Many human intervention studies in nutrition measure a change in blood biomarkers as a surrogate estimation of health. One of the biomarkers that became popular over two decades ago was general ‘antioxidant activity’ employing methods such as oxygen radical absorbance capacity, total radical-trapping antioxidant parameter, trolox-equivalent antioxidant capacity and ferric-reducing ability of plasma(Reference Cao and Prior1). At the time, these assays promised an easy, inexpensive and accessible way to measure a potential health benefit. This approach was supported by the fact that many dietary components relevant to nutrition including vitamin E, vitamin C, carotenoids and polyphenols are chemical antioxidants when tested in vitro (Reference Rice-Evans, Miller and Paganga2). Furthermore, many other molecules, even those which have highly defined and well-established biochemical properties, such as tryptophan, have also been reported as chemical antioxidants(Reference Nayak and Buttar3). However, it is now appreciated that the molecular mechanisms contributing to antioxidant effects in vivo are much more complex and that the health benefits of the so-called antioxidant dietary components may be due to much more specific actions. Any antioxidant activity in vivo is very site specific and remains contentious(Reference Traber and Atkinson4,Reference Brigelius-Flohe and Davies5) , and the mechanism can alternatively involve an indirect antioxidant activity including, for example, the inhibition of reactive oxygen species-producing enzymes such as xanthine oxidoreductase(Reference Kerimi and Williamson6) and NADPH oxidase(Reference Steffen, Gruber and Schewe7) by polyphenols.
The main problem for reporting ‘antioxidant activity’ in blood is interpretation, especially when an extrapolation to potential effects on health effects is claimed. In the early days of research in this field, it was originally thought that an increase in ‘antioxidant activity’ in blood was indicative of a health benefit, but it was soon questioned whether this alone was sufficient to indicate a health benefit(Reference Aviram8). Assays such as oxygen radical absorbance capacity, trolox-equivalent antioxidant capacity, ferric-reducing ability of plasma and total radical-trapping antioxidant parameter measure complex combinations of different markers (Fig. 1) which preclude simple interpretation. Uric acid is one of the main contributors to ‘antioxidant activity’ in blood as measured by all of these assays. Since increased circulating concentration of uric acid is a well-established risk factor for gout(Reference Richette and Bardin9) and for risk of developing type 2 diabetes(Reference Lv, Meng and He10), an increase in ‘antioxidant activity’ due to a higher concentration of uric acid is unlikely to indicate a health benefit. After fruit consumption, plasma ‘antioxidant activity’ increased postprandially, originally attributed to absorbed polyphenols appearing in blood(Reference Cao, Russell and Lischner11). Subsequent work showed that the increase in ‘antioxidant capacity’ was actually due to a transient increase in uric acid in plasma due to the fructose content of the fruit(Reference Lotito and Frei12). This problem even led to the suggestion that ‘antioxidant activity’ should be corrected for the nightmare of uric acid (Reference Costantini13). Moreover, an increase in albumin could also give an apparent increase in ‘antioxidant activity’, especially when using the oxygen radical absorbance capacity and trolox-equivalent antioxidant capacity assays, but an increase in albumin is seen mostly during dehydration and other adverse conditions(Reference Holliday14).
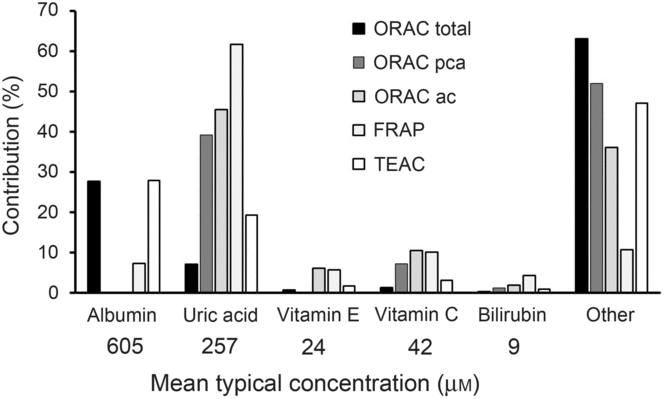
Fig. 1. Contribution of individual components to the total antioxidant capacity of plasma. Data have been taken from Cao & Prior(Reference Cao and Prior1) and adapted. Contribution is the percentage that each component contributes to the total antioxidant value for each method. The mean concentration in fasting plasma is indicated. ORAC, oxygen radical absorbance capacity; ORAC pca, ORAC measured using serum treated with perchloric acid; ORAC ac, ORAC measured using serum treated with acetone; FRAP, ferric-reducing ability of plasma; TEAC, trolox-equivalent antioxidant capacity.
The approximate mean concentrations of the main endogenous molecules that contribute to ‘antioxidant activity’ in in vitro assays have been reported in many publications and are shown in Fig. 1. After high doses of dietary phytochemicals such as polyphenols and carotenoids are consumed, the blood concentration of their combined metabolites reaches only 1–10 μM, much lower than most of the relevant endogenous plasma components. As a consequence, it is unsurprising that, under normal dietary conditions, the metabolites from these food-derived metabolites have minimal impact on the plasma total antioxidant value in any of the assays.
If it is important for the goals of a particular study, then it is recommended that each of the components in Fig. 1 should be measured individually to give a quantitative estimation of changes in discrete entities. Alternatively, it may be appropriate to measure specific biomarkers of oxidative stress(Reference Frijhoff, Winyard and Zarkovic15).
For the reasons discussed above, the British Journal of Nutrition does not accept papers that report findings from the use of general antioxidant assays such as trolox-equivalent antioxidant capacity, ferric-reducing ability of plasma, oxygen radical absorbance capacity and total radical-trapping antioxidant parameter on blood samples.




