Introduction
About 13% of the world’s bird species are threatened (IUCN 2017), and the decline of many bird species is linked to anthropogenic habitat destruction and/or fragmentation. Palearctic migrants that breed in Europe and spend the non-breeding season in sub-Saharan Africa are especially prone to decline and may be affected adversely by conditions along migration routes or in non-breeding areas as well as on their breeding sites (Sanderson et al. Reference Sanderson, Donald, Pain, Burfield and van Bommel2006, Vickery et al. Reference Vickery, Ewing, Smith, Pain, Bairlein, Škorpilová and Gregory2014). Therefore, a detailed knowledge of migration routes and location of staging areas, as well as general ecology in the non-breeding season is important for their conservation. However, for many long-distance migrants, basic knowledge of their staging sites, non-breeding areas and migratory connectivity, is still lacking. A prominent example is the Aquatic Warbler Acrocephalus paludicola (Flade et al. Reference Flade, Diop, Haase, Le Nevé, Oppel, Tegetmeyer, Vogel and Salewski2011).
The Aquatic Warbler is listed as ‘Vulnerable’ on the IUCN Red List of Threatened Species (IUCN 2017). Until the early 20th century the species was common in fen mires and wet meadows from western Central to Eastern Europe. During the 20th century, Aquatic Warblers disappeared from most of the former breeding range and the global population dropped by > 90% owing to destruction of breeding habitat (Flade and Lachmann Reference Flade and Lachmann2008).
The Aquatic Warbler is a trans-Saharan migrant. The great majority of Aquatic Warblers are presumed to migrate on a coastal route through Western Europe via the Iberian Peninsula and Morocco to moulting and staging areas in sub-Saharan West Africa (de By Reference de By1990, Schäffer et al. Reference Schäffer, Walther, Gutteridge and Rahbek2006, Flade et al. Reference Flade, Diop, Haase, Le Nevé, Oppel, Tegetmeyer, Vogel and Salewski2011). The precise location of non-breeding areas remained largely unknown until recent discoveries of the species in the vicinity of Djoudj National Park in Senegal, in Mauritania, and in the Inner Niger Delta in Mali (Flade et al. Reference Flade, Diop, Haase, Le Nevé, Oppel, Tegetmeyer, Vogel and Salewski2011, Foucher et al. Reference Foucher, Boucaux, Giraudot, André, Lorrillière and Dugué2013). Based on remote sensing of habitat and presence-absence data from field surveys, Tegetmeyer et al. (Reference Tegetmeyer, Frick and Seifert2014) estimated that only up to 20% of the global population of c.20,000–25,000 birds (in spring, Flade and Lachmann Reference Flade and Lachmann2008) could spend the boreal winter in Djoudj, and it is questionable whether the remaining birds spend this time in the Inner Niger Delta.
In a pilot study, in 2010 we equipped male Aquatic Warblers with light-level geolocators (hereafter “geolocators”) on breeding grounds in Ukraine and retrieved some geolocators in 2011 (Salewski et al. Reference Salewski, Flade, Poluda, Kiljan, Liechti, Lisovski and Hahn2013). The data revealed an unknown migration route from Ukraine to staging sites on the Iberian Peninsula along the northern Mediterranean south of the Alps, indicating the need to protect unknown staging sites on the Balkan Peninsula and in Italy. It was, however, not possible to identify the non-breeding areas as the four geolocators retrieved after the migration cycle stopped recording data as early as September. In order to identify non-breeding areas of Aquatic Warblers, we conducted a follow-up study with an increased number of geolocators attached to birds in 2012 at two breeding sites: the Dzikoje marshes in Belarus and the Supii mires in Ukraine. The aims were (1) to confirm the existence of a migration route through the northern Mediterranean south of the Alps, (2) to identify staging areas in the north Mediterranean, and (3) to identify staging areas in sub-Saharan Africa, in order to guide future conservation efforts.
Methods
Study sites
Field work was conducted at Dzikoje in Belarus (52.74°N, 24.22°E) and at the Supii marshes in central Ukraine (50.40°N, 31.74°E). The Dzikoje mire complex has an area of c.150 km2, but only 1,165 ha is suitable habitat for Aquatic Warbler, which occurred there with 150–200 singing males in the period 2008–2016 (U. Malashevich, unpubl. data). Along the 1–3 km wide Supii river valley, fen mires occur over a stretch of more than 40 km including 470 ha suitable for Aquatic Warblers. The Supii valley held 108–126 singing males in 2012 (only 11–14 in 2017; A. Poluda, unpubl. data) with the largest group of 80–90 males concentrated in our study site (Poluda et al. Reference Poluda, Flade, Krogulec, Khymyn, Iliukha and Korkh2016).
Capturing and recapturing of Aquatic Warblers in 2012/13
In 2012 we searched for singing males between 24 and 30 June in Dzikoje and between 3 and 9 July in Supii. We caught them by chasing them into mistnets or/and luring them into the nets by playing Aquatic Warbler song. Caught birds were ringed and 29 adult males in Dzikoje and 17 adult males in Supii were equipped with a geolocator (SOI-GDL 2 with a 5 mm light guide; Swiss Ornithological Institute). Only adult males were equipped with a geolocator because of the lower return rate of first year birds and due to a presumably lower site faithfulness and more inconspicuous behaviour of adult females (Dyrcz and Zdunek Reference Dyrcz and Zdunek1993, Kloskowski and Krogulec Reference Kloskowski and Krogulec1999). The geolocators were attached using a leg-loop harness (Rappole and Tipton Reference Rappole and Tipton1990), made from VMQ silicone O-rings (Johannsen AG, Switzerland). All geolocators started measuring light intensities on 1 July 2012 with an interval of 5 min. The weight of the geolocators including the harness ranged from 0.57 to 0.62 g (mean: 0.60 ± 0.01 g SD). For 45 birds (one missing body mass) the geolocators added 3.9% to 5.3% to their body mass. This is above the often used “5% rule” for tags. It is, however, within the range of additional mass for which Naef-Daenzer et al. (2001; additional load up to 5.8%) and Schmaljohann et al. (2012; mean additional load 6.1%) could find no significant effects on condition, manoeuvrability, flight behaviour, clutch size and range use in small passerines equipped with similar devices. Therefore, and in line with Blackburn et al. (Reference Blackburn, Burgess, Freeman, Risely, Izang, Ivande, Hewson and Cresswell2016), we felt confident that the geolocators would not affect the behaviour of the respective birds. We used twenty-three and five adult males in Dzikoje and in Supii, respectively, as a control group to evaluate potential differences in return rates, these were ringed but no geolocator attached.
In the subsequent year, both breeding areas were checked for singing males with rings between 26 May and 1 June 2013 (Dzikoje) and between 25 and 31 May 2013 (Supii). We used the same method to recapture these individuals and to retrieve geolocators. For details about capturing and recapturing of birds on Supii in 2010/11 see Salewski et al. (Reference Salewski, Flade, Poluda, Kiljan, Liechti, Lisovski and Hahn2013).
Data analysis
Estimating locations
Light-level geolocation data for the eight Aquatic Warblers were analysed using the R (R Core Team 2017) package GeoLight ver. 2.01 (Lisovski and Hahn Reference Lisovski and Hahn2012). Daily sunrise and sunset times were determined using a light intensity threshold of 1 (arbitrary light value). We used the post-deployment periods, e.g. light recordings from the breeding site, to calibrate (to get a reference sun elevation angle; see Lisovski et al. Reference Lisovski, Hewson, Klaassen, Korner-Nievergelt, Kristensen and Hahn2012). In addition, we performed a Hill-Ekstrom calibration using the relatively long stationary periods during the boreal winter. However, in three individuals (RR, QX, PP) these periods where either missing or very short. In these cases we used the Hill-Ekstrom calibration result from the individual with the longest non-breeding residence period available for the particular year (RB, OY, see Table S1 in the online Supplementary Material, for details on calibration and sun elevation angles used). To estimate staging locations we used the siteEstimate function following the protocol introduced by Hiemer et al. (Reference Hiemer, Salewski, Fiedler, Hahn and Lisovski2018). In brief, periods of residency were distinguished from periods of movement based on changes in the sunrise/sunset times using the function changeLight: a switch in behaviour was considered to have occurred if the probability associated with a change (change point probability) in the timing of sunrise and sunset was higher than the 90th percentiles of all probabilities calculated within the entire time series. Additionally, staging sites with less than four twilights (i.e. two days) were regarded as potential artefacts and classified as movements. Next, long periods of residency that included at least one equinox were used to derive a sun elevation angle independent from breeding site calibration. The location of each detected period of residency was then estimated using the sun elevation angle of the breeding site as well as the independently calculated sun elevation angle for the major staging site during the boreal winter. To provide an estimate of uncertainty, we also estimated the location for each site with a sun elevation angle of plus/minus one degree. Furthermore, we reanalysed our previous data set (Salewski et al. Reference Salewski, Flade, Poluda, Kiljan, Liechti, Lisovski and Hahn2013) of the geolocators retrieved in 2011 as described above to examine our previous results with a more precise analytical method. Raw light-level data and analysis scripts (R scripts) are available upon request from ‘Movebank’ (www.movebank.org, project: Aquatic Warbler Migration).
Results
Recapturing Aquatic Warblers
At Dzikoje, four (14%) of the 29 Aquatic Warblers fitted with a geolocator in 2012 were recaptured in 2013, but one of the retrieved geolocators contained no data. None of the control birds (n = 23) was recaptured in 2013, but three birds with rings from other projects were, indicating that capture efforts did not concentrate on birds with visible geolocators. Attempts to capture two additional ringed birds that could have been members of the control group failed. At Supii, four (24%) out of the 17 Aquatic Warblers fitted with a geolocator in 2012 were recaptured in 2013. However, one individual had lost the geolocator and one retrieved geolocator had not collected any data. Three (60%) of the five control birds were recaptured. Combining birds fitted with a geolocator in 2010 and 2012, 10 (21%) out of 47 Aquatic Warblers were recaptured in the following year. Nine (43%) of the 21 control birds were recaptured.
Post-nuptial migration
All individuals left the breeding grounds between 9 and 24 July (Table S1) and after moving initially in a south-west or southerly direction all birds (no data for geolocator HK) moved west through south-central or southern Europe towards the Iberian Peninsula. Individual staging sites were in southeast Europe (Bulgaria, Greece), northern Italy and southern France (Figure 1). EN may have migrated from northern Italy directly to North Africa, possibly via the Balearic Islands (Figure 1a).
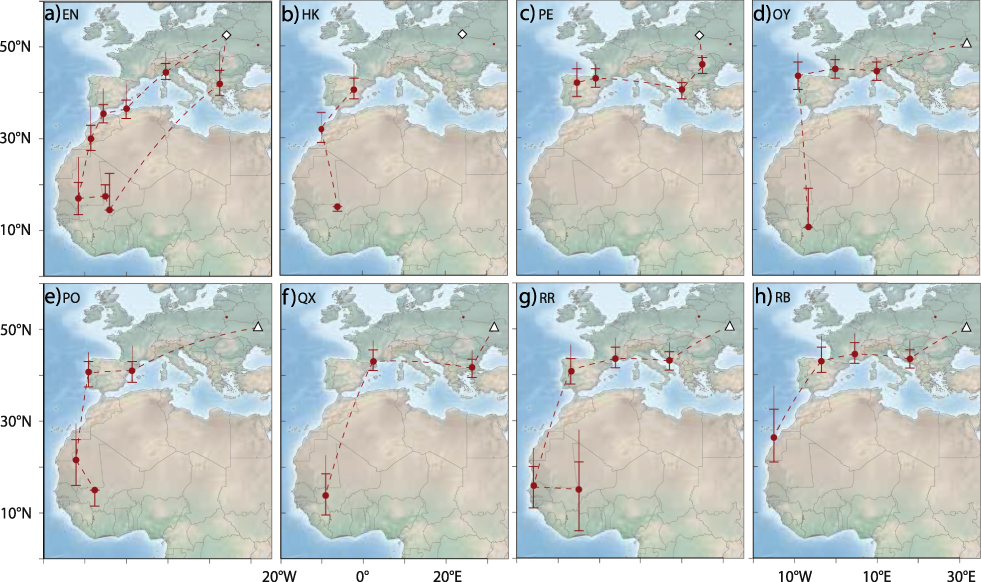
Figure 1. Migration routes of Aquatic Warblers from two breeding areas, Dzikoje in Belarus (white diamond) and Supii in Ukraine (white triangle). QX, RR and RB were equipped with geolocators in 2010 and EN, HK, PE, OY and PO in 2012. Staging sites are shown as circles with error bars indicating location estimates using different sun elevation angles around the winter site calibration (± 1 degree). The upper extension to the error bar indicates the location estimate using the breeding site calibration (see methods for more details). Dashed lines connect consecutive staging areas, but do not necessarily indicate the true migration routes as these cannot be estimated with high confidence from the light intensity records.
All birds stopped over in the western Mediterranean where they arrived between 24 July and 04 August. The time spent in the western Mediterranean varied between 12 and 40 days with a median of 22 days (no data for HK). These stop-overs were either situated in south-western France, on the Iberian Peninsula, or in coastal north-west Africa. Aquatic Warblers used one to two staging sites in the western Mediterranean with the exception of EN that had three stopovers, most likely all in north-west Africa (Figure 1).
All birds left their last staging sites in Europe or North Africa between 12 August and 10 September (median: 24 August) to migrate to sub-Saharan Africa. One geolocator (PE) stopped recording data after leaving its last European staging site. The results indicate that the seven remaining birds flew without a longer stop-over to their first staging area south of the desert. While the analysis indicate that the birds may have required one or two days only to cross the Sahara, the data should be treated as approximations only (e.g. at least ± 1 day).
Sub-Saharan staging areas
Seven Aquatic Warblers reached first staging sites south of the Sahara that were mostly situated in Mauritania or Mali. For RB, the first staging area was at the western border of the desert, and for OY it was most likely in northern Côte d’Ivoire (Figure 1d, 1g). First staging areas in sub-Saharan Africa were reached between 13 August and 11 September (median: 23 August, no arrival data for PE that left Spain relatively late). At the first sub-Saharan staging areas two geolocators failed to collect further data in August and in early September (QX, RB) and two in late October (HK, OY) respectively. Three birds, however, continued to migrate, moving to the east (EN, RR) or southeast (PO) after a stopover of ten days (PO, RR) and twelve days (EN) to second sub-Saharan staging areas in Mali. EN left the second staging area in Mali in early November to reach a third sub-Saharan staging area approximately 300 km to the south of the second one. There EN stayed until 10 April. EN therefore spent most of the boreal winter in the region of the Inner Niger Delta in Mali (Figure 1a).
With the exception of one coastal and the southernmost site the median estimates of all sub-Saharan staging sites were between 4°W and 9°W and between 10°N and 17°N, mostly in the Sahel (Figure 2). Two of these sites were in the area of the Inner Niger Delta in Mali, whereas the others were not in the vicinity of larger water bodies.
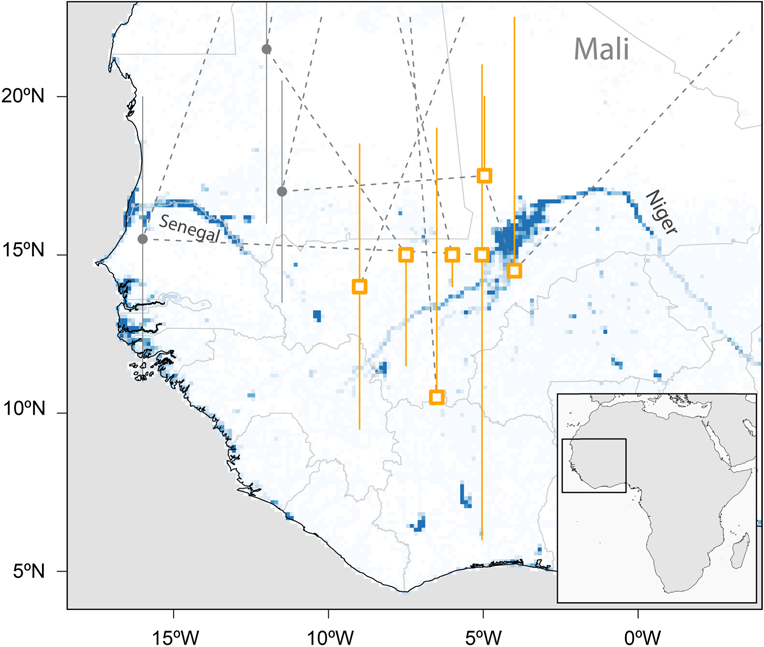
Figure 2. Major sub-Saharan staging sites (rectangles) of six tracked Aquatic Warblers (EN, HK, OY, PO, OX, RR). Grey circles indicate short-term staging sites en route. The locations are plotted on a map showing year-round water availability downloaded and scaled to lower resolution (0.125×0.125degrees) from https://global-surface-water.appspot.com (Pekel et al. Reference Pekel, Cottam, Gorelick and Belward2016).
Pre-nuptial migration
The only bird (EN) with data available for the entire annual cycle migrated on a more easterly route in spring than on autumn migration and flew to its breeding area (Dzikoje) with a single stopover of five days in south-east Europe (Figure 1a). The entire c.4.900 km journey from Mali to Belarus took approximately 18 days with arrival on the breeding grounds on 28 April.
Discussion
The application of geolocators provided insights into adult male Aquatic Warbler migration phenology, routes and staging areas that have implications for conservation. However, this study may also rise concerns about potential effects of geolocators on the survival of individual male Aquatic Warblers. The recapture rate of geolocator birds in Supii was conspicuously lower (21%) than the recapture rate of the control group (43%). Yet, at Dzikoje, we only recaptured geolocator birds (14%) but none of the control birds, despite an unbiased effort to recapture birds from both groups. While these results can be interpreted in both directions (no vs. strong effects of geolocators on survival), we can only recommend to thoroughly monitor return rates of tagged and control birds in future studies and weigh the potential negative effects against the benefits for conservation in this rare and threatened species.
Focusing on the phenology of migration, timing of departure from the breeding areas was in line with the results of previous studies: the majority of male Aquatic Warblers from other breeding areas were reported to also depart in the second half of July. However, some females may stay longer due to late breeding in which males are not involved (Heise Reference Heise1970, Wawrzyniak and Sohns Reference Wawrzyniak and Sohns1977, Vergeichik and Kozulin Reference Vergeichik and Kozulin2006). The latter may lead to later migration periods in central and northern central Europe compared to the males of this study (reviewed by Wawrzyniak and Sohns Reference Wawrzyniak and Sohns1977). As earlier reported for Aquatic Warblers breeding in Supii (Salewski et al. Reference Salewski, Flade, Poluda, Kiljan, Liechti, Lisovski and Hahn2013) and confirmed with a re-analysis of that data, all birds migrated through southern Europe, where the species is considered a very rare passage migrant e.g. in Italy (Spina and Volponi Reference Spina and Volponi2008, but see Brichetti and Fracasso Reference Brichetti and Fracasso2010).
Almost all Aquatic Warblers used staging sites in southern France or the Iberian Peninsula for several days. No bird migrated along the North Sea and Atlantic coasts or used the species’ well-known staging sites at the French Atlantic coast (de By Reference de By1990, Julliard et al. Reference Julliard, Bargain, Dubos and Jiguet2006, Provost et al. Reference Provost, Kerbiriou and Jiguet2010, Jiguet et al. Reference Jiguet, Chiron, Dehorter, Dugué, Provost, Musseau, Guyot, Latraube, Fontanilles, Séchet, Laignel, Gruwier and Le Nevé2011). OY might have been an exception, staging in the area of the Gironde estuary. That birds stopping over in France may not take on sufficient fuel to reach sub-Saharan Africa was suggested by Jakubas et al. (Reference Jakubas, Wojczulanis-Jakubas, Foucher, Dziarska-Palac and Dugué2014), and this study indicates that staging sites in France may not be important for all Aquatic Warblers. Further studies are needed to reveal possible population-specific migration routes. Nevertheless, the entire world population, including birds migrating south of the Alps, may use staging sites on the Iberian Peninsula during autumn migration, and staging is presumably critical to provide fuel for the flight across the Sahara (Atienza et al. Reference Atienza, Pinilla and Justribó2001, Miguélez et al. Reference Miguélez, Zumalacárregui, Fuertes, Astiárraga, González-Jáñez, Roa and de la Calzada2009, Neto et al. Reference Neto, Encarnacao and Fearon2010, Jiguet et al. Reference Jiguet, Chiron, Dehorter, Dugué, Provost, Musseau, Guyot, Latraube, Fontanilles, Séchet, Laignel, Gruwier and Le Nevé2011).
Migration through the Iberian Peninsula was in August and therefore early compared to previous studies (Atienza et al. Reference Atienza, Pinilla and Justribó2001, Julliard et al. Reference Julliard, Bargain, Dubos and Jiguet2006, Neto et al. Reference Neto, Encarnacao and Fearon2010). Atienza et al. (Reference Atienza, Pinilla and Justribó2001) reported two migration peaks in Spain: One in late August and a second one in late September. The first of these peaks coincides almost with migration of adult males fitted with geolocators. The second peak could be explained by differential migration of different sex and age cohorts (Wawrzyniak and Sohns Reference Wawrzyniak and Sohns1977, Wojczulanis-Jakubas et al. Reference Wojczulanis-Jakubas, Chrostek, Jiguet, Zumalacárregui Martínez, Miguélez and Neto2017). Adult males are known to migrate before adult females and first-year birds (Wojczulanis-Jakubas et al. Reference Wojczulanis-Jakubas, Jakubas, Foucher, Dziarska-Palac and Dugué2013, see also Neto et al. Reference Neto, Encarnacao and Fearon2010 for differential migration of age classes in Portugal). Early migration by adult males could also explain the relatively early arrival in sub-Saharan Africa in late August documented in this study as this was slightly earlier than expected from a compilation of African records (Schäffer et al. Reference Schäffer, Walther, Gutteridge and Rahbek2006).
First staging areas in sub-Saharan Africa were not situated along the coast as suggested from an assumed coastal migration route (Schulze-Hagen Reference Schulze-Hagen, Blotzheim and Bauer1991, Atienza et al. Reference Atienza, Pinilla and Justribó2001). With the exception of RR, all first sub-Saharan staging sites were at least 500 km inland in Mauritania and Mali and did not include the known staging areas in and around Djoudj National Park in Senegal (Flade et al. Reference Flade, Diop, Haase, Le Nevé, Oppel, Tegetmeyer, Vogel and Salewski2011, Tegetmeyer et al. Reference Tegetmeyer, Frick and Seifert2014). Therefore, the birds staying in Djoudj are presumably from other populations than the ones studied by us. Anecdotal support of this view comes from ring recoveries: an Aquatic Warbler colour-ringed in Djoudj, Senegal, was later observed on its breeding area at Biebrza in Poland, and a bird ringed in the Inner Niger Delta was later recaptured by our project in Supii in central Ukraine (Poluda et al. Reference Poluda, Flade, Foucher, Kiljan, Tegetmeyer and Salewski2012).
The three birds whose geolocators collected data after arriving at the first sub-Saharan staging area stayed there for 10–12 days before continuing migration for about 700–900 km to the next staging area. This migration step included an abrupt shift in migratory direction from south-south-west to almost due east (EN, RR) or to the south-east (PO). A similar change in migratory direction after the Sahara-crossing has also been shown for the Common Swift Apus apus and Common Redstart Phoenicurus phoenicurus (Åkesson et al. Reference Åkesson, Klaassen, Imgren, Fox and Hedenström2012, Kristensen et al. Reference Kristensen, Tøttrup and Thorup2013).
The final staging area of EN was close to the Inner Niger Delta in Mali about 20 km east of Mopti. Additionally, HK stayed in Mali at a side arm of the Niger River. However, PO stayed in Mali quite distant from the Niger River (c.170 km to the side arm where HK stayed) and OY stayed in northern Côte d’Ivoire close to a dam near Tingrela in late October. Aquatic Warblers are assumed to start moulting remiges in October or November (Tegetmeyer et al. Reference Tegetmeyer, Thoma and Arbeiter2012). Therefore, it is unlikely that they undertake prolonged migrations after October. Thus, it can be expected that the last detected staging areas of HK and OY were also the areas in which they stayed at least during the largest part of the non-breeding season. Although speculative and ignoring the relatively short trip of c.350 km by EN, this may indicate that Aquatic Warblers stay in a single area throughout the non-breeding season. The latter is reached shortly after arrival in sub-Saharan Africa without showing ‘itinerancy’, i.e. using different staging areas for prolonged times during the non-breeding season (Moreau Reference Moreau1972).
Our study suggests, though relying on sparse data, that for male Aquatic Warblers from Dzikoje and Supii the Niger River valley is an important staging area during the non-breeding season, but the Senegal River system is not. Different studies have approached the questions of the whereabouts of Aquatic Warbler’s sub-Saharan staging areas using a compilation of observations (Schäffer et al. Reference Schäffer, Walther, Gutteridge and Rahbek2006), maximum entropy models combining observations of Aquatic Warblers in the Djoudj area with land cover and climate data (Buchanan et al. Reference Buchanan, Lachmann, Tegetmeyer, Oppel, Nelson and Flade2011), or stable isotope analyses (Oppel et al. Reference Oppel, Pain, Lindsell, Lachmann, Diop, Tegetmeyer, Donald, Anderson, Bowden, Tanneberger and Flade2011). They all identified the Senegal and Niger River valleys as suitable areas. Buchanan et al. (Reference Buchanan, Lachmann, Tegetmeyer, Oppel, Nelson and Flade2011) as well as Oppel et al. (Reference Oppel, Pain, Lindsell, Lachmann, Diop, Tegetmeyer, Donald, Anderson, Bowden, Tanneberger and Flade2011) indicate more potential Aquatic Warbler sites at wetlands isolated from the two main inundation zones, and our study also suggests the use of such isolated and often temporary wetlands.
OY probably spent the boreal winter south (10.5°N) of the potential non-breeding area as predicted by using remote sensing analyses (southern limit about 13.3°N; Buchanan et al. Reference Buchanan, Lachmann, Tegetmeyer, Oppel, Nelson and Flade2011) and bioclimatic and land transformation variables (southern limit about 11.8°N at the longitude of OY but about 11°N further east; Walther et al. Reference Walther, Schäffer, van Niekerk, Thuiller, Rahbek and Chown2007) at least at this longitude. Analysis of stable isotopes of rectrices grown on the wintering grounds revealed that some Aquatic Warblers may spend the boreal winter in northern Côte d’Ivoire or adjacent areas (Oppel et al. Reference Oppel, Pain, Lindsell, Lachmann, Diop, Tegetmeyer, Donald, Anderson, Bowden, Tanneberger and Flade2011). OY therefore indicates that the African range of Aquatic Warblers may extend further south than previously understood.
There is an apparent discrepancy between being a wetland specialist and stopping over and staying for months at the southern fringe of the Sahara and in the Sahel zone (Figure 2), areas known for their severe droughts. It is obvious that Aquatic Warblers therefore rely on relatively small wetlands in an otherwise dry climate. In addition to the large Sahel wetlands (Djoudj, Inner Niger Delta), scattered small water bodies in the desert and Sahel may play a crucial role in successful migration for Aquatic Warblers. Their loss may add a serious threat for birds searching for a staging area in an otherwise hostile environment after the Sahara crossing. Like the large wetlands (Zwarts et al. Reference Zwarts, Bijlsma, van der Kamp and Wymenga2009) small water bodies are also under pressure because of anthropogenic use and climate change (Mullié et al. Reference Mullié, Brouwer, Codjo, Decae, Beintema and van Vessern1998, Brouwer Reference Brouwer, Herrera, Davies and Manzano Baena2014, Brouwer et al. Reference Brouwer, Abdoul Kader and Sommerhalter2014) and their number is declining: Niger, for instance, has lost more than 80% of its wetland resources during recent decades (Haas et al. Reference Haas, Bartholomé and Combal2009). Detecting (Combal et al. Reference Combal, Haas, Andigué, Nonguiema and Bartholomé2009, Gal et al. Reference Gal, Grippa, Hiernaux, Peugeot, Mougin and Kergoat2016) and preserving small water bodies that rely on local rainfall (Gond et al. Reference Gond, Bartholomé, Ouattara, Nonguierma and Bado2004) may therefore be of utmost importance for the conservation of Aquatic Warblers and for biodiversity in the Sahel in general (Mullié et al. Reference Mullié, Brouwer, Codjo, Decae, Beintema and van Vessern1998, Brouwer Reference Brouwer, Herrera, Davies and Manzano Baena2014, Wetlands International 2017).
Only one geolocator (EN) recorded data for the entire migration cycle. In spring EN crossed the Sahara and the Mediterranean apparently on a more easterly route than in autumn (Figure 1a). This example is consistent with a loop migration as suggested by previous authors (Mester Reference Mester1967, de By Reference de By1990, Atienza et al. Reference Atienza, Pinilla and Justribó2001, Poulin et al. Reference Poulin, Duborper and Lefebvre2010). The bird arrived in the breeding area approximately 18 days after leaving the last sub-Saharan staging site; this included a staging period of four days in south-east Europe. Spring migration was therefore almost two times faster than autumn migration, when the time spent between the breeding area and the first sub-Saharan staging site was approximately 35 days (09 July–13 August). More rapid northern migration has already been suggested for Aquatic Warblers based on migration phenology (Atienza et al. Reference Atienza, Pinilla and Justribó2001), and fits the general pattern for Palearctic migrants to sub-Saharan Africa (Tøttrup et al. Reference Tøttrup, Klaassen, Strandberg, Thorup, Willemoes Kristensen, Søgaard Jørgensen, Fox, Afanasyev, Rahbek and Alerstam2011, Åkesson et al. Reference Åkesson, Klaassen, Imgren, Fox and Hedenström2012, Schmaljohann et al. Reference Schmaljohann, Buchmann, Fox and Bairlein2012, Ouwehand et al. 2015).
In conclusion, we confirmed the existence of a ‘regular’ but previously unknown migration route along the northern Mediterranean via south-east Europe and Italy for adult male Aquatic Warblers, and conservation of staging sites along it is important for south-eastern populations of the species. In the western Mediterranean, all adult male Aquatic Warblers from Dzikoje and Supii used staging sites on the Iberian Peninsula or in north-west Africa before crossing the Sahara. These sites are of utmost importance for Aquatic Warbler conservation as probably the entire world population migrates via the Iberian Peninsula in autumn. Staging sites in West Africa may consist of relatively small wetlands. Although explicit small water bodies cannot be identified using geolocators our study presents data indicating the need to protect small wetlands in general. These areas may be either under threat by similar factors as on the breeding grounds. Large scale transformation of suitable natural freshwater habitats and floodplains into agricultural areas, such as rice paddies, through construction of dams and water reservoirs, intensive grazing by livestock as well as the spread of the invasive cattail (Typha sp.) has led to degradation and loss of potential wintering sites (Zwarts et al. Reference Zwarts, Bijlsma, van der Kamp and Wymenga2009, Flade et al. Reference Flade, Diop, Haase, Le Nevé, Oppel, Tegetmeyer, Vogel and Salewski2011, Wetlands International 2017). Preserving these wetlands is especially important as climate change scenarios predict increasing temperature and decreasing precipitation for West Africa (IPCC 2014) and as the demand for rice cultivation is still increasing (Brouwer Reference Brouwer, Herrera, Davies and Manzano Baena2014). Additionally, some Aquatic Warblers may spend the non-breeding season further south than previously assumed and wetlands south of the Sahel zone should be incorporated in conservation scenarios too. Individuals from other breeding sites may, however, use different staging sites/areas. With respect to questions how additional knowledge would have implications for the implementation of management strategies and how further efforts could increase the gain of further studies for conservation (Allan and Sing 2016, McGowan et al. Reference McGowan, Beger, Lewison, Harcourt, Campbell, Priest, Dwyer, Lin, Lentini, Dudgeon, McMahon, Watts and Possingham2017), future geolocator studies should concentrate on other populations to close gaps in knowledge and to provide missing information crucial for successful management.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270918000357
Acknowledgements
We thank S. Baumung, K. Heinicke, T. Heinicke, V. Hrudzinskaja, O. Keiss, B. Kiesewetter, N. Kulikova, T. Langgemach, I. Leheida, V. Losieva, A. Prott, T. Ryslavy, R. Shkabara, D. Shymanchuk, S. Sidaruk, L. Sitkevich, Z. Slizh, G. Viol, K. Wesolowski and L. Winter for their enthusiastic help in the field and APB-BirdLife Belarus and the scientific department of the “Bielaviežskajapušča” National Park for their support of field work in Belarus. D. Franklin kindly improved our English. SL was partly supported by the National Science Foundation [grant numbers NSF ARC-1147289 to Marilyn Ramenofsky].The Swiss Federal Office for Environment financially supported the development of the geolocators (UTF-Nr. 254, 332, 363, 400).




