmune diseases among human population as research proves that helminth infection in early stages of life are important contributors to the proper development of the immune system (Rook, Reference Rook2012). With an increasing trend of anthelmintic resistance among different helminths, immunological studies among small ruminants in the direction of identifying potential antigenic proteins for the development of a vaccine against Trichostrongylus and other economically important nematodes in general are necessary. Pathogenicity has not been investigated in humans due to minimal number of infections. However, in sheep, Trichostrongylus causes high morbidity ranging from diarrhoea, severe degradation of intestinal wall to even mortality, which is more obvious in lambs. With the advent of new zoonotic diseases in the world, studies are needed to be conducted over these neglected mild pathogenic parasites to better understand their zoonotic potential and accordingly devise management strategies in the future.
Conclusion
The purpose of this review was to compile data regarding human trichostrongylosis in order to gain insights into the epidemiological, immunological and pathogenic aspects of Trichostrongylus species among humans. It was found that trichostrongylosis occurs among pastoral communities across the globe particularly in tropical countries. The majority of cases have been identified by traditional faecal examination techniques such as FEAC, however with the development of highly precise PCR-based approaches, it is important to combine these advanced techniques with traditional ones to accurately identify Trichostrongylus and other zoonotic helminths infecting humans. Most of the cases have been linked to the close association with the small ruminants or consumption of contaminated food. More investigations on human trichostrongylosis have led to an upsurge in instances being reported in recent years. With the recent finding of Trichostrongylus longispicularis among humans in Iran, the number of species associated with humans in this genus has increased to 11.
Further investigations are needed to be done to know the zoonotic potential and disease status of trichostrongylosis around the world. With meagre pathogenic studies and lack of information regarding the mechanism of immune response against Trichostrongylus spp., these two fields have remained unexplored; further research is needed in this direction to gather insights about the histological and physiological changes associated with human trichostrongylosis. Ruminants being an important source of food and other products, close association of humans with these domesticated animals is inevitable. In light of the emergence of new species of Trichostrongylus in humans, it is crucial to study their pathogenicity and zoonotic potential. Furthermore, it is recommended to devise proper management strategies in order to check the transmission of such zoonotic parasites in the future.
Financial support
The authors acknowledge financial support for this study from the Human Resource Development Group Council of Scientific and Industrial Research (HRDGCSIR), India and University of Kashmir.
Conflicts of interest
None.
Ethical standards
We, as authors of this review article, have made every effort to conduct a thorough and unbiased evaluation of the literature on the topic at hand. We have taken care to ensure that all sources are properly cited. We have also strived to maintain objectivity and accuracy in our interpretation of the data, and have not allowed personal biases to influence our conclusions. It is our hope that this review article will provide readers with a fair and comprehensive overview of the current state of knowledge on the subject.