Infections often precipitate a substantial acute- phase response (APR), which leads to the sequestration of Fe, Fe-limited erythropoiesis and anaemia of inflammation. In such patients, it is difficult to assess to what extent Fe deficiency contributes to the anaemia.
Ferritin, an Fe-storage protein that circulates in small quantities proportional to the amount of Fe in the stores is the best measure of storage Fe(Reference Cook and Skikne1, 2), but is not valid as a measure of storage Fe during conditions eliciting an APR(Reference Witte3). Soluble transferrin receptors (sTfR), expressed on the cell membranes to receive Fe from circulating transferrin, are up-regulated when Fe is lacking in the cytosol. Since sTfR are present in serum, proportional to the amount expressed on cell membranes, serum sTfR is considered a useful marker of Fe deficiency(Reference Cook, Skikne and Baynes4). It has been argued that serum sTfR is unaffected by the APR(Reference Cook, Skikne and Baynes4), but recent studies suggest the opposite(Reference Kasvosve, Gomo and Nathoo5, Reference Beesley, Filteau and Tomkins6).
The serum concentration of the acute- phase reactants, such as C-reactive protein, α1-acid glycoprotein and α-antichymotrypsin (ACT), has been used to adjust for the effect of the APR on the markers of status with respect to Fe and other micronutrients, when assessing the prevalence of deficiency in an apparently healthy population(Reference Thurnham, Mburu and Mwaniki7). The acute- phase reactants differ with respect to magnitude, timing and duration of the response, with ACT and α1-acid glycoprotein having a more delayed response, and a combination of the acute- phase reactants may be needed to account for different stages of the response(Reference Thurnham, Mburu and Mwaniki8).
However, it may also be of interest to assess the Fe status in a clinical setting, among the patients with more massive APR. As part of a trial among pulmonary tuberculosis patients in Tanzania(Reference Range, Andersen and Magnussen9, Reference Range, Changalucha and Krarup10), we collected cross-sectional data on nutritional status. In the present paper, we present data on the role of the APR and other predictors of serum ferritin and sTfR.
Subject and methods
Study area and population
The study was conducted in Mwanza region, Tanzania, between August 2001 and July 2002, within the framework of the National Tuberculosis and Leprosy Programme(11). In brief, tuberculosis suspects attending in- and out-patient clinics at each of five recruitment centres were examined, diagnosed and treated in accordance with the recommended standard procedures(12). Those diagnosed as smear-positive or smear-negative pulmonary tuberculosis (PTB) cases, based on the routine examination of three sputum specimens (spot, morning and spot) for acid-fast bacilli using the Ziehl–Neelsen staining technique, were all offered anti-tuberculosis treatment. Before the start of the treatment, these patients were offered inclusion in the study if aged 15 years or above and if considered a new or relapse case. After informed consent, all the participants were requested to submit a morning sputum specimen in a sterile universal bottle for confirmation at the Zonal tuberculosis Reference Laboratory at Bugando Medical Centre, using microscopy after fluorochrome (auramine O) staining and culture on Lowenstein–Jensen solid media(Reference Githui, Kitui and Juma13). For the purpose of the present study, only those found positive by microscopy (auramine O staining) or culture at the Zonal Tuberculosis Reference Laboratory were considered sputum-positive pulmonary tuberculosis patients (PTB+). Those not found positive by microscopy after auramine O staining or by culture at the Zonal Tuberculosis Reference Laboratory were considered sputum-negative tuberculosis patients (PTB − ) if history, clinical manifestations and X-ray were indicative of tuberculosis according to the WHO guidelines(12).
Methods
Before the start of the tuberculosis treatment, questionnaire data on socio-demography, smoking, alcohol and soil consumption, and medical history were obtained from each patient.
Stool and urine samples were also taken for two consecutive days. Duplicate 50 mg Kato thick smears were made from each stool sample, covered with cellophane soaked in glycerine and malachite green, and examined for Schistosoma mansoni and geohelminths (i.e. hookworm, Ascaris lumbricoides, Trichuris trichiura, Strongyloides stercoralis) eggs(Reference Peters, El Alamy and Warren14). The mean egg counts of the two slides were computed and then multiplied by 20 to obtain the number of eggs per gram (epg) of faeces, which is referred to as the intensity of infection. Urine specimens were collected and examined for Schistosoma haematobium using the filtration technique with Nuclepore® membranes(Reference Peters, Mahmoud and Warren15). The predominant hookworm species in the area was known to be Necator americanus (Reference Lwambo, Siza and Brooker16).
A blood sample was taken from the cubital vein between 08.00 and 12.00 hours, and Hb was determined using a portable haemoglobinometer (HemoCue™; Angelholm, Sweden). Anaemia was defined as Hb < 120 g/l for women and < 130 g/l for men(Reference Stoltzfus and Dreyfuss17). Furthermore, thick and thin blood smears were prepared and stained with Giemsa's stain, and examined for malaria parasites. HIV testing was done using two enzyme immunological assays. Samples found negative using UNIFORM I Vironostika HIV-MIXT (Organon, Boxtel, The Netherlands) were considered negative, whereas those found positive or indeterminate were tested with a confirmatory enzyme immunological assays, UNIFORM II, Enzygnost anti-HIV-1/HIV-2 (Behring, Marburg, Germany). The samples found anti-HIV positive were then tested for HIV-1 using a modification of an in-house-developed RT-PCR(Reference Krarup, Drewes and Madsen18).
From the remaining blood, serum was separated and kept at − 70°C until further analyses. Serum ferritin, ACT and sTfR were determined at the Department of Human Nutrition, University of Copenhagen, Denmark.
Serum ACT was measured by automated turbidimetry (Cobas Mira Plus, Roche, Montclair, NJ, USA). Rabbit anti-human ACT (catalogue no. Q 0367, DAKO, Glostrup, Denmark) was used to precipitate ACT and turbidity was measured at 345 nm after incubation for 8·3 min at 37°C. The results are given as g/l serum based on a standard curve in the range 0·05–1·24 g/l (DAKO, cat. no. X0908). The samples with values above the reference range were diluted appropriately. The inter-run variation was 3 % CV. The accuracy was validated against high and low commercial controls (DAKO, cat. no. X0940 and X0939). The values above 0·4 g/l were considered elevated(Reference Friis, Gomo and Koestel19). Serum ferritin was measured by a fluoroimmunoassay kit (DELFIA Ferritin; Wallac, Turku, Finland). Detection limit of the method was 0·5 μg/l and inter-run variation was 5 % CV.
Serum concentration of sTfR was measured by an automated turbidimetry kit (IdeA sTfR-IT, cat. no. 67 968, Orion Diagnostica, Espoo, Finland) analysed with Cobas Mira Plus, Roche. Serum sTfR was precipitated with sheep anti-human sTfR and turbidity was measured at 600 nm after incubation for 9 min at 37 °C. The results are given as mg/l serum based on a standard curve in the range 0·3–8·5 mg/l Inter-run variation was 8 % CV. The accuracy was validated against high and low commercial controls (Orion Diagnostica no. 67 975 and 67 976). According to the manufacturer, the values above 1·9 mg/l were considered elevated.
Ethical consideration
The Ethics Committee of the National Institute for Medical Research in Tanzania granted permission to conduct the study, and the Danish Central Medical Ethics Committee in Denmark approved it. Oral informed consent was obtained from all study participants before inclusion. The patients diagnosed with helminth or malaria infections and anaemia were referred for the treatment. Pre-HIV test counselling was given to all participants; post-test counselling was offered to those who wanted to know their HIV results.
Statistical analysis
Normal probability plots were used to assess the distribution of continuous variables. Data on serum ferritin was log(x) transformed to achieve approximate normal distributions. The two-sample t test or one-way ANOVA were used to test for the differences in the means between two or more groups, respectively, while the χ2 test was used to test for the differences in the proportions. Linear regression analyses were used to identify the predictors of serum ferritin and serum sTfR. The variables assessed were sex, smoking, consumption of alcohol and soil, PTB and HIV status, and S. mansoni and hookworm infections, with and without adjustment for elevated serum ACT using dummy variables based on the categories 0·4–0·6, 0·6–0·8, 0·8–1·0 and above 1·0 g/l, using the values < 0·4 g/l as reference. Age was adjusted for using dummy variables with age < 20 years as reference, but not shown. The variables with P < 0·10 were kept in the model, and those with P < 0·05 were considered significant. Normal and residual v. fitted plots were examined to assess the normality and homoscedasticity of residuals. Stata version 9.0 (StataCorp, College Station, TX, USA) was used for all analyses.
Results
Of 655 PTB patients, 81·2 % were PTB+ and 18·8 % PTB − (Reference Range, Magnussen and Mugomela20). The prevalence of HIV co-infection was 47·2 %, with 43·6 % among the PTB+ patients and 62·6 % among the PTB − patients. Half (49·5 %) of the males and 4·8 % of the females reported smoking. Regular alcohol consumption was reported by 25·1 % of the females and 42·2 % of the males. Regular soil eating was reported by 10·0 % of the females and 0·8 % of the males.
Schistosoma mansoni egg output was found in 34·4 % of the patients. In those infected, the geometric mean egg output was 34 epg, and 24 % had intensities above 100 epg, indicating moderate and heavy intensities(21). The prevalence of hookworm infection was 18·0 %. In those infected, the geometric mean egg output was 88 epg, and only 2 % had intensities above 2000 epg, used to define moderate or heavy infections(21). Other helminth infections (S. haematobium, A. lumbricoides, T. trichiura, S. stercoralis) as well as malaria parasitaemia were less common, with prevalences below 5 %.
Acute- phase response
The mean serum ACT was 0·72 g/l, with only 8·9 % of the patients having values below 0·4 g/l, and with 16·6 % above 1·0 g/l. There was no difference between the males and females (0·74 v. 0·73 g/l, P = 0·37). The PTB+ patients had 0·15 g/l (95 % CI 0·10, 0·20) higher mean serum ACT compared with the PTB − ones (0·76 v. 0·61 g/l, P < 0·001): among the PTB − patients 21·7 % had serum ACT below 0·4 g/l and 11·6 % above 1·0 g/l, compared with only 4·2 % and 20·2 %, respectively, among the PTB+ ones. Furthermore, among the PTB+ patients, serum ACT increased with culture intensity as it was 0·70 (95 % CI 0·56, 0·65), 0·73 (95 % CI 0·68, 0·78) and 0·80 (95 % CI 0·78, 0·82) g/l among those with mild, moderate and severe culture intensities (P for trend < 0·001). Current smokers had 0·07 g/l (95 % CI 0·01, 0·12) higher serum ACT than non-smokers, but previous smokers did not have higher serum ACT (0·006, 95 % CI − 0·4, 0·06). There was no difference in serum ACT between the patients with or without HIV co-infection (0·02 g/l, 95 % CI − 0·02, 0·06).
Hb and markers of iron status
Hb was 16·5 g/l (95 % CI 12·8, 20·3) higher among the males compared with the females (Table 1). Similarly, geometric mean serum ferritin was 1·9 (95 % CI 1·6, 2·3) times higher in the males, and mean serum sTfR was 0·47 mg/l (95 % CI 0·24, 0·71) higher in the females. As shown in Fig. 1, serum sTfR declined with increasing log serum ferritin, and levelled off when the latter was above 1·5 log or 31·6 μg/l, similarly for the females and males (not shown).
Table 1 Markers of Hb and iron status among 655 tuberculosis patients by sex
(Mean values and 95 % confidence intervals)

sTfR, soluble transferrin receptor.
* Defined as Hb < 120 g/l for females and < 130 g/l for males.
† Geometric mean (95 % CI).
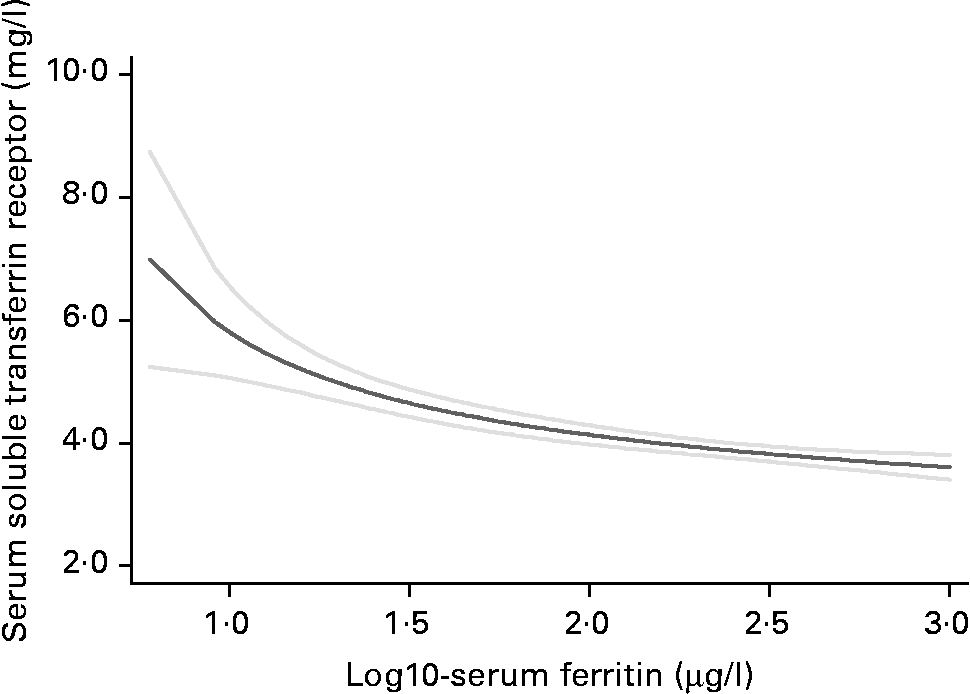
Fig. 1 Fractional polynomial plot of the relationship between serum soluble transferrin receptor by log10 serum ferritin (![]() , mean;
, mean; ![]() , 95 % CI).
, 95 % CI).
Among the males, Hb was 6·0 g/l (95 % CI − 0·4, 12·5) lower and the prevalence of anaemia was 7·9 % (95 % CI 2·6, 18·5) higher among the PTB+ patients compared with the PTB − ones, whereas there were no differences among the females (Table 2). Similarly, among the males, geometric mean serum ferritin was 1·4 (95 % CI 1·1, 1·9) times higher among the PTB+ patients compared with the PTB − ones, whereas there were no differences among the females (95 % CI 0·6, 1·3). The PTB+ patients compared with the PTB − ones had higher mean serum sTfR in both the females (0·68 mg/l, 95 % CI 0·20, 1·15) and the males (0·41 mg/l, 95 % CI 0·03, 0·78). HIV+ compared with HIV − had lower Hb among both the females (7·6 g/l, 95 % CI 2·2, 13·0) and the males (15·6 g/l, 95 % CI 10·8, 20·4; Table 2). Similarly, geometric mean serum ferritin was 1·8 times (95 % CI 1·4, 2·4) higher among the HIV+ compared with the HIV − in the females, and 2·0 times (95 % CI 1·6, 2·5) higher in the males. Accordingly, serum sTfR was lower among the HIV+ compared with the HIV − among the females (0·68 mg/l, 95 % CI 0·30, 1·05), but not among the males ( − 0·03, 95 % CI − 0·33, 0·26).
Table 2 Markers of Hb and iron status among 655 tuberculosis patients by tuberculosis and HIV status
(Mean values and 95 % confidence intervals)

sTfR, soluble transferrin receptor.
* Tuberculosis patients are categorised as PTB+ if mycobacteria were detected in sputum, and PTB − if not.
† Geometric mean (95 % CI).
Hb declined and geometric mean serum ferritin increased with increasing serum ACT (Table 3), both among the females and males, whereas there was no significant difference in serum sTfR across the categories of serum ACT. Log serum ferritin was approximately 1·8 or 63·1 μg/l when serum ACT was below 0·4 g/l, but then increased linearly with increasing serum ACT. By contrast, serum sTfR increased with serum ACT increasing up to 0·4 g/l and reached a plateau (not shown).
Table 3 Markers of Hb and iron status among 655 tuberculosis patients by serum α1-antichymotrypsin category
(Mean values and 95 % confidence intervals)

sTfR, soluble transferrin receptor.
* Geometric mean (95 % CI).
The role of smoking, soil eating, alcohol consumption, as well as infection with hookworm and S. mansoni were also assessed. Among the males, current smokers had significantly lower serum sTfR (3·4 mg/l) than non-smokers (3·9 mg/l) and previous (3·9 mg/l) smokers (one-way ANOVA, P = 0·009). By contrast, there were no associations between smoking status and Hb and serum ferritin. Among the females, those eating soil had 0·44 (95 % CI 0·28, 0·69) times lower serum ferritin than those not eating soil (66·1 v. 151·4 μg/l, P < 0·001), whereas there were no differences in Hb and serum sTfR. Due to the low numbers, the effect of smoking and soil eating could not be assessed in the females and males, respectively. Alcohol consumption was associated with 4·1 g/l (95 % CI 0·1, 8·2, P = 0·046) higher Hb, 1·55 (95 % CI 1·29, 1·86, P < 0·001) times higher serum ferritin and 0·4 mg/l (95 % CI 0·2, 0·7, P < 0·001) lower serum sTfR, both among the males and females (not shown). Hookworm infection was associated with 6·1 (95 % CI 1·0, 11·1, P = 0·02) g/l lower Hb, 0·63 (95 % CI 0·50 0·80, P < 0·001) times lower serum ferritin and 0·37 (95 % CI 0·06, 0·70, P = 0·02) mg/l higher serum sTfR. By contrast, S. mansoni infection was associated with 0·80 (95 % CI 0·66, 0·96, P = 0·02) times lower serum ferritin (176 v. 221 μg/l), but not Hb and serum sTfR.
Predictors of serum ferritin and soluble transferrin receptor
The role of sex, infectious disease (PTB, HIV, hookworm, S. mansoni) status, alcohol consumption, smoking and soil eating as the predictors of serum ferritin and sTfR was assessed in linear regression models, without and with adjustment for elevated serum ACT. Since there were no interactions between sex and other potential predictors, the analyses were done for the females and males combined.
For serum ferritin (Table 4), negative predictors were female sex, soil eating, S. mansoni and hookworm infection, and positive predictors were alcohol consumption and smoking, sputum-positive tuberculosis and HIV infection. With these predictors in the model (model 1), 24 % of the variation in serum ferritin was explained. Compared with the overall geometric mean serum ferritin of 205·7 μg/l, the geometric mean serum ferritin among the individuals falling into all the reference categories (the intercept), i.e. females, not smoking, drinking alcohol and eating soil, and PTB − and uninfected with HIV, S. mansoni and hookworm, was 137·4 μg/l. Elevated serum ACT was strongly associated with serum ferritin, in that serum ferritin was 1·44, 2·75, 4·00 and 6·50 times higher in those with serum ACT between 0·4–0·6, 0·6–0·8, 0·8–1·0 and above 1·0 g/l. Introducing elevated serum ACT to the model (model 2) explained the effect of sputum-positive tuberculosis (PTB+; 10B fell from 1·51 to 1·06), but not of HIV infection. Furthermore, it seemed to have explained some of the effects of S. mansoni, but none of the effects of hookworm infection. Also, some of the effects of current, but not past, smoking seemed to have been explained. The intercept fell to 60·3 μg/l and the proportion of the variation explained increased from 0·24 to 0·47. If tuberculosis culture intensity were included, instead of PTB as a dichotomous variable, then mild, moderate and severe intensities were associated with 1·32 (95% CI 1·03, 1·70), 1·53 (95% CI 1·12, 2·04) and 1·62 (95% CI 1·30, 2·00) times higher geometric mean serum ferritin compared with the PTB − patients (linear trend, P < 0·001) and 1·04 (95% CI 0·84, 1·30), 1·13 (95% CI 0·86, 1·48) and 1·00 (95% CI 0·82, 1·22; linear trend, P = 0·87) in models 1 and 2, respectively.
Table 4 Predictors of serum ferritin (μg/l, log10 transformed) with regression coefficients (10B), 95 % CI and the corresponding P values. Elevated serum α1-antichymotrypsin (ACT) was adjusted for in model 2, but not in model 1*

* Age was controlled for but not shown. Model 1: n 651; intercept 137·4 (95 % CI 102·3; 184·5); R 2 0·24. Model 2: n 650; intercept 60·3 (95 % CI 44·3; 82·2); R 2 0·47.
† Coded 1 if yes, and 0 if no.
‡ Patients categorised as pulmonary tuberculosis positive (PTB+) if mycobacteria were detectable in sputum, and PTB − if not. PTB − was used as reference.
§ Serum α1-antichymotrypsin (ACT) < 0·4 g/l used as reference.
For serum sTfR, positive predictors were female sex, soil eating and PTB+, and negative predictors were current smoking and HIV infection (Table 5, model 1). Age, alcohol consumption and S. mansoni and hookworm infection were not predictors. Serum ACT levels above 0·4 g/l were associated with 0·4–0·6 mg/l higher serum sTfR levels (model 2). If expressed as a dichotomous variable, serum ACT above 0·4 was associated with 0·46 (95 % CI 0·04, 0·89) mg/l or 1·12 (95% CI 1·01, 1·25) times higher serum sTfR (not shown).
Table 5 Predictors of serum soluble transferrin receptor (mg/l) with regression coefficients (B), 95 % CI and the corresponding P values. Elevated serum α1-antichymotrypsin (ACT) was adjusted for in model 2, but not in model 1*

* Variables tested but not found significant were age, alcohol consumption and S. mansoni infection. Hookworm was a predictor, but not with HIV in the model. Model 1: n 651; intercept 3·7 (95 % CI 3·3; 4·0); R 2 0·07. Model 2: n 651; intercept 3·3 (95 % CI 2·9; 3·8); R 2 0·08.
† Coded 1 if yes, and 0 if no.
‡ Patients were categorised as pulmonary tuberculosis positive (PTB+) if mycobacteria were detectable in sputum, and PTB − if not. PTB − was used as reference.
§ Serum α1-antichymotrypsin (ACT) < 0·4 g/l used as reference.
The inclusion of elevated serum ACT seemed to explain some of the effects of sputum-positive tuberculosis. Low, moderate and heavy culture intensities were associated with 0·54, 0·22 and 0·56 mg/l higher serum sTfR compared with the PTB − patients (model 1). However, with serum ACT controlled for, the corresponding regression coefficients declined to 0·45, 0·07 and 0·41.
Discussion
We found that serum ACT between 0·4–0·5 and above 0·5 g/l was associated with approximately 1·5 and 3 times higher serum ferritin, and 6 and 15 g/l lower Hb, as in previous studies(Reference Friis, Gomo and Koestel19). Serum ACT is an acute- phase protein produced in hepatocytes in response to infections, and mediated by IL-6(Reference Castell, Gomez-Lechon and David22). By adjusting for elevated serum ACT, we attempted to explain the variation in serum ferritin and Hb due to infection, through IL-6-mediated hepcidin production. The recently discovered peptide hepcidin is a key Fe-regulatory hormone(Reference Nemeth and Ganz23), which is released from hepatocytes in response to Fe and oxygen. It degrades the membrane Fe exporter, ferroportin, thereby blocking the release of Fe from enterocytes, macrophages and hepatocytes. By contrast, Fe deficiency, anaemia and hypoxaemia suppress hepcidin production, there by increasing dietary Fe absorption and release of Fe to plasma. Interestingly, inflammation induces hepcidin production, mediated by the inflammatory cytokine IL-6. This results in sequestration of Fe in the stores and Fe-limited erythropoiesis, and eventually anaemia of inflammation(Reference Nemeth and Ganz23).
The serum concentration of the acute- phase reactants, such as C-reactive protein, α1-acid glycoprotein and ACT, has been used to adjust for the effect of the APR on the markers of micronutrient status when assessing the prevalence of deficiency in an apparently healthy population(Reference Thurnham, Mburu and Mwaniki7). The acute- phase reactants differ with respect to magnitude, timing and duration of the response, with ACT and α1-acid glycoprotein having a more delayed response, and a combination of the acute- phase reactants may be needed to account for different stages of the response(Reference Thurnham, Mburu and Mwaniki8).
We found that serum ACT was highly elevated, with a mean of 0·72 g/l and 81·9 % above 0·4 g/l. This can be compared with 0·32 g/l and 8·6 %, respectively, found in a community-based study among almost 1500 apparently healthy adults from the same area(Reference Malenganisho, Magnussen and Vennervald24).
While serum ACT was generally high among the PTB patients, it was the highest among the PTB+ patients in whom it increased with bacterial intensity. There are several explanations for the lower serum ACT among the PTB − patients, which is a heterogeneous group of patients. Most of the PTB − patients probably had mild TB, and a correspondingly mild APR. Some may have had advanced HIV infection, with severe immune suppression, which makes them unable to expectorate mycobacteria(Reference Ellner25), as well as raising an appropriate APR. Still others may not have had PTB, but Pneumocystis carinii/jirovecii or other atypical pneumonia, probably due to advanced HIV infection. This is conceivable, since the prevalence of HIV was higher among the PTB − patients compared with the PTB+ ones, and viral load was significantly higher among the HIV-infected PTB-patients compared with the PTB+ ones. The lack of difference in serum ACT by HIV infection was also seen in the community-based study in the same area(Reference Malenganisho, Magnussen and Vennervald24), and among pregnant Zimbabwean women(Reference Friis, Gomo and Koestel26).
Although serum ferritin is considerably elevated among PTB patients, our data suggest that it may be elevated in a predictable manner. As shown, log serum ferritin increased linearly with serum ACT above 0·4 g/l. With other predictors in the model, serum ACT between 0·4–0·6, 0·6–0·8, 0·8–1·0 and above 1·0 g/l was associated, respectively, with 1·4, 2·8, 4·0 and 6·5 times higher serum ferritin. The effect of serum ACT between 0·4–0·6 g/l (1·44, 95 % CI 1·10, 1·86) on serum ferritin was roughly similar to that estimated based on the data from a community-based study in the same area (1·98, 95 % CI 1·68, 2·32)(Reference Malenganisho, Magnussen and Vennervald24).
Serum sTfR has been considered to be unaffected by the APR as assessed by serum C-reactive protein(Reference Asobayire, Adou and Davidsson27, Reference Verhoef, West, Ndeto, Burema, Beguin and Kok28). However, other studies suggest that also serum sTfR is affected by the APR(Reference Beesley, Filteau and Tomkins6). A recent cross-sectional study among Zimbabwean children found that serum sTfR increased with increasing serum C-reactive protein, but also serum IL-6(Reference Kasvosve, Gomo and Nathoo5). We used serum ACT, induced by IL-6(Reference Berninger29), as a measure of the APR, and found a threshold effect, i.e. that serum ACT above 0·4 g/l was associated with 0·46 mg/l higher serum sTfR. Since different assays give different results, it may be more useful to refer to a 1·12 times increase with serum ACT above 0·4 g/l. Elevated serum ACT partially explained the effect of PTB+, but not of the other predictors in the model, and did not increase the proportion of the variance explained.
Pulmonary tuberculosis, HIV and helminth infections
Compared with the PTB − patients, serum ferritin was 50 % higher in the PTB+ patients, and increased with bacterial intensity. However, these relationships were fully explained by elevated serum ACT. By contrast, HIV infection was associated with almost twice as high levels of serum ferritin, which was not mediated by the APR, as HIV infection per se may not precipitate an APR(Reference Friis, Gomo and Koestel26). The effect of HIV infection on serum ferritin probably reflects a genuine and slower effect on Fe status, as Fe becomes sequestered in the stores as a consequence of the depressed red cell synthesis in the bone marrow. S. mansoni and hookworm infection were both associated with lower serum ferritin. An established hookworm infection results in the loss of blood, and subsequently depletion of Fe stores, although the initial infection may cause an immune/acute-phase-response-mediated anaemia. S. mansoni infection is caused by a blood-dwelling fluke, and its effects on Hb could be mediated through haemolysis and suppressed erythropoiesis as well as the loss of blood, which will have opposite effects on serum ferritin.
Higher serum sTfR was seen among the PTB+ patients, and this did not change with elevated serum ACT accounted for in the analysis, although elevated serum ACT itself was associated with 0·46 mg/l higher concentrations. HIV infection, by contrast, was associated with marginally significantly lower serum sTfR.
Other predictors
The females had serum ferritin levels that were two-thirds that of the males, and higher serum sTfR, reflecting the higher requirement of women of reproductive age, due to menstrual losses, and the losses due to frequent reproductive cycles.
The higher serum ferritin in smokers was similar to what was reported from a study among the PTB+ patients in South Africa(Reference Plit, Theron and Fickl30). Smoking is a source of Fe(Reference Mateos, Brock and Perez-Arellano31), but not likely to affect the total body Fe. However, Fe may accumulate in lung macrophages and promote the growth of mycobacteria(Reference Boelaert, Gomes and Gordeuk32), and thus worsen the outcome of the infection. In addition, smoking per se may cause an APR(Reference Das33). This may explain the association we found, in that the higher serum ferritin was partially explained by the APR. However, this does not explain the higher levels in previous smokers, which were independent of serum ACT. By contrast, the considerably lower serum sTfR in current smokers seemed to be independent of serum ACT.
Consumption of alcoholic beverage was widespread both in the female and male patients, mainly as traditional beer brewed from maize, cassava, millet and sorghum. Alcohol consumption was a positive predictor of serum ferritin, but not of serum sTfR, and this was neither mediated nor confounded by the APR. Although socio-economic confounding cannot be excluded, the higher serum ferritin could also be due to alcohol-induced liver damage(Reference Fletcher, Halliday and Powell34), which may not necessarily be mediated by an APR. Alternatively, it could reflect larger Fe stores, due to the enhancing effect of alcohol on Fe absorption(Reference Hallberg and Hulthen35), or the beverage could contain Fe from the raw material or the fermentation vessel. In the same community, alcohol consumption has previously been shown to be associated with not only higher serum ferritin but also higher Hb(Reference Malenganisho, Magnussen and Vennervald24), which may support the hypothesis that the association reflects a higher intake of Fe.
The prevalence of soil eating among PTB patients, 10 % in the females and 0·8 % in the males, was lower than in the 18·8 and 0·4 %, respectively, found in the community-based study in the same study area(Reference Malenganisho, Magnussen and Vennervald24). In accordance with the findings from a number of studies among children(Reference Geissler, Mwaniki and Thiong'o36) and pregnant women(Reference Geissler, Mwaniki and Thiong37), we found that soil eating was associated with lower serum ferritin. However, we also found an association with higher serum sTfR. Yet, it is not clear whether low Fe stores cause a craving for soil or whether soil reduces Fe absorption(Reference Harvey, Dexter and Darnton-Hill38, Reference Moore and Sears39).
Conclusion
Although the substantial APR among PTB patients affects the direct measures of storage and tissue Fe, a number of known or biologically plausible predictors still explain a considerable proportion of the variation. As suggested previously(Reference Northrop-Clewes40), it may be possible to develop an algorithm, based on one or several acute- phase proteins, which will allow to assess the Fe status during infections.
Acknowledgements
DBL Centre for Health Research and Development and DANIDA (grant no. 91 072) funded the study. The authors would like to acknowledge all health staff from the health facilities, laboratories and National Institute for Medical Research, Mwanza, for their contribution to the study. We thank the PTB patients for consenting to participate in the study.
H. F., N. R., P. M., A. B. A., W. M. and J. C. designed the study and were responsible for the planning, coordination and supervision of the field activities. P. K. and H. K. were responsible for the laboratory analyses. H. F. and C. B. K. did the initial data analyses. H. F. did the final data analyses and wrote the first draft of the manuscript. All authors were involved in the interpretation of the results and contributed to the final version of the manuscript.
None of the authors have conflicts of interests.








