Introduction
Giardia duodenalis, a flagellated protozoan, is a major cause of diarrhoeal illness worldwide [Reference Huang and White1]. In the USA, Giardia is the most commonly identified enteric parasite of humans [Reference Kappus2], with an estimated 1.2 × 106 episodes of illness occurring annually [Reference Scallan3] and Giardia identified in 4–7% of stool specimens from patients with diarrhoeal illness tested by clinical diagnostic laboratories [Reference Kappus2]. Giardia infection typically results in self-limited gastrointestinal (GI) illness characterised by diarrhoea, abdominal cramps, bloating, malaise, nausea, weight loss and malabsorption [Reference Huang and White1, Reference Nash4]. Asymptomatic infection also occurs frequently [Reference Huang and White1, Reference Nash4]. Additionally, giardiasis has been associated with the development of chronic diarrhoea or irritable bowel syndrome [Reference Nakao, Collier and Gargano5], fatigue, reactive arthritis and allergies [Reference Nash4, Reference Cantey6, Reference Painter, Collier and Gargano7]. Cases of giardiasis are estimated to result in 1918 emergency department visits, 3581 hospitalisations and $34 million in hospitalisation costs annually in the USA [Reference Collier8, Reference Adam9].
The infectious dose of Giardia is low, with ingestion of as few as ten cysts needed to cause infection [Reference Rendtorff10]. Cysts are immediately infectious upon being excreted in feces [Reference Rendtorff10] and infected individuals can shed 1 × 108 to 1 × 109 cysts in their stool each day for several months [Reference Rendtorff10–Reference Danciger and Lopez12]. Giardia cysts are environmentally hardy and moderately chlorine tolerant and can, therefore, survive in water, food, or on surfaces for several weeks to several months [Reference Huang and White1, Reference Xiao and Fayer13]. Giardia infection is transmitted through the faecal-oral route and results from the ingestion of Giardia cysts. Transmission occurs indirectly through the consumption of faecally contaminated water or food and directly through animal-to-person transmission and person-to-person transmission. Additional host-level factors such as illnesses and medications might impact individuals’ susceptibility to infection [Reference Williamson14].
Most information on risk factors for domestically-acquired giardiasis in the USA has come from outbreak investigations [Reference Stuart15]. From 1971–2011, 242 US giardiasis outbreaks were reported to the Centers for Disease Control and Prevention (CDC). These outbreaks resulted from waterborne (74.8%), foodborne (15.7%), person-to-person (2.5%) and zoonotic (1.2%) transmission [Reference Adam16]. However, >99% of giardiasis cases are sporadic (i.e., not associated with a recognised outbreak) [Reference Yoder17] and only a few case-control studies in the USA have investigated risk factors for sporadic giardiasis [Reference Chute, Smith and Baron18–Reference Wright20]. The CDC collaborated with the Colorado and Minnesota public health departments to conduct a case-control study of sporadic giardiasis in Colorado and Minnesota aimed at evaluating a wider range of risk factors among more participants across an expanded geographic area compared with the relatively small, single-state studies that preceded it.
Methods
Data collection
Persons with laboratory-confirmed Giardia infection were identified by the Colorado and Minnesota public health departments in 2003 and 2004. Under a protocol approved by the CDC and the states’ Institutional Review Boards, public health departments identified case-patients over a 12-month period by monitoring reports of laboratory-confirmed Giardia infections not associated with known outbreaks under investigation by the state or local health departments. Colorado monitored the Colorado Electronic Disease Reporting System for laboratory-confirmed cases in their catchment area, consisting of seven counties within the Denver metro area. Minnesota received reports from the entire state through a passive reporting system. Due to the higher incidence of giardiasis and larger catchment area in Minnesota, every second laboratory-confirmed case was eligible for the study.
Cases were defined as individuals with symptomatic, non-outbreak-associated, laboratory-confirmed giardiasis and an onset of illness within 6 weeks of their public health interview date. For each case-patient, up to two matched controls were enrolled. Controls were matched to case-patients by site (Colorado and Minnesota) and age group (⩽11 months, 1–4 years, 5–11 years, 12–17 years, 18–44 years, 45–64 years and ⩾65 years). Controls were recruited using progressive digit dialing anchored on the case-patient's phone number, where one number was added to the case-patient's phone number until someone who met the inclusion criteria was reached. Only one control was recruited per household.
Investigators contacted case-patients and controls by telephone, obtained informed consent (from parents or guardians for participants aged <18 years) and enrolled them in the study. A structured telephone questionnaire was administered by trained staff to participants ⩾12 years of age or to the parent or guardian of participants <12 years of age. Participants were asked about demographic information, medical conditions, illness symptoms and possible exposures occurring in the 2 weeks prior to the onset of GI symptoms in the matched case-patient. Risk factor questions addressed health status and medications, drinking-water consumption, recreational water exposure, travel and outdoor activity, food and drink consumption, contact with young children and persons with diarrhoea, contact with animals, and sexual practices (only among persons ⩾18 years of age).
Case-patients and controls were excluded if they lived outside of the states’ surveillance catchment areas, were not reached after 15 telephone attempts, had unavailable telephone numbers, were physically or emotionally unable to answer questions, were non-English speakers, or were recent immigrants, adoptees, or refugees. Additional exclusion criteria that applied only to case-patients included asymptomatic infection, unknown illness onset date, previous Giardia infection within 3 months, and not having the earliest symptom onset date within a household cluster of laboratory-confirmed giardiasis. Additional exclusion criteria that applied only to controls included diarrhoea during the 4 weeks before the illness onset date of the matched case-patient, or diarrhoea in a household contact during the 4 weeks before the illness onset date of the matched case-patient.
Statistical analysis
Over 500 exposure questions were included in the questionnaire; to prioritise potential risk factors for model building, we developed a framework to organise host factors and transmission pathways for Giardia infection (Fig. 1). We subdivided transmission pathways into direct faecal contact and indirect faecal contact. We defined direct faecal contact as risk factors for animal-to-person transmission and person-to-person transmission. We defined indirect faecal contact as activities that could lead to faecal ingestion without contact with the human or animal source, including drinking-water consumption, recreational water exposures, food consumption, travel and outdoor activity. Host factors included illnesses and medications that might impact susceptibility to infection. Several analysis exposures were derived by combining multiple items from the questionnaire and recoding free text responses. We selected potential giardiasis risk factors from the framework for analysis based on biological plausibility, previously reported associations and those that contained at least 25 exposed individuals.
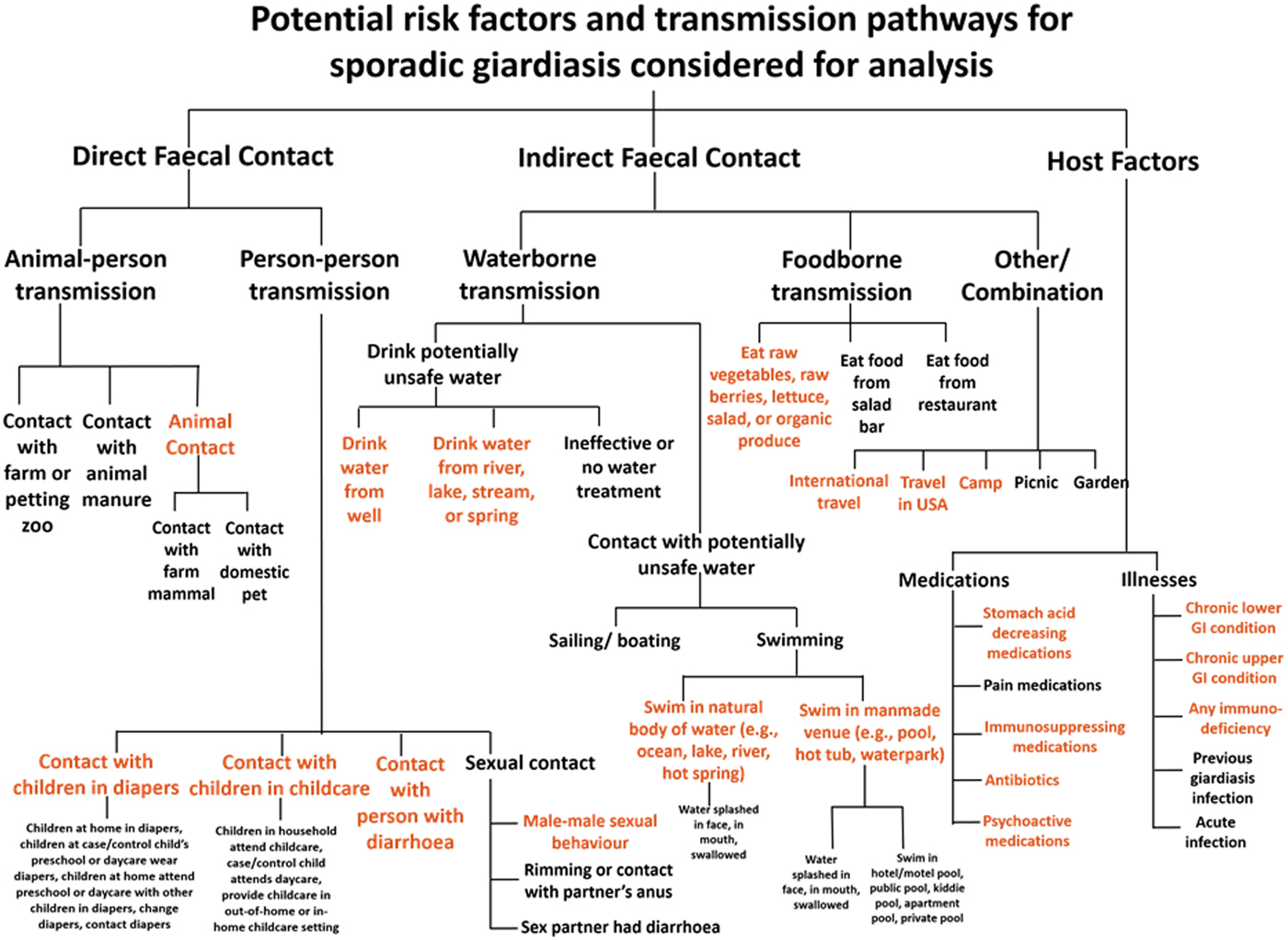
Fig. 1. All risk factors and transmission pathways considered for analysis in the Colorado and Minnesota giardiasis case-control study. Items written in orange text in the figure represent potential risk factors selected for multivariable analysis based on biological plausibility, previously reported associations and those that contained at least 25 exposed individuals.
Data were analysed using SAS software (Version 9.3; SAS Institute, Cary, NC) with multivariable logistic regression. Crude odds ratios (OR) and 95% confidence intervals (CI) were estimated for each exposure variable of interest. ORs were also calculated for each exposure, controlling for the matching variables age and site; these values were essentially equivalent to the crude estimates, so results from the unadjusted models were considered in further analyses. Exposures associated with disease status with P < 0.20 in the crude models or those that had a previously reported association with giardiasis were included in the multivariable logistic regression model. This model estimated adjusted ORs (aOR) and 95% CIs, controlling for age, site and sex. The interaction between male or female sex and having a sex partner of the same sex was assessed in this model. Population attributable fractions (PAF), the estimates of the fraction of the total disease in the population that would not have occurred if the effect associated with the risk factor of interest were absent, were calculated for the factors positively associated with Giardia infection in the multivariable model [Reference Bruzzi21].
To evaluate risk factors for domestically-acquired giardiasis, we repeated analyses after excluding individuals with a history of international travel during the 2-week exposure period. This multivariable model, referred to as the domestic-only model, also controlled for sex, age and site.
Statistical analysis: semi-Bayes shrinkage methods
Given the large number of potential risk factors assessed in the questionnaire, a separate analysis applied semi-Bayes shrinkage methods to the distribution of ORs for 70 exposures that have not previously been shown to have an association with giardiasis, or those that had too few cases and controls exposed to result in stable estimates in the conventional multivariable regression model that evaluated 18 potential risk factors. After excluding all associations that we expected a priori, we applied semi-Bayes shrinkage methods to the distribution of the remaining ORs [Reference Steenland22, Reference Greenland and Robins23]. Semi-Bayes shrinkage narrows the distribution of observed ORs and improves their precision by applying a regression-derived shrinkage estimator [Reference Lash24]. When this method is applied, imprecisely measured associations that are well above or below the null are drawn towards the center of the distribution, thereby reducing the potential to overestimate true associations and allowing us to identify new associations with improved validity [Reference Lash24]. To implement this method, it was assumed that 95% of the true ORs would fall between 0.25 and 4. Each OR was adjusted for age, site and sex. We also adjusted each OR for international travel and drinking water from a river, lake, stream, or spring because these exposures are previously reported strong risk factors and therefore could be confounders [Reference MacLehose25].
Results
Study population
During the 12-month study period, 653 cases of laboratory-confirmed Giardia infection were identified in Colorado and Minnesota. A total of 213 case-patients were enrolled, of which 199 were matched with 381 controls; 14 case-patients were excluded because no matched controls were found (Fig. 2).
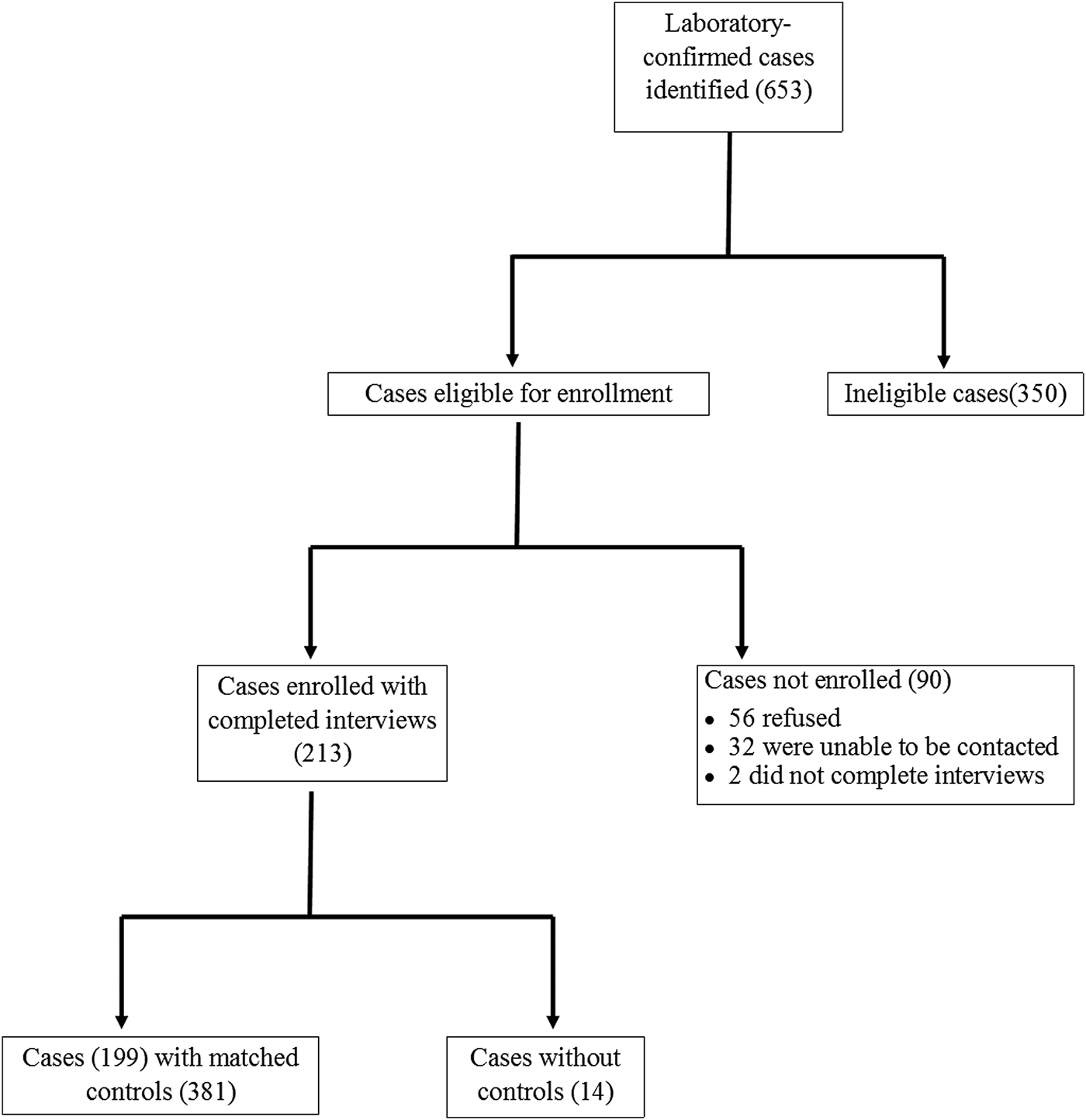
Fig. 2. Recruitment of reported laboratory-confirmed Giardia cases and matched controls in the Colorado and Minnesota giardiasis case-control study. A total of 213 case-patients were enrolled, of which 199 were matched with controls. The 440 case-patients not enrolled were most commonly excluded from enrollment because they had onset of gastrointestinal symptoms greater than 6 weeks (43.6%), refused to participate (12.7%), were recent immigrants, adoptees, or refugees (12.3%), or were asymptomatic (9.3%). Other reasons for non-enrollment included inability to be reached (7.3%), non-English speaking (6.6%), failure to have the earliest illness onset within a family cluster of laboratory-confirmed giardiasis (2.3%), incomplete interview (0.5%) and other miscellaneous reasons (5.5%).
Demographic characteristics
Case-patients were mostly white (97.0%) and non-Hispanic (92.9%) (Table 1). The median age of enrolled case-patients and controls was 37 years. Case-patients and controls were generally similar with regard to the distribution of race, ethnicity, income, site and age, but more case-patients were male compared with controls (56.3% of case-patients vs. 39.1% of controls; OR = 2.0; 95% CI 1.4–2.8; P < 0.0001).
Table 1. Demographic characteristics for giardiasis cases and controls in the Colorado and Minnesota giardiasis case-control study
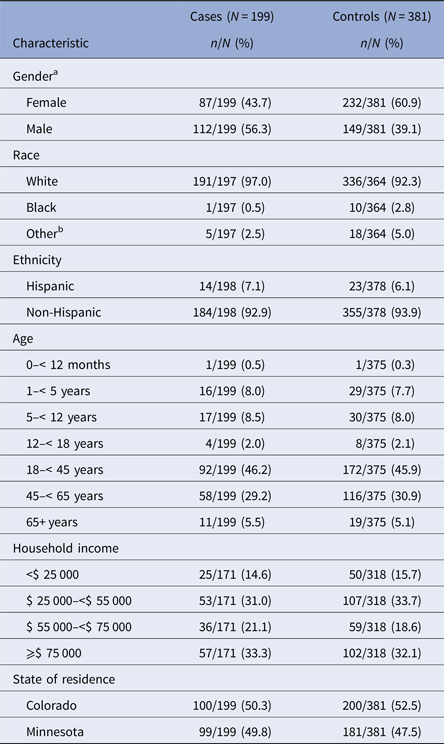
a A statistically significant association between gender and giardiasis was identified (odds ratio = 2.0; 95% confidence interval = 1.4, 2.8; P < 0.0001).
b Other race includes American Indian/Alaskan Native, Asian/Pacific Islander, mixed, other.
Risk factor analysis
In the bivariate and multivariable analyses, international travel, having contact with children in diapers, drinking water from a river, lake, stream, or spring, swimming in a natural body of water, taking antibiotics and having a chronic GI condition or taking medication indicated for such a condition (hereafter referred to as chronic GI condition), were associated with a statistically significant increased odds of giardiasis, and eating raw vegetables or fruit was associated with a decreased odds of giardiasis (Table 2). In addition to these main effects, an interaction between having a sex partner of the same sex and being male/female was detected (Wald χ 2 = 7.3; P = 0.007). Male–male sexual behaviour was associated with significantly increased odds of giardiasis (aOR = 45.7; 95% CI 5.8–362.0), but female–female sexual behaviour was not (aOR = 0.6; 95% CI 0.1–6.4). Factors that were associated with giardiasis in the bivariate analysis but not after multivariable adjustment included camping, animal contact, swimming in a manmade aquatic venue, and taking psychoactive medication.
Table 2. Bivariate and multivariable analysis of exposures in the 2 weeks before illness onset caused by Giardia infection in the Colorado and Minnesota giardiasis case-control study
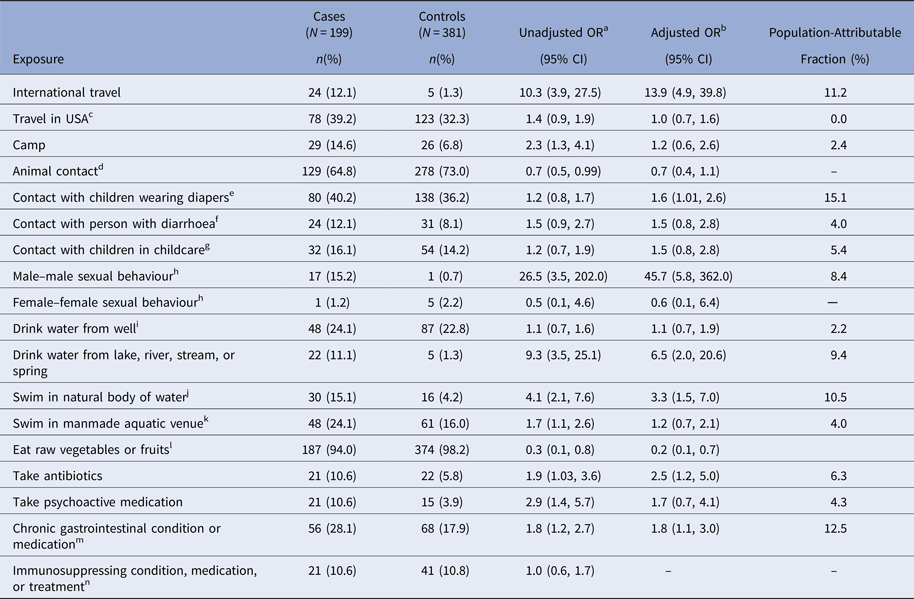
a Unadjusted odds ratio.
b Odds ratio from full multivariable model adjusted for age, site, sex and all exposures that have been previously reported to be associated with giardiasis and showed an association with disease status with P < 0.2 in the bivariate models. Given these criteria, immunosuppressing condition, medication, or treatment was excluded from the multivariable analysis.
c The variable ‘travel in the United States’ was derived by grouping travel within home state more than 100 miles and travel outside of home state within the USA.
d The variable ‘animal contact’ was derived by grouping any contact with domestic pets (i.e., kitten, cat, puppy, dog) and contact with farm mammals (i.e., calf, cow, goat, sheep, horse, pig).
e The variable ‘contact with children wearing diapers’ was derived by grouping children in home wear diapers, children at child's childcare location wear diapers, contact with children in diapers and change diapers.
f The variable ‘contact with person with diarrhoea’ was derived by grouping direct contact with person with diarrhoea and provide care for person with diarrhoea.
g The variable ‘contact with children in childcare’ was derived by grouping attends childcare, children in home attend out-of-home childcare, provide childcare outside of home, provide childcare in home.
h Statistically significant interaction was identified between sex (male vs. female) and having a same-gender sex partner; this is presented stratified by individual sex.
i The variable ‘drink water from well’ was derived by grouping well as source of drinking water at home and well as source of drinking water outside of home.
j The variable ‘swim in natural body of water’ was derived by grouping swim, wade, or enter an ocean, lake, pond, river, stream, or hot spring.
k The variable ‘swim in manmade aquatic venue’ was derived by grouping swim, wade, or enter a swimming pool, hot tub/spa, or waterpark.
l The variable ‘eat raw vegetables or fruits’ was derived by grouping eat lettuce, garden salad, raw vegetables, raw berries, raw fruits with skin/peel and any organic produce.
m The variable ‘chronic gastrointestinal (GI) condition’ was derived by grouping chronic lower GI conditions (i.e., chronic diarrhoea, Crohn's disease, irritable bowel syndrome), chronic upper GI conditions (i.e., stomach ulcer disease), chronic lower GI medications (i.e., laxatives, enemas, antidiarrhoeal medications) and chronic upper GI medications (i.e., stomach-acid reducing medications).
n The variable ‘immunosuppressing condition, medication, or treatment’ was derived by grouping immunosuppressing conditions (i.e., diabetes, cancer, HIV/AIDS, other immunodeficiency), medications (i.e., steroids, cyclosporine) and treatments (i.e., organ transplant, chemotherapy, radiation).
Results were similar after excluding individuals with a history of international travel, except that an additional significant crude association between giardiasis and travelling in the USA was found (Supplementary Table S1; Supplementary Material is available on the Cambridge Core website).
PAFs
Among all case-patients, the proportion whose illness could be attributed to foreign travel was 11.2% (Table 2). The largest PAFs resulted from contact with children wearing diapers (15.1%), having a chronic GI condition (12.5%), swimming in a natural body of water (10.5%), drinking water from a river, lake, stream, or spring (9.4%) and male–male sexual behaviour (8.4%).
Semi-Bayes shrinkage methods
Supplementary Table S2 shows the conventional and semi-Bayes-adjusted ORs and 95% CIs for 70 exposures that do not have a previously reported association with giardiasis, or those that had too few cases and controls exposed to result in stable estimates in the conventional multivariable regression model. Prior to semi-Bayes adjustment, the conventional ORs ranged from 0.1 (95% CI 0.01–0.5) for swimming in or entering a recreational waterpark to 35.8 (95% CI 4.6–276.2) for male–male sexual behaviour. After semi-Bayes adjustment, the ORs ranged from 0.5 (95% CI 0.2–1.2) for taking pain medication to 3.9 (95% CI 1.2–13.1) for same-gender sexual behaviour. Of the 70 exposures considered, 11 were associated with an increased odds of giardiasis and four were associated with a decreased odds of giardiasis prior to semi-Bayes adjustment. After the adjustment, the only exposure that remained significantly associated with giardiasis was same-gender sexual behaviour.
Discussion
This case-control study represents the largest and most extensive assessment of risk factors for sporadic Giardia infection in the USA to date. The results from this study demonstrate the importance of domestic transmission and the diversity of risk factors for giardiasis across multiple transmission pathways. Among these pathways, person-to-person transmission was responsible for the largest proportion (32.9%) of illness among case-patients, representing an opportunity for intervention through modifiable, individual-level changes in behaviour.
Giardia infection was strongly associated with international travel (aOR = 13.9), supporting the results of previous case-control studies in the USA [Reference Chute, Smith and Baron18] and other developed countries including the UK [Reference Gray, Gunnell and Peters26, Reference Minetti27], Canada [Reference Gagnon28] and New Zealand [Reference Hoque29]. Despite this strong association, only 11.2% of the illness in this group of case-patients was attributed to international travel, underscoring the importance of domestic risk factors for giardiasis. The actual transmission pathways that result in infection associated with international travel likely include drinking local water, eating undercooked food and direct faecal contact due to inadequate sanitation. Therefore, individuals should practice extra caution when traveling and avoid consuming undercooked food and inadequately treated water and ice [Reference Watson, Hlavsa, Griffin, Brunette, Kozarsky, Cohen, Gershman, Magill, Ostroff, Ryan, Shlim, Weinberg, Wilson, O'Sullivan and Henry30]. In the unadjusted analyses, travelling in the USA (in the domestic-only model) and camping were associated with giardiasis. Camping has been frequently reported as a risk factor for giardiasis [Reference Chute, Smith and Baron18, Reference Wright20, Reference Gray, Gunnell and Peters26, Reference Minetti27] and one study in New Zealand reported domestic travel as a risk factor [Reference Hoque31]. Like international travel, domestic travel and camping might confer an elevated risk through behaviours across multiple transmission pathways including eating undercooked food, practicing poor hygiene, drinking untreated surface water and drinking water from new sources with different pathogens to which individuals lack immunity.
Our study also provides evidence for transmission through consumption of contaminated water and food. Drinking water from a river, lake, stream, or spring was identified as a strong risk factor for giardiasis. Waterborne outbreaks and sporadic cases of giardiasis have been previously associated with consuming untreated water from natural bodies of water including rivers and streams [Reference Adam16, Reference Dennis19, Reference Wright20, Reference Isaac-Renton and Philion32–Reference Weiss34]. Studies in Colorado [Reference Wright20], Minnesota [Reference Weiss34] and New Hampshire [Reference Dennis19] identified consumption of untreated water as an important cause of sporadic giardiasis in the USA. In addition to direct consumption of contaminated water, recreational activities in lakes, rivers and swimming pools have been implicated as risk factors in numerous outbreak investigations and studies of sporadic giardiasis [Reference Stuart15, Reference Adam16, Reference Hoque29, Reference Porter35, Reference Snel36]. In our study, swimming in a natural body of water was associated with Giardia infection. Natural recreational waters are not treated with disinfectants, such as chlorine or bromine, the primary barrier to the transmission of infectious pathogens in aquatic venues [Reference Graczyk37]. Swimming in a manmade aquatic venue (i.e., swimming pool, waterpark, or hot tub/spa) was a risk factor in the unadjusted analysis (although not in the multivariable analysis), emphasising the importance of public health efforts to promote healthy swimming behaviours (e.g., staying out of the water if one has diarrhoea) to improve swimmer hygiene and prevent water contamination in all recreational water venues.
As with water, food can become contaminated by Giardia cysts and links between giardiasis and various types of produce have been reported [Reference Stuart15, Reference Hoque31]. We found that eating raw vegetables or fruit was inversely associated with giardiasis. A similar protective effect was reported in a sporadic giardiasis case-control study in the UK [Reference Minetti27]. It is possible that repeated exposure via contaminated raw produce could provide protective immunity [Reference Hunter and Thompson38]. Fruits and vegetables can become contaminated during irrigation and fertilisation activities, by water used during processing or packing, or by unwashed hands during harvesting or any point in the production chain [Reference Bello39]. Since fruits and vegetables are often eaten raw, preventing produce contamination and washing produce before use are particularly important [Reference Burnett and Beuchat40]. The addition of serologic approaches to future studies could provide more data on the possible role of fresh produce for protective immunity. However, it is possible that this result might reflect increased healthful behaviours among controls compared with case-patients; individuals who frequently consume fruits and vegetables might have better general health than non-produce-consumers and could be less likely to contract giardiasis or develop a symptomatic infection.
Direct person-to-person transmission represented the most important transmission pathway in our study, as the risk factors within this pathway were responsible for the largest proportion of illness among case-patients. Contact with children in diapers represented the largest PAFs among all risk factors (15.1% in the main model and 19.3% in the domestic-only model), suggesting that diaper contact could represent an important risk factor for giardiasis in the USA as it is likely a relatively common exposure. An increased risk of infection associated with diaper contact has also been reported in New Zealand [Reference Hoque31, Reference Hoque41] and the UK [Reference Minetti27]. Our results indicate that many Giardia infections could be prevented by targeting diaper-handling hygiene practices in home and childcare settings.
Another form of person-to-person transmission, sexual transmission among men who have sex with men (MSM), was quantified in our study. Since the late 1970's, high Giardia prevalence has been reported among MSM, suggesting transmission through male-male sexual behaviour [Reference Escobedo42, Reference Phillips43], but this is the first epidemiological study to document a statistically significant association between giardiasis and male–male sexual behaviour. Further, same-gender sexual behaviour remained associated with giardiasis after semi-Bayes adjustment, which strengthened the evidence of risk because this method reduces the potential to overestimate true associations. Female–female sexual behaviour was not a risk factor for giardiasis, suggesting that riskier sexual contact among MSM (e.g., engaging in oral-anal contact) or sexual networks among MSM with an increased prevalence of giardiasis might be driving the elevated risk. Further, other enteric infections have been frequently identified among MSM [Reference Bowen44]. Transmission of giardiasis and other enteric illnesses through sexual contact could be reduced by using barriers between the mouth and a partner's genitals or rectal area and washing hands after touching the anus, rectal area, condom, or other barrier used during anal sex [Reference Painter45].
Despite person-to-person transmission representing the largest PAFs in our study, it is likely that the estimates for risk factors within this pathway were underestimated as case-patients were excluded from the study if they did not have the earliest symptom onset date within a household cluster. Accordingly, it is likely that our study underestimated the risk associated with childcare centres, as transmission to close contacts of persons attending or working in childcare centres has been frequently reported [Reference Chute, Smith and Baron18, Reference Dennis19]. The relatively high PAFs associated with person-to-person transmission (32.9%), in spite of the exclusion of ill household members, suggest that person-to-person transmission represents an important pathway for sporadic giardiasis transmission in the USA and prevention efforts should target behaviours associated with the direct faecal contact.
Our study was novel in its consideration of the role of host factors (e.g., illnesses and medications that might influence host susceptibility) in Giardia transmission. We identified a previously unreported risk for giardiasis associated with taking antibiotics during the 2 weeks prior to symptom onset. Disruption of the microbiota with antibiotics is known to precede the emergence of several enteric pathogens including Salmonella spp. and Clostridium difficile [Reference Doorduyn46–Reference Pavia48]. Antibiotic use may lead to prolonged alterations in the microbiota and decreased colonisation resistance, lowering the dose of pathogens needed for colonisation and infection and therefore increasing the risk of illness [Reference Pavia48]. While antibiotics are beneficial when clearly indicated, our result underscores the importance of avoiding unnecessary use. In addition to antibiotics, we found a previously unreported host-level risk associated with taking psychoactive medication in the bivariate analysis. Psychoactive medications, including medications for anxiety, depression and psychosis, have antimicrobial activity and might influence gut motility and sensation; this could also change the composition of gut microbiota and increase infection susceptibility [Reference Costedio, Hyman and Mawe49, Reference Munoz-Bellido, Munoz-Criado and Garcia-Rodriguez50]. Further, we identified an additional novel host-level association between giardiasis and having a chronic GI condition. This was an important risk factor, with a PAF of 12.5%. Elevated giardiasis prevalence has been reported in patients with GI conditions including Crohn's disease [Reference Scheurlen51], irritable bowel syndrome [Reference Minetti27], celiac disease [Reference Raizman52] and other GI diseases [Reference Scheurlen51]. It has been suggested that GI conditions might influence Giardia infection susceptibility by disturbing the protective function of the mucosa in inflammatory states [Reference Scheurlen51]. However, it is not clear whether individuals with these conditions are more susceptible to infection or whether they are more likely to develop symptomatic disease. In addition, conditions with symptoms resembling those of giardiasis (e.g., chronic GI conditions, co-infection with other enteric pathogens) might increase the probability of a Giardia test being performed, thereby increasing the probability of misclassifying asymptomatic infection as symptomatic giardiasis. While diarrhea caused by other conditions is possible, the finding of this association warrants further investigation in studies with more extensive clinical data [Reference Cantey6].
Our study has several limitations. The study design was a retrospective case-control in which exposure information was ascertained via subject interview after disease status was known, potentially resulting in recall bias. The study design might have also led to a selection bias in favour of controls who were more likely to be at home and, thus, perhaps not representative of the general population. In addition, persons with asymptomatic infections might have been misclassified as controls since stool samples were not collected to confirm the absence of infection in the controls. Further, our data were collected in two states over 1 year, thereby limiting generalisability. Finally, PAFs from case-control studies should be interpreted with caution because ORs might not represent causal effects. Although the data for this study were collected in 2003 and 2004, the main giardiasis transmission pathways have likely remained commonplace in the USA. Rates in the overall population have declined, but it is unclear if this is related to changes in disease transmission or changes in surveillance, given the 2011 modification of the case definition clarifying that clinical symptoms are necessary for categorising giardiasis cases as confirmed [Reference Painter45]. Changes in surveillance capacity and a decreased emphasis on giardiasis surveillance might have also occurred [Reference Painter45].
In summary, this study represents the largest, most recent and most comprehensive case-control study of sporadic giardiasis in the USA to date. Our results highlight the diversity of risk factors for sporadic giardiasis and the importance of domestic risk factors, particularly those that are related to direct transmission from person-to-person. We also identified novel risk factors and host-level factors associated with infection, including male–male sexual behaviour, taking antibiotics and having a chronic GI condition. Educational efforts to prevent direct faecal contamination and transmission and to decrease exposure to unsafe drinking and recreational water have the potential to considerably reduce sporadic giardiasis transmission in the USA. In addition, the low percentage of illnesses associated with international travel (11%) emphasises the importance of domestic giardiasis transmission and the need for healthcare providers and the general public to have improved awareness of non-international-travel-associated infection through multiple transmission pathways.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268818001073
Acknowledgements
The authors thank Timothy L. Lash, DSc, MPH for assistance in applying the semi-Bayes analysis. The authors also thank state and local public health agency staff in Colorado and Minnesota for interviewing case-patients and controls.
Declaration of interest
None.







