Patients with depression who do not respond well to initial treatment trials are commonly referred to as having treatment-resistant depression (TRD). Although definitions of treatment resistance have varied over time, in several recent studies it has been defined as failure to achieve remission with two or more adequate antidepressant treatment trials.Reference Conway, George and Sackeim1,Reference Reutfors, Andersson, Brenner, Brandt, DiBernardo and Li2 TRD is associated with increased mortality, loss of function and lower quality of life, as well as high societal costs.Reference Reutfors, Andersson, Brenner, Brandt, DiBernardo and Li2,Reference Johnston, Powell, Anderson, Szabo and Cline3
Electroconvulsive therapy (ECT) is an effective treatment for depression4 and can be considered for patients with TRD, as reflected in guidelines from several countries.5,Reference Milev, Giacobbe, Kennedy, Blumberger, Daskalakis and Downar6 In patients with psychotic depression, ECT is often considered a first-line treatment regardless of treatment resistance.Reference Milev, Giacobbe, Kennedy, Blumberger, Daskalakis and Downar6 Although some observational studies have reported a significantly worse ECT outcome in patients with treatment resistance,Reference Prudic, Haskett, Mulsant, Malone, Pettinati and Stephens7–Reference Dombrovski, Mulsant, Haskett, Prudic, Begley and Sackeim9 others have not.Reference Pluijms, Birkenhager, Huijbrechts and Moleman10–Reference Heijnen, van den Broek and Birkenhager16 Some of these studies were small and patients were treated mainly with tricyclic antidepresssants,Reference Prudic, Sackeim and Devanand8,Reference Heijnen, van den Broek and Birkenhager16 differing from today's practice in which selective serotonin reuptake inhibitors are more commonly used. In two meta-analyses, history of medication failure was predictive of a lower response rate after ECT,Reference Heijnen, Birkenhager, Wierdsma and van den Broek17,Reference Haq, Sitzmann, Goldman, Maixner and Mickey18 with one study reporting response rates of 58% and 70%Reference Haq, Sitzmann, Goldman, Maixner and Mickey18 and the other reporting remission rates of 48% and 65%Reference Heijnen, Birkenhager, Wierdsma and van den Broek17 for patients with and without previous failure of an adequate antidepressant trial, respectively. In the included studies, treatment resistance was defined by at least one failed antidepressant trial of adequate dose and duration, not using today's more common definition of two failed antidepressant trials. Moreover, many studies included patients with psychotic depression. Thus, previous studies have not addressed the particularly relevant clinical question of response rates after ECT in non-psychotic, unipolar depression resistant to multiple previous pharmacological treatment attempts. The aim of the present study was to assess and compare the response rate of ECT for non-psychotic, unipolar TRD (defined by at least two failed antidepressant trials of adequate dose and duration), in relation to the response rate among patients with non-TRD, in a nationwide patient sample.
Method
Data sources
The Swedish National Quality Register for ECT (Q-ECT) has had national coverage since 2011, and around 90% of all patients treated with ECT in Sweden accept inclusion onto the register. Data are registered after completed treatment, separately for index and continuation series, and include information on patient characteristics, severity of symptoms, indications for therapy, electrical stimulus parameters and seizure characteristics, and scores on the Clinical Global ImpressionsReference Guy19 – Severity (CGI-S; before and after treatment) and Improvement (CGI-I; after treatment) scales. For the CGI-S and CGI-I, a clinician compares the patient's condition after treatment to that of before treatment, according to a seven-point scale ranging from 1 (very much improved) to 7 (very much worse). Evaluations of ECT effect are usually performed the day after, or at most a week after, the final session in the treatment series. The National Patient Register (NPR) contains patient data including main and secondary diagnoses and procedures according to the International Statistical Classification of Diseases and Related Health Problems (ICD), with revision 10 used since 1997, as well as dates for in-patient episodes. It has near complete coverage of both in-patient and out-patient specialised care, with out-patient care gradually included since 2001. The Prescribed Drug Register (PDR) contains data on all dispensed prescribed drugs in Sweden since July 2005. Drugs that are administered in hospitals are not included. The Swedish Longitudinal Integration Database for Health Insurance and Labour Market Studies (LISA) is held by Statistics Sweden and includes integrated demographic data.
Study population
From the Q-ECT, we identified patients who were aged ≥18 years and had been treated for a unipolar, non-psychotic depressive episode (ICD-1020 codes of F32 for depressive episode or F33 for recurrent depression, excluding F32.3 and F33.3 for psychotic depression) with at least one ECT session as part of a first-time, index ECT series in Sweden between 1 January 2011 and 31 December 2017 (Fig. 1). When registration of treatment indication was lacking or unclear in the Q-ECT, diagnostic information was added from the NPR. This resulted in 6615 eligible patients with a diagnosis of depression treated with ECT. Patients with any registered lifetime diagnosis of bipolar disorder, manic or hypomanic episode, psychotic disorder or dementia were excluded, as were patients lacking data on the primary outcome measure (CGI-I score). After these exclusions, a total of 4244 patients were included in the final analyses.
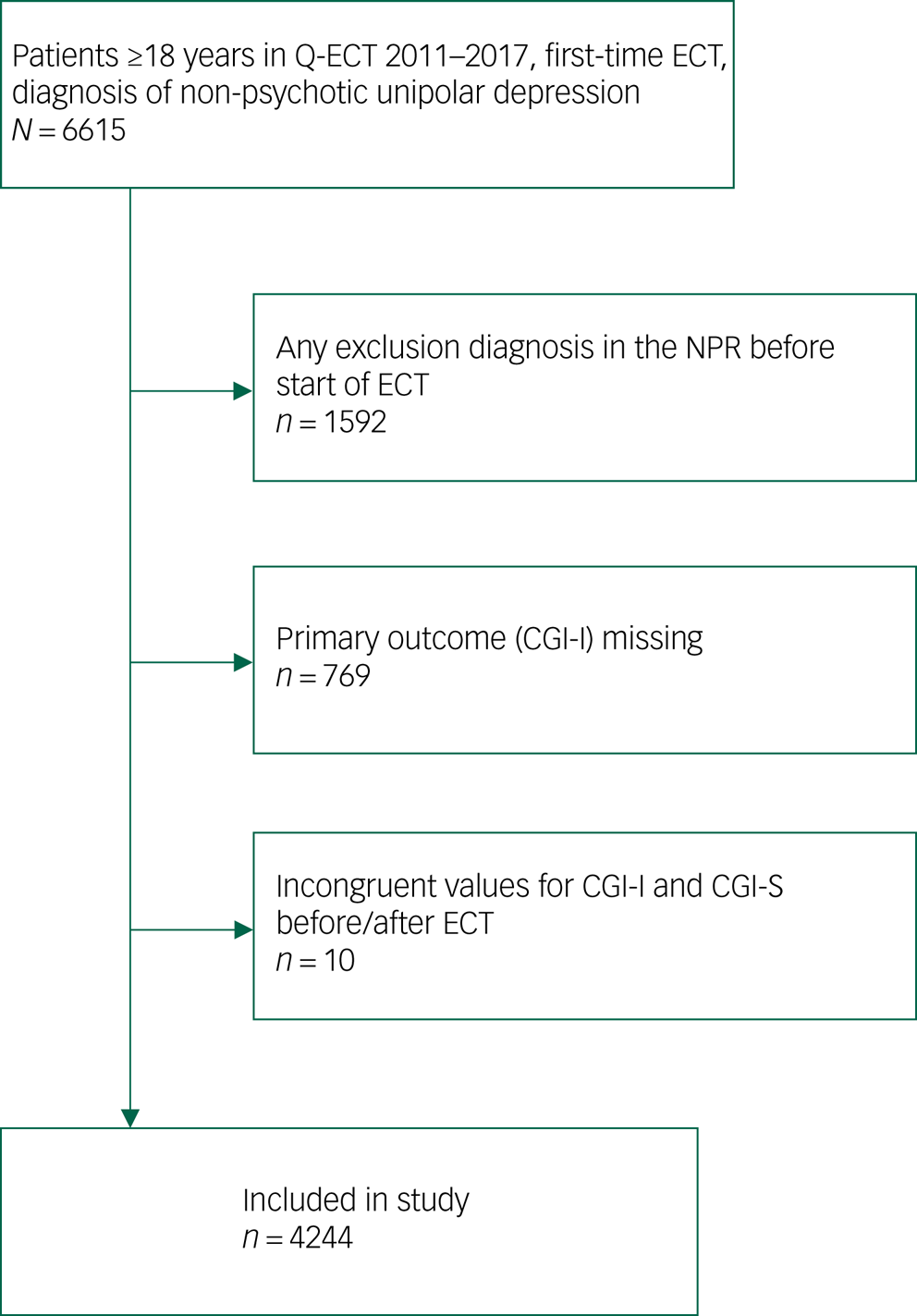
Fig. 1 Flow chart of study inclusion. CGI-I, Clinical Global Impressions – Improvement Scale; CGI-S, Clinical Global Impressions – Severity Scale; ECT, electroconvulsive therapy; Q-ECT Swedish National Quality Register for ECT; NPR, Swedish National Patient Register.
Exposure
Patients were classified as having TRD if at least two subsequent pharmacological treatment trials for depression (a different Anatomic Therapeutic Chemical classification code, or an antidepressant add-on medication) were recorded in the PDR during the 2 years before ECT. To be considered adequate, a treatment trial needed to be at least 28 days long, reflecting a duration commonly required for antidepressants to have effect. The duration of each filled prescription was estimated from package size, dosage and prescription text. Add-on medications were defined as lithium, risperidone, olanzapine, aripiprazole and quetiapine, which are recommended for the treatment of TRD by guidelines.5 Patients not meeting criteria for TRD were classified as ‘non-TRD’. A validation studyReference Hägg, Brenner, Reutfors, Li, DiBernardo and Bodén21 has shown that patients identified as TRD with this register-based method have similar characteristics as adaptations of clinical methods. In patients with depression, incidence rates of TRD as identified by register-based methods are similar in SwedishReference Reutfors, Andersson, Brenner, Brandt, DiBernardo and Li2 and DanishReference Gronemann, Jorgensen, Nordentoft, Andersen and Osler22 studies.
Additionally, we categorised exposure according to the number of pharmacological treatment trials before ECT, ranging from zero to five or more.
Covariates
Income, education level and marital status were collected from Statistics Sweden. Any lifetime diagnosis of personality disorder (ICD-10 codes F60–F61), anxiety disorder (ICD-10 codes F40–F42) or substance use disorder (ICD-10 codes F10–F16, F18 and F19) was noted from the NPR, as were previous admissions for depression (ICD-10 codes F32–F33). Treatment setting was collected from the Q-ECT except when unavailable, in which case NPR data was used. Use of benzodiazepines, pregabalin and other anti-epileptics (except clonazepam and pregabalin) at the time of ECT was assessed with data on prescription fills from the PDR. Technical parameters of ECT were attained from the Q-ECT.
Outcome measures
Outcome variables were retrieved from the Q-ECT. The main outcome measure was response to ECT, defined as CGI-I scores of 1–2 (very much improved or much improved). The secondary outcome measure was remission, defined as patients who were assessed as very much improved (CGI-I score of 1).
Statistical analysis
Social and clinical characteristics, as well as technical parameters of administered ECT, were categorised and tabulated. Subsequently, patients with TRD were compared with patients with non-TRD by χ 2-test. P-values of <0.05 were considered statistically significant. Further, outcome measures for the two groups were compared using logistic regression to calculate crude and adjusted odds ratios, together with their corresponding 95% confidence intervals and P-values, for response in patients with TRD compared with non-TRD. The following covariates were included simultaneously in the adjusted model, selected because of their relevance as potential confounders based on the literature: age, gender, anxiety disorder, personality disorder, substance use disorder and depression severity (CGI-S score before treatment). We also explored the impact of all of the available independent variables. Covariates were added one at a time to a base model consisting of age and gender. None of the covariates changed the estimated odds ratio by >10% for TRD versus non-TRD. Subsequently, unadjusted and adjusted odds ratios of ECT response were calculated stratified by TRD status for each of the variables included in the model, comparing each category to a reference category. We further compared ECT response for exposure categories of zero to five or more antidepressant trials in the same 4244 patients, using the adjusted model.
Preregistration
The methodology and statistical approach for this study were preregistered on the Open Science Framework (available at https://osf.io/ay6bk).
Ethical approval
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human patients were approved by the Regional Ethical Vetting Board in Uppsala (approval number 2014/174). Individual informed consent was not required because this was a register-based study of anonymised data.
Results
Study population
Out of the 4244 included patients, 1121 patients were categorised as having TRD based on previous prescription fills in the PDR, with the remaining 3123 patients categorised as having non-TRD. As presented in Table 1, the proportion of women was significantly higher in patients with TRD. Patients with TRD were more often married, whereas differences in age, education level and income were non-significant. Higher proportions of patients with TRD had anxiety disorder (64.8% v. 42.9%), personality disorder (11.6% v. 7.4%) and substance misuse disorder (23.4% v. 17.9%). An out-patient ECT treatment setting was more common in patients with TRD (16.3% v. 6.5%). There were also significantly more previous hospital admissions for depression in the TRD group, as well as recent prescription fills of benzodiazepines and anti-epileptics. The majority of patients with TRD or non-TRD received six to ten sessions of ECT (64.5% v. 67.4%, respectively; see Table 1), but a higher proportion of patients with TRD received more than ten sessions (19.2% v. 15.9%; P = 0.01). In the first session, no significant difference between groups was found for electrode placement, whereas seizure length was slightly shorter in patients with TRD.
Table 1 Sociodemographic and clinical characteristics of patients with versus without treatment-resistant depression at start of electroconvulsive therapy, and parameters of administered electroconvulsive therapy
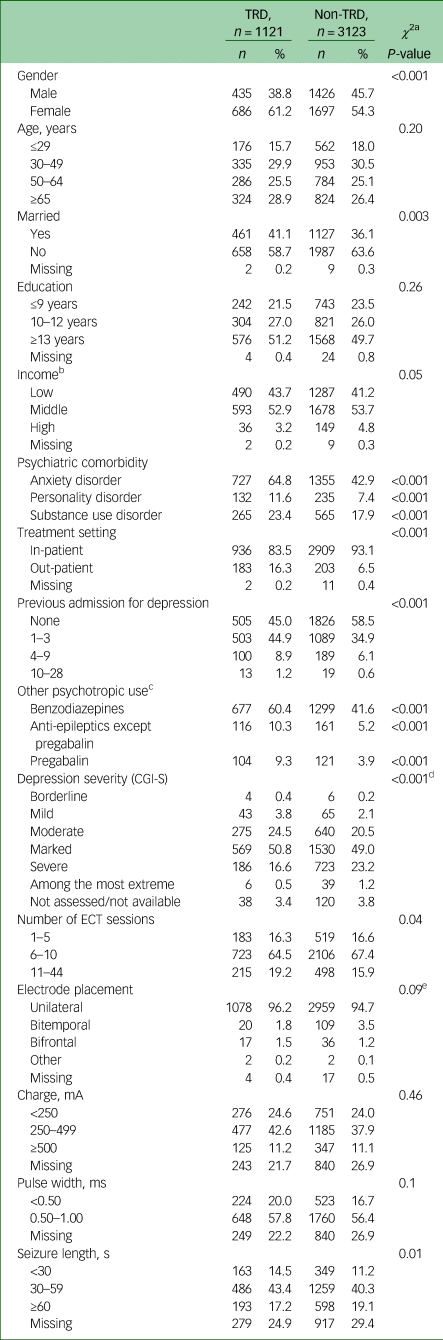
TRD, treatment-resistant depression; CGI-S, Clinical Global Impression – Severity Scale; ECT, electroconvulsive therapy; mA, milliampere; ms, millisecond.
a. Missing excluded from analysis. P-values refer to a null hypothesis that all categories of the variable are equal.
b. Low- and high-income groups defined as bottom and top 20 percentiles of income.
c. Prescription fills within 90 days before ECT.
d. CGI-S scores of 2–3 analysed as one group.
e. Comparing unilateral to remaining placements.
Outcomes
Patients with TRD had a lower CGI-I response rate compared with patients with non-TRD (65.9% v. 75.9%), corresponding to an adjusted odds ratio of 0.64 (95% CI 0.54–0.75) (Table 2). The CGI-I remission rate was 16.4% for TRD and 25.7% for non-TRD (adjusted odds ratio 0.64, 95% CI 0.53–0.77).
Table 2 Electroconvulsive therapy response in patients with versus without treatment-resistant depression

TRD, treatment-resistant depression; CGI-I, Clinical Global Impressions – Improvement Scale.
When response rates were examined separately by stratified covariate categories (Table 3), they were found to increase by age, ranging from 47.7% (≤29 years) to 75.6% (≥65 years) in patients with TRD, and from 58.2% (≤29 years) to 85.3% (≥65 years) in patients with non-TRD, corresponding to adjusted odds ratios between the oldest and youngest patients of 3.74 (95% CI 2.47–5.68) in TRD and 3.74 (95% CI 2.88–4.86) in non-TRD. There was no significant difference in response related to anxiety disorder or personality disorder in patients with TRD, whereas in patients with non-TRD, response rates were lower among patients with either of these diagnoses. Stratifying for depression severity, response rates were significantly higher in severely ill patients than in those who were markedly ill, with an adjusted odds ratio of 1.49 (95% CI 1.02–2.19) in patients with TRD and 1.40 (95% CI 1.11–1.76) in patients with non-TRD. Remission was similarly higher with increasing age and more severe depression in both groups, whereas other covariates had no significant effect on remission in patients with TRD (Supplementary Table 1 available at https://doi.org/10.1192/bjo.2023.5).
Table 3 Electroconvulsive therapy response in patients with versus without treatment-resistant depression, by covariate categories
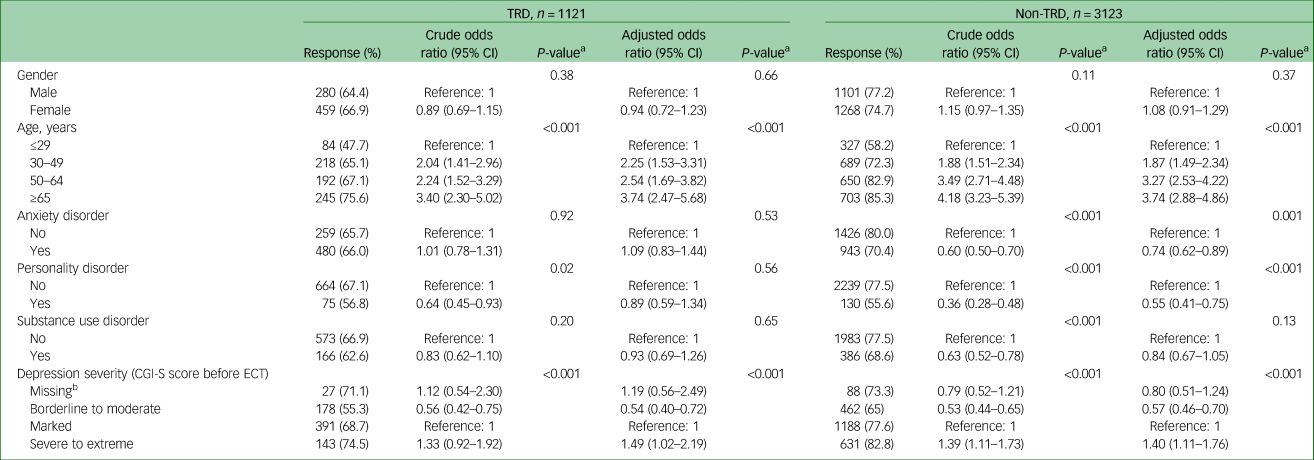
TRD, treatment-resistant depression; CGI-I, Clinical Global Impressions – Severity Scale; ECT, electroconvulsive therapy.
a. P-values refer to a null hypothesis that all categories of the variable are equal.
b. Missing included as a separate category.
Response rates were 74.6% of 1976 patients receiving benzodiazepines, 63.5% of 277 patients receiving anti-epileptics and 63.6% of 225 patients receiving pregabalin, compared with 73.2% of all 4244 patients. Adding benzodiazepine use to the base model did not change the odds ratio for response, whereas anti-epileptics and pregabalin use decreased the odds ratios by 1.6% and 2.3%, respectively.
Comparing outcomes across different numbers of antidepressant treatment trials before ECT (Supplementary Table 2) in the same 4244 patients, we found that response rates gradually declined from 82.1% in patients with no previous treatment trial, to 63.4% in those with five or more treatment trials before ECT; remission rates declined from 34.8% in previously untreated patients, to 13.4% after five or more treatment trials. Adjusted odds ratios were 0.43 (95% CI 0.29–0.64) for response and 0.35 (95% CI 0.22–0.55) for remission in patients with five or more trials compared with those with no previous treatment trials.
Discussion
Main findings
In this large, nationwide study of patients treated with ECT for unipolar, non-psychotic depression, we found high response rates in both groups, with 65.9% of patients with TRD assessed as much or very much improved after treatment, compared with 75.9% of patients with non-TRD. Lower proportions of patients had remission defined as the highest possible improvement assessment after treatment, with 16.4% compared with 25.7% for TRD and non-TRD, respectively. The differences in response and remission remained significantly lower in TRD even after statistical adjustment for a number of potential confounders. Nearly all of the difference in response rates could be attributed to the lower remission in patients with TRD, as evident from the 10% difference in response rates juxtaposed with the 9.3% difference in remission rates. We identified age and depression severity as factors associated with higher response rates in both patients with TRD and non-TRD, whereas anxiety or personality disorders were only associated with odds of response in patients with non-TRD.
Comparison with previous findings
Our study is the first to primarily investigate resistance to multiple medication trials as a predictor of ECT outcome, as well as the largest sample yet to assess the association between any treatment resistance and ECT outcome. Response and remission were defined by CGI-I score after ECT, whereas most previous studies used definitions of response or remission based on depression rating scales.Reference Heijnen, Birkenhager, Wierdsma and van den Broek17,Reference Haq, Sitzmann, Goldman, Maixner and Mickey18 Despite this, response rates after ECT were similar to a meta-analysis including a total of 1175 patients from 11 studies, finding response in 58% of patients with resistance to at least one medication compared with 70% in patients without medication resistance before ECT.Reference Haq, Sitzmann, Goldman, Maixner and Mickey18
Although lower than for non-TRD, our finding of an ECT response rate of 65.9% in patients with TRD is high when compared with the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, where treatment steps 3 and 4 – consisting of either switching to another class of antidepressant or augmentation treatments with lithium or T3 after two previous failed treatment trials – each prompted response in <17% of patients.Reference Rush, Trivedi, Wisniewski, Nierenberg, Stewart and Warden23 Given that patients in our study were selected for ECT, more often were in-patients (in contrast to the out-patient setting of STAR*D) and likely had more severe depressive symptoms with higher likelihood of ECT response, our results may not necessarily be inferable to patients with less severe depression. To our knowledge, no studies have been conducted directly comparing antidepressant treatment and ECT for TRD.
Although we are not aware of any previous study stratifying ECT response by the number of previous treatment trials before ECT, the gradually declining response and remission rates by each increase in the number of trials found here is in line with observations made in earlier studies,Reference Prudic, Haskett, Mulsant, Malone, Pettinati and Stephens7,Reference Prudic, Sackeim and Devanand8 where ECT response rates were lower with higher levels of previous antidepressant trial potency; however, results from a smaller studyReference Pluijms, Birkenhager, Huijbrechts and Moleman10 did not show a similar trend.
Various mechanisms that might explain previous findings from studiesReference Pluijms, Birkenhager, Huijbrechts and Moleman10–Reference Heijnen, van den Broek and Birkenhager16 where response rates were not lower in patients with treatment resistance are plausible. First, previous studies may have been underpowered. In line with that explanation, two meta-analyses of predictors of ECT outcomeReference Heijnen, Birkenhager, Wierdsma and van den Broek17,Reference Haq, Sitzmann, Goldman, Maixner and Mickey18 showed significant differences comparing patients with and those without treatment resistance. The largest of these included a total of 1175 patients compared with the 4244 included in this study.Reference Haq, Sitzmann, Goldman, Maixner and Mickey18 Second, some studies that did not find a lower response in patients with treatment resistance used bilateral ECT,Reference Husain, Kevan, Linnell and Scott12,Reference de Vreede, Burger and van Vliet14,Reference Rasmussen, Mueller, Knapp, Husain, Rummans and Sampson15 which might explain the more similar response rates in patients with and those without treatment resistance. Finally, several studies included patients with psychotic features, unlike ours, which may be of relevance since such features have been found to predict a better ECT response, with a meta-analysis finding response rates of 78% in psychotic depression compared with 71% in non-psychotic depression.Reference van Diermen, van den Ameele, Kamperman, Sabbe, Vermeulen and Schrijvers24 Response rates in patients with TRD and non-TRD may accordingly become more similar when this condition is included, and with psychotic depression there is a risk of ‘pseudo-TRD’, since an adequate previous pharmacological treatment of psychotic depression has been shown to be rare among patients treated with ECT.Reference Mulsant, Haskett, Prudic, Thase, Malone and Mann25
In previous studies on predictors of ECT response, higher age and depression severity, as well as absence of anxiety and personality disorders, have been found to be significantly associated with a better ECT effect in depression,Reference Haq, Sitzmann, Goldman, Maixner and Mickey18,Reference Steinholtz, Reutfors, Brandt, Nordanskog, Thornblom and Persson26 but this has not been studied specifically among patients with TRD. In the present study, we demonstrated that patients with TRD did not have significantly higher ECT response or remission depending on anxiety or personality disorders, whereas patients with non-TRD did. Older age and higher depression severity, however, were significantly associated with better odds of treatment response and remission in both patients with TRD and non-TRD.
The proportion of patients with TRD in this study (26.4%, 1121 out of 4244) was lower than patients with medication resistance in previous studies of patients treated with ECT. This may partly be because of the stricter requirement of two previous trials used in the study, and partly because of differences in treatment guidelines.
Limitations
Diagnoses in the registers used for this study were recorded in regular clinical settings, meaning that diagnostic methods may have varied, although the validity of psychiatric diagnoses has been found to be high in both the NPRReference Ludvigsson, Andersson, Ekbom, Feychting, Kim and Reuterwall27 and Q-ECT.Reference Ahmad, Sandberg, Brus, Ekman, Hammar and Landén28 The outcome was measured with the CGI-I instead of depression rating scales, and assessments were non-blinded. The method used for defining TRD does not include non-pharmacological treatments, does not require switching between antidepressant classes and makes assumptions about medication failure when a new prescription is filled, meaning that some patients may have been misclassified as having TRD if they did not actually take the collected medication or dropped out because of side-effects. Related to this, because the PDR does not include data on in-hospital medication, there is a possibility that some patients who had TRD when treated as in-patients were misclassified in our study as having non-TRD. However, data from the Q-ECT show that the mean length of stay before starting ECT is 4 days (with an average total length of stay of 29 days for patients treated with ECT), and that only 5% of patients start ECT after more than 27 days of in-patient care (enabling 28-day trials of antidepressants). Add-on medications such as quetiapine may sometimes have been prescribed for other indications than for treatment of depression, such as for anxiety or sleep problems. Also, although only the previous 2 years of antidepressant fills were considered, the method did not exclude antidepressant use for preceding depressive episodes or indications other than depression. Further, although patients with any previous registration of ECT were excluded, there may have been cases where patients had previous ECT that was not registered in the Q-ECT or NPR, but who were selected for treatment because of previous response, likely increasing chances of a positive outcome. Such patients would likely be treated with ECT early and not go on to develop TRD, and may thus have contributed somewhat to the higher response rates in non-TRD. Moreover, data on the technical ECT parameters were limited to the first session, meaning any change in electrode placement or stimulus charge during the treatment course would not be detected. Because charge titration is not used in Sweden, we were also not able to provide data on stimulus charge exceeding the seizure threshold. Finally, our study does not address duration of treatment effects. A recent Swedish trial comparing ECT to racemic ketamine infusions for unipolar depression showed that after remission achieved by ECT, around 64% of patients relapsed within 12 months, with most of these being in the first 6 months.Reference Ekstrand, Fattah, Persson, Cheng, Nordanskog and Åkeson29 There are previous results to support a higher rate of relapse in patients with preceding treatment resistance than in those without.Reference Sackeim, Prudic, Devanand, Nobler, Lisanby and Peyser30
Interpretation and conclusion
The association between TRD and a lower ECT response may represent a true effect of TRD on positive ECT outcome, such as if patients with TRD have a subtype of depression that is inherently more difficult to treat across treatment modalities. However, another possibility is that there is residual unmeasured confounding, which could contribute to the association. First, because of limitations in the available data in the Q-ECT, patients were already treated with ECT at study inclusion and may thus have been selected as suitable for ECT based on unmeasured clinical factors. Patients who were treated with ECT before they had developed TRD may have had characteristics possibly associated with improved ECT effect, such as rapidly worsening symptoms or a clear episodic character of depression, whereas for patients with TRD, a perceived lack of options may have resulted in them being given ECT despite possible negative factors such as a less clear-cut diagnosis of depression. Second, the rate of personality disorder in this material was relatively low compared with other studies, such as a European multicentre studyReference Souery, Oswald, Massat, Bailer, Bollen and Demyttenaere31 where personality disorder was present in 50.5% of patients with TRD and 37.1% of patients with non-TRD. If there were more undiagnosed patients with personality disorder in the TRD group in our study, this could explain the lower response in patients with TRD to some extent, given the lower response rates we found in patients with non-TRD and diagnosed personality disorder. Although the possible mechanisms above cannot be excluded, TRD status should be seen as a prognostic clinical marker associated with somewhat lower ECT response.
In conclusion, results of this study of real-world data on unipolar, non-psychotic depression indicate that a clear majority of patients with depression respond to ECT. Response rates to ECT were found to be somewhat lower in the TRD group than in the non-TRD group, but were still encouraging relative to available alternatives in TRD, such as further antidepressant trials. In patients with TRD, as well as patients with non-TRD, older age and depression severity were corroborated as ECT response markers.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjo.2023.5
Data availability
The data used for this study may not be shared by the authors to a third party, according to the ethical permission granted for its use. It is accessible by application to the Swedish authorities (The National Board of Health and Welfare and Statistics Sweden).
Author contributions
M.T., J.R. and R.B. conceptualised the study, with contributions from all other authors. L.B. performed registry data management and statistical analysis. A. Nygren drafted the manuscript. A. Nordenskjöld provided data from the Q-ECT, and assisted in data management. All authors provided critical feedback, contributed to the interpretation of the results and revised the manuscript.
Funding
R.B. was supported by a Swedish Research Council grant (number 2016-02362). M.T. was supported by Region Stockholm (clinical research appointment). A. Nygren was supported by a project grant from Stiftelsen Professor Bror Gadelius Minnesfond. A. Nygren, J.R. and L.B. were supported by the public–private real-world evidence collaboration between Karolinska Institutet and Janssen Pharmaceuticals (contract 5-63/2015).
Declaration of interest
A. Nygren, J.R. and L.B. are affiliated with/employed at the Centre for Pharmacoepidemiology at Karolinska Institutet, which receives grants from several entities (pharmaceutical companies, regulatory authorities, contract research organisations) for the performance of drug safety and drug utilisation studies, and are part of a research collaboration between Karolinska Institutet and Janssen Pharmaceutica NV, for which Karolinska Institutet has received/receives grant support. R.B., A. Nordenskjöld and M.T. have no competing interests to declare.








eLetters
No eLetters have been published for this article.