Research has clearly established a link between n-3 PUFA and general health( Reference Connor 1 , Reference Calder 2 ). Their longer-chain derivatives (n-3 LCPUFA) are significant structural components of cell membrane phospholipids throughout the body and are especially rich in the central nervous system( Reference Uauy, Mena and Rojas 3 ). The large accretion of DHA observed in the fetal brain and retina during the second half of gestation is required to promote their optimal growth and development( Reference Lauritzen, Hansen and Jørgensen 4 , Reference Clandinin, Chappell and Leong 5 ). More recently, several studies have shown that DHA supply in early life is a major determinant of visual and neurocognitive functions in the offspring( Reference Innis 6 , Reference Shulkin, Pimpin and Bellinger 7 ).
Additional evidence supports the hypothesis that n-3 PUFA positively affect numerous maternal and child health outcomes. Lower incidence of low birth weight and preterm birth( Reference Salvig and Lamont 8 ), reduced risk of pre-eclampsia( Reference Burchakov, Kuznetsova and Uspenskaya 9 ) and perinatal depression( Reference Lin, Chang and Chong 10 ) as well as the primary prevention of asthma and allergies in early childhood and childhood( Reference Best, Gold and Kennedy 11 ) have been associated with n-3 LCPUFA.
Since neither the placenta nor the fetus possesses sufficient desaturase activity to synthesize long-chain PUFA, the primary source of these fatty acids for the fetus comes from maternal stores and dietary intake( Reference Hanebutt, Demmelmair and Schiessl 12 ). Guidelines advise pregnant women to consume 250–500 mg of EPA plus DHA daily, including at least 200 mg of DHA daily( 13 , 14 ). The European Food Safety Authority recommends an additional intake of 100–200 mg of DHA daily( 15 ). However, the Western diet is relatively deficient in n-3 PUFA. Food surveys have also reported insufficient dietary intakes of n-3 LCPUFA among a large portion of European pregnant women( Reference de Groot, Hornstra and van Houwelingen 16 – Reference Sioen, van Lieshout and Eilander 19 ). The Belgian food-based dietary guidelines appear to specifically encourage the general population to consume dietary sources of n-3 PUFA, including two portions of fish per week of which at least one portion is oily fish( 20 ). Longitudinal studies focusing on biochemical fatty acid markers indicate that pregnancy is associated with a significant decrease in maternal DHA status( Reference Al, van Houwelingen and Kester 21 – Reference Hornstra, Al and van Houwelingen 23 ). These observations suggest that under present dietary conditions, mothers may be unable to meet these needs due to the high fetal DHA requirements.
Although seafood is the main dietary source of n-3 LCPUFA, it may also be contaminated by methylmercury, a neurotoxin that is particularly harmful to the developing fetal brain( 24 ). Warning messages about the adverse health impact of mercury exposure may have led to women dramatically reducing their fish consumption( Reference Oken, Kleinman and Berland 25 – Reference Forbes, Graham and Berglund 27 ). A survey in Belgium highlighted that consumers’ awareness of the potential toxicity of fish consumption is higher than their awareness of the potential health benefits( Reference Verbeke, Sioen and Pieniak 28 ).
Several nutrients are of utmost importance during pregnancy and need to be discussed during antenatal care. Pregnant women reported receiving much less information regarding the importance and role of n-3 PUFA during pregnancy than regarding other nutrients such as folate, iron and calcium( Reference Sinikovic, Yeatman and Cameron 29 ). Health-care providers are a trusted source of pregnancy-related dietary information( Reference Aaronson, Mural and Pfoutz 30 ) and can influence the eating practices of their pregnant patients( Reference May, Suminski and Berry 31 ). According to the study of Bloomingdale et al., pregnant women might indeed be willing to increase their fish consumption if they received appropriate advice from their obstetrician( Reference Bloomingdale, Guthrie and Price 32 ).
Although some studies have focused on women’s awareness of the importance of n-3 LCPUFA or fish consumption during pregnancy( Reference Sinikovic, Yeatman and Cameron 29 , Reference Bloomingdale, Guthrie and Price 32 , Reference Emmett, Akkersdyk and Yeatman 33 ), there have been no studies investigating this topic among front-line health-care professionals. Therefore, the aims of the present study were to: (i) provide an insight into the knowledge, attitude and practices of pregnant women and gynaecologists–obstetricians regarding n-3 PUFA; and (ii) study the relationship between knowledge, attitude and dietary practices regarding n-3 PUFA of pregnant women and their n-3 PUFA status measured by the omega-3 index.
Materials and methods
Study design and populations
The recruitment of the pregnant women took place between February and August 2016 in the Obstetrics and Gynecology Department of the CHR Liège Hospital, Liège, Belgium. The women were invited to complete a self-administered questionnaire while waiting for their first antenatal appointment. Inclusion criteria were: (i) being between the 7th and 18th week of gestation; (ii) being free from any chronic diseases such as hypertension and diabetes; and (iii) presenting with a singleton pregnancy. All women were informed about the purpose of the study and written consent was obtained prior to completing the survey.
The second study population consisted of all gynaecologists–obstetricians with an email address (n 452) from the Belgian Group of French Language Gynecologists–Obstetricians (GGOLFB). They were invited to complete an anonymous online survey via email between February and May 2016. The investigators have not been able to link email address and sociodemographic data. The completion of the survey indicated the consent from gynaecologists–obstetricians to participate in the study.
The pregnant women and the gynaecologists–obstetricians were recruited independently.
The study protocol was reviewed and approved by the Ethics Committee of the CHR Liège Hospital, Liège, Belgium (B412201526650).
Study instruments
The pregnant women completed a self-administered questionnaire on sociodemographic characteristics as well as knowledge, attitude and dietary practices regarding n-3 PUFA during pregnancy. Maternal characteristics included age, parity, nationality and level of education. Level of education was expressed as low (primary and lower and upper secondary) and high (non-university degree and university degree). General beliefs about n-3 PUFA and sources of received information were investigated at the beginning of the survey by two open-ended questions. Specifically, one question on pregnant women’s beliefs was: ‘What did you know about omega-3 fatty acids?’ Pregnant women who cited one or several correct facts about n-3 PUFA without prompting were classified as having good knowledge. After a brief definition of ‘omega-3 fatty acids’, closed-ended questions were used to assess participants’ knowledge about the health benefits of n-3 PUFA during pregnancy and dietary sources of n-3 PUFA. Simply stating that ‘These are good fats, most of which must be provided by the diet’ allowed participants to make a connection with their prior knowledge. As a result, more people were able to answer to the closed-ended questions about health benefits and dietary sources of n-3 PUFA. Attitude was assessed by the question: ‘What importance do you attach to omega-3 fatty acids during pregnancy?’ The answers were classified as ‘moderate or high importance’ and ‘no or little importance’. One question also explored what participants considered would be the best source of information about n-3 PUFA during pregnancy. Finally, the pregnant women were invited to communicate any dietary changes made since pregnancy, with a focus on fats. Specifically, the questions were: ‘Since pregnancy, have you modified your diet? – If yes, how?’ and ‘Since pregnancy, have you paid particular attention to your fat intake? – If yes, how?’ Detailed information on use of n-3 PUFA supplements was also collected, including product name, type of medication, dose, frequency and duration of use.
The online survey created for gynaecologists–obstetricians included questions about demographic characteristics as well as knowledge, attitude and clinical practices regarding n-3 PUFA during pregnancy. Knowledge was assessed by questions asking about the frequency of low n-3 PUFA status and existing guidelines on n-3 PUFA. For example, the first question was: ‘In your opinion, pregnant women have suboptimal omega-3 polyunsaturated fatty acid levels in blood: never, sometimes, often or always?’ Suboptimal n-3 PUFA status was then described as ‘non-existent or occasional’ (‘never’ or ‘sometimes’) or ‘common’ (‘often’ and ‘always’). The questionnaire also explored the need and the desire for further education on the topic. Attitude was assessed using the same question as for the pregnant women. Concerning nutritional care practices, questions were related to the monitoring of n-3 PUFA status in pregnant women, the implementation of preventive actions in relation to a possible suboptimal n-3 PUFA status, and the transmission of information on the importance and/or the role of n-3 PUFA during pregnancy. The opinion of gynaecologists–obstetricians on routine n-3 LCPUFA supplementation was also researched. The question was: ‘Do you think that the provision of systematic n-3 LCPUFA supplementation to pregnant women would be necessary? – For what reasons?’
Erythrocyte n-3 PUFA analysis
Fasting blood specimens were collected from each pregnant woman at recruitment using an EDTA tube for testing for omega-3 index. Erythrocyte fatty acid composition was analysed by GC as previously described( Reference Hoge, Bernardy and Donneau 34 ). The omega-3 index (IOM3) was defined as erythrocyte EPA plus DHA expressed as weight percentage of total fatty acids. It reflects the long-term intake of EPA and DHA( Reference Harris and Von Schacky 35 ). In cardiology, IOM3 was proposed as a marker, and even as a risk factor, for cardiovascular events (e.g. sudden cardiac death). IOM3 ≥ 8 % was suggested as optimal for reducing risk( Reference Harris 36 , Reference Harris, Del Gobbo and Tintle 37 ).
Statistical analysis
Open-ended questions were coded before analysis. Classical descriptive statistics were used to describe the general characteristics of the study participants. For quantitative variables, results were expressed as mean and sd. Frequency tables were used to summarize categorical variables. The χ 2 test was applied to compare proportions of categorical variables between groups. Multiple regression analysis was used to test the effect of the pregnant women’s dietary practices on omega-3 index. A logarithmic transformation was applied to omega-3 index values to normalize their distribution. The statistical analysis also investigated the direct, indirect and total effects of knowledge and attitude on omega-3 index in the pregnant women, with dietary practices as mediating variable (Fig. 1). The mediation effect was tested using the method described by Valeri and Vanderweele( Reference Valeri and Vanderweele 38 ), which allows dichotomous mediators. The bootstrapping method was used to test for the significance of the direct, indirect and total effect using 95 % CI. A statistically significant indirect effect implies the presence of mediation. There is ‘partial mediation’ when the direct effect is significantly different from 0 and ‘complete mediation’ if it is not. Both regression and mediation models were adjusted for the pregnant women’s characteristics (age, parity, nationality and level of education). P<0·05 was considered statistically significant. Statistical analysis was performed using the statistical software package SAS version 9.4 and mediation analysis was performed by using the macro %mediation( Reference Valeri and Vanderweele 38 ).
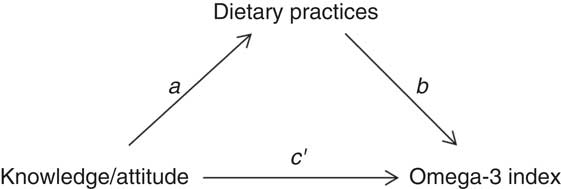
Fig. 1 Path diagram of simple mediation, investigating dietary practices as a mediator between knowledge and attitude and omega-3 index (a, knowledge/attitude v. dietary practices relationship; b, dietary practices v. omega-3 index relationship, controlling for knowledge/attitude; c′, direct effect; function of a and b, indirect effect; total effect=direct + indirect effects)
Results
Characteristics of populations
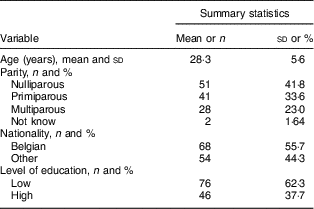
A total of 122 pregnant women participated in the study. Their characteristics are summarized in Table 1. The women were 28·3 (sd 5·6) years old and 62·3 % had a low educational level. There were sixty-eight (55·7 %) Belgian Caucasians, nineteen (15·6 %) came from North Africa or the Middle East, nine (7·4 %) from Central Africa, eight (6·6 %) from Asia, seven (5·7 %) from West Africa, seven (5·7 %) from South Europe, three (2·5 %) from West Europe and one (0·8 %) from East Africa. Fifty-one (41·8 %) participants were nulliparous (no previous birth). The median (interquartile range) omega-3 index was 5·51 (4·73–6·67) %. A majority of participants (91·8 %) exhibited a low n-3 LCPUFA status (IOM3<8 %).
Table 1 Sociodemographic characteristics of pregnant women (n 122) sampled in CHR Liège Hospital, Liège, Belgium, February–August 2016
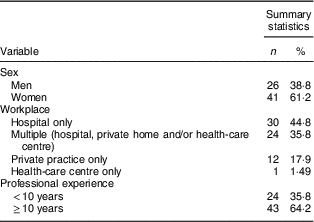
Sixty-seven gynaecologists–obstetricians (15 % participation rate) completed the survey. Their characteristics are displayed in Table 2. Thirty (44·8 %) professionals worked in a hospital and twenty-four (35·8 %) had multiple workplaces (hospital, private home and/or health-care centre). Almost two-thirds of participants (64·2 %) had a professional experience of over 10 years.
Table 2 Demographic characteristics of gynaecologists–obstetricians (n 67) sampled from the Belgian Group of French Language Gynecologists-Obstetricians, February–May 2016
Knowledge, attitude and dietary practices of the pregnant women
Among respondents, seventy-six (62·3 %) had already heard of or read something about n-3 PUFA of whom forty-six (60·5 %) had good knowledge about n-3 PUFA. The answers to these open-ended questions are summarized in Table 3. A quarter of the women correctly reported that n-3 PUFA are essential fats because the body cannot synthesize them and that they must be obtained from foods. The same proportion recognized that n-3 PUFA are beneficial nutrients for health, particularly for the heart, the nervous system or the brain. Approximately 20 % of the women accurately cited fish/oily fish and fish oil as dietary sources and 17·3 % correctly emphasized that n-3 PUFA positively affect maternal and child health outcomes. Among wrong beliefs, the women claimed that n-3 PUFA acids are antioxidant vitamins or that they are involved in muscle regeneration. Information was primarily derived from the media, Internet, books or magazines (56·0 %), from health-care providers (37·3 %) or school (26·7 %). Among the women who cited health-care providers as source of information, information was given by the sole gynaecologist–obstetrician for sixteen out of twenty-eight (57·1 %) women, by the gynaecologist–obstetrician plus other professionals for five (17·9 %) women, by the midwife for one (3·6 %) woman and by other professionals not related to the pregnancy for six (21·4 %) women. The women who received information from their gynaecologist–obstetrician were significantly more likely to demonstrate knowledge related to health benefits of n-3 PUFA during pregnancy than the uninformed women (33·3 v. 5·6 %; P=0·0015).
Table 3 Pregnant women’s answers to the question ‘What did you know about omega-3 fatty acids?’ (n 122) and sources where pregnant women get their information (n 76), Belgium, February–August 2016
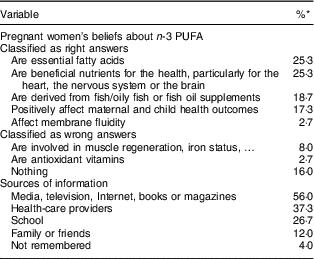
* Percentages do not add up to 100 %, because multiple answers were accepted.
From the semi-closed questions, sixty-nine (56·6 %) participants replied that they did not know the health benefits of n-3 PUFA during pregnancy. The three best-known benefits were optimal development of the fetal brain (71·7 %), decreased risk of hypertension/pre-eclampsia (43·4 %) and enhanced visual function of the child (39·6 %). Thirty-six (29·5 %) women did not know any dietary source of n-3 PUFA. The three best-known dietary sources were oily fish (75·6 %), rapeseed, walnut or linseed oil (45·4 %) and fish oil supplement (38·4 %).
Knowledge about n-3 PUFA was not influenced by the information source.
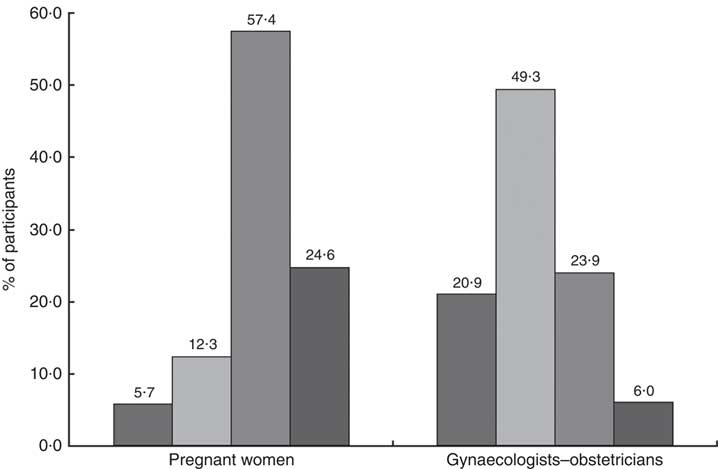
One hundred (82·0 %) pregnant women attached moderate or high importance to n-3 PUFA during pregnancy (Fig. 2). They would prefer receive information about n-3 PUFA from the gynaecologist–obstetrician (53 %), the general practitioner (17 %), the nutritionist (10 %), the midwife (3 %) or the media (1 %). Sixteen per cent did not have any preference.
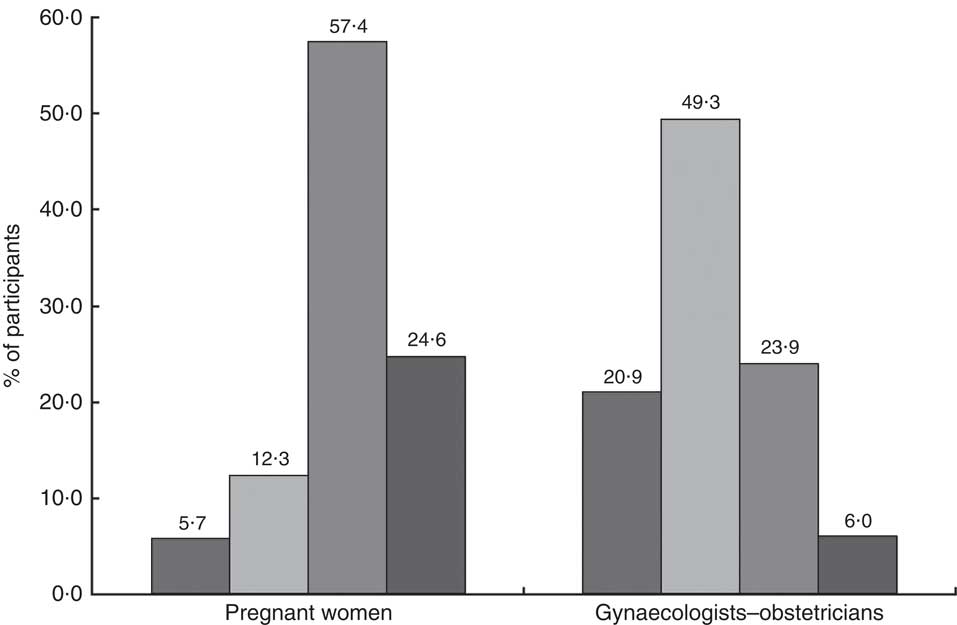
Fig. 2 Importance of n-3 PUFA during pregnancy (
![]() , no importance;
, no importance;
![]() , little importance;
, little importance;
![]() , moderate importance;
, moderate importance;
![]() , high importance) according to pregnant women (n 122) and gynaecologists–obstetricians (n 67) sampled in Belgium, February–August 2016
, high importance) according to pregnant women (n 122) and gynaecologists–obstetricians (n 67) sampled in Belgium, February–August 2016
The women who had already heard of or read information about n-3 PUFA were significantly more likely to perceive n-3 PUFA as important than women who did not (88·2 v. 71·4 %; P=0·02).
More than half (52·5 %) of all women reported changing their diet since they had become pregnant. Forty-two (34·4 %) declared paying particular attention to their consumption of n-3 PUFA, by including foods naturally rich in or enriched with n-3 PUFA in their diet (n 12) or by using n-3 LCPUFA supplements (n 23) or both (n 7). The proportion with favourable dietary practices regarding n-3 PUFA was significantly higher in women who had already heard of or read information about n-3 PUFA than in women who had not (P=0·02), in women with good knowledge than in other women (P=0·002), as well as in women who perceived n-3 PUFA as moderately or highly important than in women who perceived these nutrients as not or little important (P=0·006; see Table 4). Women who had received information about n-3 PUFA from their gynaecologist–obstetrician were also more likely to report favourable dietary practices than women who had not (66·7 v. 32·7 %; P=0·0074).
Table 4 Number and proportion of pregnant women (n 122) with favourable dietary practices regarding n-3 PUFA intake according to knowledge and attitude, Belgium, February–August 2016
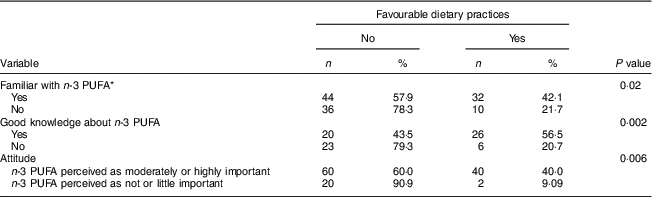
* Women familiar with=women who have already heard of or read about n-3 PUFA.
Knowledge, attitude and dietary practices of pregnant women were not influenced by sociodemographic characteristics.
Association between knowledge, attitude and dietary practices of the pregnant women and their omega-3 index
After adjustment for sociodemographic features, the omega-3 index was significantly higher in pregnant women with favourable dietary practices regarding n-3 PUFA than in others (β=0·10 (se 0·05); P=0·04).
Mediation analysis showed that dietary practices significantly mediated the relationship between knowledge and omega-3 index. The indirect effect was 0·323 (95 % CI 0·011, 0·722) and the mediation was complete. Dietary practices were not a mediator of the relationship between attitude and omega-3 index (indirect effect=0·174 (95 % CI −0·141, 0·549)). Moreover, there was no significant direct effect of attitude on the omega-3 index. More results regarding the fatty acid profile of the participants can be found elsewhere( Reference Hoge, Bernardy and Donneau 34 ).
Knowledge, attitude and clinical practices of gynaecologists–obstetricians
While twenty-six (38·8 %) of the sixty-seven health-care professionals claimed that a suboptimal n-3 PUFA status is common in pregnant women, forty-one (61·2 %) reported this situation as non-existent or occasional. Fifty-four (81·8 %) recognized that guidelines on n-3 PUFA for pregnant women are needed. Health-care providers for whom low n-3 PUFA status is common were more likely to ask for guidelines than those for whom this situation is non-existent or occasional (96·2 v. 72·5 %; P=0·02). Fifty-three (79·1 %) of the sixty-seven participants reported that their training was insufficient to inform their pregnant patients about n-3 PUFA. Forty-one (61·2 %) would be rather or very interested in receiving information.
Twenty (29·9 %) gynaecologists–obstetricians declared attaching moderate or high importance to n-3 PUFA during pregnancy (Fig. 2). This proportion was significantly higher in professionals for whom a suboptimal n-3 PUFA status is common in pregnant women than in those for whom this situation is non-existent or occasional (53·9 v. 14·6 %; P=0·0006). Attitude did not differ according to demographic characteristics (sex, workplace and professional experience). More than half (58·2 %) of the sixty-seven participants declared having one or several clinical practices regarding n-3 PUFA during pregnancy. Thirty-eight (56·7 %) claimed to give information about n-3 PUFA to their pregnant patients. Of the professionals, 53·7 % reported implementing preventive actions when low n-3 PUFA levels were suspected: dietary advice for 18·2 % of them, supplementation for 57·6 % or the two combined strategies for 24·2 %. One gynaecologist–obstetrician systematically determined biomarkers of n-3 PUFA status in her patients, and six did it depending on the women’s diet. The results are summarized in Table 5.
Table 5 Detailed clinical practices of gynaecologists–obstetricians (n 67) regarding n-3 PUFA during pregnancy, Belgium, February–May 2016
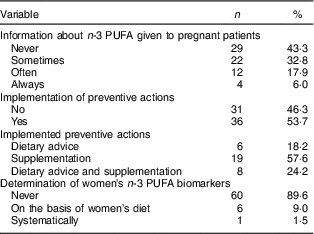
The professionals’ practice did not differ according to demographic characteristics or estimated frequency of suboptimal n-3 PUFA status. Gynaecologists–obstetricians who attached moderate or high importance to n-3 PUFA during pregnancy were more likely to have nutritional care practices than those who saw them as little or not important (90·0 v. 55·3 %; P=0·006). For one-third of obstetricians (33·3 %), a routine supplementation should be set up to ensure an adequate n-3 PUFA status in pregnant women. The reasons given included a healthy pregnancy and the optimal development of the fetus, or a diet low in n-3 PUFA. For 47 %, this strategy is not relevant for the following reasons: there is not enough evidence yet, nutritional support should be provided on a case-by-case basis, priority must be given to the n-3 PUFA from foods, or supplements are too expensive. The remaining 19·7 % declared they could not respond due to a lack of knowledge. Health-care providers for whom a suboptimal n-3 PUFA status is common were more likely to be in favour of supplements (59·1 v. 29·0 %; P=0·03) than those for whom this situation is non-existent or occasional. The same trend was observed with health-care providers who attached moderate or high importance to n-3 PUFA during pregnancy (66·7 v. 28·6 %; P=0·008).
Discussion
Over the past years, n-3 PUFA and pregnancy has been a topic of much research interest and controversy. While there is a lot of evidence supporting a beneficial role of n-3 LCPUFA in multiple maternal and child health outcomes, the typical Western diet has been found to be deficient in n-3 PUFA, with a relatively ratio of high n-6 PUFA to n-3 PUFA( Reference Stark, Van Elswyk and Higgins 39 , Reference Micha, Khatibzadeh and Shi 40 ). Pregnant women do not achieve the recommended intake of at least 200mg of DHA daily( Reference Koletzko, Lien and Agostoni 41 ). Although there are two major ways for increasing n-3 LCPUFA intake, there is no clear message on the best strategy to adopt. On the one hand, fish consumption may increase the maternal and fetal exposure to contaminants such as methylmercury, dioxins and polychlorinated biphenyls. Advice on fish consumption has consequently been found to be confusing and conflicting( Reference Forbes, Graham and Berglund 27 ). On the other hand, trials on fish or algal oil supplementation in pregnancy have shown some benefits for both mother and child; however, the results are still mixed and inconclusive( Reference Imhoff-Kunsch 42 , Reference Rogers, Valentine and Keim 43 ). The discussion is definitely still ongoing, particularly in the light of recent scientific papers reporting no cardiovascular benefits from n-3 PUFA supplementation( Reference Elagizi, Lavie and Marshall 44 – Reference Abdelhamid, Brown and Brainard 46 ).
Several conditions are reminiscent of vitamin D: large media coverage, early and non-definitive research on health benefits, and the absence of consensual guidelines for the management of nutrient insufficiency. These have been shown to create a situation wherein both patients and health-care providers experience uncertainty( Reference Bennett, Frisby and Young 47 , Reference Bianchi, Huneau and Le Goff 48 ). In this context, the present study aimed at exploring knowledge, attitudes and practices of both pregnant women and gynaecologists–obstetricians regarding n-3 PUFA during pregnancy.
Our findings clearly highlighted a lack of knowledge among pregnant women. Only 38 % (forty-six out of 122) gave one or several correct facts about n-3 PUFA without prompt. Television, the Internet, books and magazines were reported to be the most common source of information. Only one-third of the pregnant women reported an increase in their n-3 PUFA intake, by including foods naturally rich in or enriched with n-3 PUFA in their diet and/or by using n-3 LCPUFA supplements. A positive association was observed between knowledge and attitude, and favourable dietary behaviours. This finding is consistent with others who found that awareness and nutrition knowledge have the potential to contribute to improving dietary behaviours( Reference Emmett, Akkersdyk and Yeatman 33 , Reference Wardle, Parmenter and Waller 49 ), even if a cause-and-effect relationship cannot be determined here. Statistical analyses were taken one step further; we investigated the relationship between self-declared dietary practices and an objective measure of n-3 PUFA status. The results confirmed that women with favourable dietary practices regarding n-3 PUFA were more likely to have higher omega-3 index. Furthermore, testing dietary practices as mediator revealed its role in the knowledge–omega-3 index relationship.
The current study revealed that only a limited number of pregnant women (twenty-two participants out of 122, 18 %) had already heard about n-3 PUFA during preconception or antenatal care. Australian pregnant women were not more privileged, since only 23 % reported having received information( Reference Sinikovic, Yeatman and Cameron 29 ). From the limited number of studies, it is a major concern that the benefits of n-3 LCPUFA are being communicated to no more than one-quarter of pregnant women. Consistent with this finding, pregnant women were found to be relatively unaware of the health benefits of n-3 PUFA for both mother and fetus.
Pregnant women show a great interest in nutrition during their pregnancy( Reference Szwajcer, Hiddink and Koelen 50 , Reference Bookari, Yeatman and Williamson 51 ) and antenatal care provides a real opportunity for discussion around these topics. The participants in the present survey reported gynaecologists–obstetricians as a preferred channel of receiving information about n-3 PUFA. This source was associated with better knowledge related to n-3 PUFA and pregnancy, as well as with favourable dietary behaviours. This confirms the key role played by health-care providers in the education of their patients( Reference Kreuter, Chheda and Bull 52 , Reference Quader, Cogswell and Fang 53 ). Assessment of the knowledge, attitude and practices of gynaecologists–obstetricians was seen as a prerequisite to improve n-3 PUFA intake in pregnant women.
Despite the well-documented roles of n-3 PUFA in pregnancy, related health-care professionals’ awareness (knowledge and attitude) was rather poor. The current study highlighted the fact that gynaecologists–obstetricians underestimated the extent of suboptimal n-3 PUFA status in pregnant women. While two-thirds of health-care providers reported that this situation is non-existent or occasional, the determination of maternal erythrocyte n-3 PUFA levels revealed that almost all pregnant women (92 %) exhibited an IOM3<8 %( Reference Hoge, Bernardy and Donneau 34 ). An IOM3≥8 % was suggested as optimal for cardiovascular protection( Reference Harris 36 , Reference von Schacky 54 ). Precise levels of n-3 LCPUFA for a healthy pregnancy are unknown; however, some studies have already observed an association of omega-3 index with the risk of postpartum depression( Reference Markhus, Skotheim and Graff 55 ) and with low birth weight and preterm delivery( Reference Ogundipe, Johnson and Wang 56 , Reference Klebanoff, Harper and Lai 57 ). While pregnant women placed high importance on n-3 PUFA during pregnancy, such an attitude was observed in only one-third of gynaecologists–obstetricians. While a lack of time/survey burden is often the main reason for not responding to a survey( Reference Cook, Wittich and Daniels 58 , Reference Dykema, Jones and Piché 59 ), the low participation rate could also reflect the lack of interest professionals have for the topic.
It is worth noting that 82 % of the professionals showed interest in receiving guidelines on n-3 PUFA in pregnant women. This result questions the availability and accessibility of information materials and guidelines for gynaecologists–obstetricians. Very few resources about n-3 PUFA, or even fish consumption, exist in Wallonia, Belgium. Seventy-nine per cent of the professionals reported that their training regarding n-3 PUFA was insufficient. The present study sheds light on a lack of adequate physician training in nutrition; a worrying situation if we consider that early-life nutrition has long-term implications on the offspring’s future health( Reference Berti, Cetin and Agostoni 60 ). The uncertainty situation (as reported above), coupled with limited health professional training, could represent key barriers for gynaecologists–obstetricians to start advising their pregnant patients about the importance of n-3 LCPUFA.
Despite weak awareness regarding the importance of n-3 PUFA during pregnancy, a little more than half of the gynaecologists–obstetricians reported taking preventive actions with pregnant patients with low n-3 PUFA intake. The most common strategy was n-3 LCPUFA supplementation. This finding can be surprising considering the conflicting scientific information. Unfortunately, no information on the recommended type of supplements and dose were collected in the current study. For one-third of the gynaecologists–obstetricians, a routine supplementation should even be implemented to ensure an adequate n-3 PUFA status in pregnant women. While supplementation can be a relatively simple way of increasing n-3 LCPUFA, some studies have found a significant variation in the quality of supplements( Reference Jordan 61 ). Even if no serious deleterious effects were observed in pregnant women taking prenatal marine oil supplements( Reference Freeman and Sinha 62 ), benefits for both mother and child should be investigated further( Reference Rogers, Valentine and Keim 43 ).
Pregnant women are a priority public health target group. They should benefit from the promotion of the importance of n-3 LCPUFA consumption early in pregnancy (and ideally in preconception care) for optimal pregnancy outcomes. As specialists at the front line of antenatal care, gynaecologists–obstetricians could play multiple roles regarding n-3 LCPUFA. They could provide information, identify deficient diet, assess biomarkers of nutritional status and encourage behaviour changes. Increasing education of health-care providers about n-3 LCPUFA during pregnancy is clearly warranted. It is encouraging to note that two-thirds of the professionals expressed a desire for further information in the present study. Besides health-care providers’ intervention, communication materials exist and could have the potential to assist in increased consumption of n-3 PUFA by pregnant women( Reference Emmett, Akkersdyk and Yeatman 33 ). Further research is needed to identify the most effective strategy concerning how antenatal care providers could promote all essential nutrients in pregnancy and enhance nutritional care provided to pregnant women.
Some limitations of the current study have to be noted. First, pregnant women and gynaecologists–obstetricians who agreed to participate in the survey may have a greater interest in the subject matter and therefore better knowledge, attitudes and practices related to n-3 PUFA. As previously discussed( Reference Hoge, Bernardy and Donneau 34 ), pregnant women from the CHR Liège Hospital have some different sociodemographic features in comparison with the Walloon population. Considering knowledge, attitude and dietary practices of pregnant women were not influenced by sociodemographic characteristics, we could be confident in our conclusions. As the study was restricted to gynaecologists–obstetricians, we have no data on knowledge, attitude and clinical practices of other health-care providers involved in antenatal care such as midwives. Finally, as the survey conducted among gynaecologists–obstetricians was anonymous, no direct link could be established between the pregnant women and their specialist (e.g. specialist advice and behaviours of the patient). However, the number of specialists included would have been very limited since the women were from the same hospital.
Conclusions
In conclusion, the present study evidenced a need for pregnant women to receive adequate nutrition education and effective communication about n-3 PUFA. n-3 LCPUFA consumption during pregnancy is important. Gynaecologists–obstetricians play an important role in the education of their patients and could discuss this issue during routine prenatal care, preferably during the first trimester. Evidence-based guidelines, refreshment training and communication tools could improve the professionals’ awareness and clinical practices regarding n-3 LCPUFA, contributing to the health of mothers and their children. The important results of the present study should stimulate researchers to conduct more comprehensive research that reflects the scale of this major health problem in Belgium and elsewhere.
Acknowledgements
Acknowledgements: The authors would like to thank Elisabetha Prato and Marie Timmermans from the CHR Liège Hospital for their assistance in the data collection. They are also grateful to Professor Adelin Albert from the University of Liège for the critical revision of the manuscript. Financial support: This research was funded by the medical analysis laboratory Roman Païs (Belgium) (http://www.rplab.be); Metagenics Europe (https://www.metagenics.eu); and the University of Liège (Belgium) (https://www.uliege.be). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. Conflict of interest: None declared. Authorship: M.G. and V.C. are joint senior authors. A.H. was involved in the research design and the coordination of the survey, performed the statistical analyses and drafted the manuscript. F.B. conducted the data collection under the supervision of M.N. and S.D. M.N. and S.D. were also involved in the research design. A.-F.D. and N.D. took part in the statistical analyses and the critical discussion of the results. M.N. contributed to the critical revision and intellectual content of the manuscript. M.G. and V.C. as the promoters of the study supervised the entire work and finalized the manuscript. All authors read and approved the final version. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethics Committee of the CHR Citadelle Hospital of Liège, Belgium (B412201526650). Written informed consent was obtained from all pregnant women.