1. Introduction
Dementia is a clinical syndrome characterized by cognitive decline and impairment in activities of daily living Reference van der Flier and Scheltens[1]. Although the etiology is not well known, risk factors of dementia have been studied to determine the basic mechanisms leading to dementia. Prior studies on the risk factors of dementia mainly focused on Alzheimer’s dementia, as it is the most common type of dementia. An older age Reference Fratiglioni, Launer, Andersen, Breteler, Copeland and Dartigues[2, Reference Miech, Breitner, Zandi, Khachaturian, Anthony and Mayer3], cardiovascular risk factors (smoking, hypertension, diabetes mellitus, and heart disease) Reference Breteler[4], and genetic factors Reference Strittmatter and Roses[5] are well-known risk factors of dementia. By influencing these risk factors, it is hoped that clinicians can modify the course of the disease.
Schizophrenia, affecting approximately 1% of the population, is associated with disturbances in perception, communication, and thought processes as well as abnormalities in behavior Reference Fratiglioni, Launer, Andersen, Breteler, Copeland and Dartigues[6, Reference Laursen, Agerbo and Pedersen7]. Schizophrenia is also characterized by cognitive impairment Reference Keefe and Fenton[8]. Robust evidence of impairment was reported across a multitude of cognitive domains, including current IQ, category fluency, verbal memory, abstract thinking, language, sustained attention, response inhibition, and symbol coding tasks Reference Miech, Breitner, Zandi, Khachaturian, Anthony and Mayer[9–Reference Holden11]. Nevertheless, the association between schizophrenia and the risk of subsequent dementia remains unclear. Studies of the relationship between schizophrenia and dementia are few, and their results are inconsistent. Several studies reported cognitive impairment or even dementia in patients with schizophrenia Reference Brodaty, Sachdev, Koschera, Monk and Cullen[12–Reference Rabins and Lavrisha14]. However, one cross-sectional study revealed no evidence of accelerated decline in cognitive abilities in schizophrenics compared to five age-derived cohorts Reference Hyde, Nawroz, Goldberg, Bigelow, Strong and Ostrem[15]. Discrepancies among those studies may be due to short follow-up periods, small sample sizes Reference Harvey, White, Parrella, Putnam, Kincaid and Powchik[16, Reference Palmer, Bondi, Twamley, Thal, Golshan and Jeste17], and the selection of chronically institutionalized older patients Reference Harvey, White, Parrella, Putnam, Kincaid and Powchik[16, Reference Harvey, Silverman, Mohs, Parrella, White and Powchik18, Reference Harvey, Parrella, White, Mohs, Davidson and Davis19]; these issues reduce the validity of research on dementia risk and schizophrenia. Despite one recent nationwide Danish cohort study with a sufficient size and follow-up time revealing a strong association between schizophrenia and dementia risk Reference Ribe, Laursen, Charles, Katon, Fenger-Grøn and Davydow[20], it lacked adjustment for medication effects on the dementia risk for patients with schizophrenia. In addition, most aforementioned studies did not adjust for dementia-related risk factors, and whether there is an interaction between schizophrenia and several covariates and their association with dementia were not thoroughly investigated. Moreover, most of those studies were conducted in western countries, and whether such findings would be observed in Asian populations remains unknown. Therefore, we conducted a more-comprehensive assessment to evaluate the risk of dementia in schizophrenia using the Taiwan National Health Insurance (NHI) Research Database (NHIRD). We hypothesized that schizophrenia would be associated with an increased risk of subsequent dementia in later life.
2. Methods
2.1. Database
Taiwan’s NHI was established in 1995, and nearly 99% of residents are enrolled in this database Reference Cheng[21]. This study used the Longitudinal Health Insurance Database 2005 derived from Taiwan’s NHIRD. These data include registration and medical claims for 1,000,000 randomly sampled individuals from the total 25.68 million beneficiaries registered in the NHIRD in 2005. The database includes detailed information regarding the health insurance system between 2000 and 2013. Patient consent was not required to access the NHIRD as data were analyzed anonymously. Although the study was entirely based on register data, ethical permission was still required according to Taiwanese law. This study was exempted from ethical approval by the Institutional Review Board of Taipei Tzu Chi Hospital (IRB no.: 04-X36-094).
2.2. Inclusion criteria for patients with Schizophrenia and the control cohort
Since Taiwan’s NHI was launched in 1995, patient’s medical claims before 1995 are unavailable. Therefore, information with regard to patients diagnosed with schizophrenia before 1995 was not available, and thus the duration of illness could not be determined. Therefore, we chose new-onset schizophrenia patients as our study cohort to prevent survival bias. Finally, patients diagnosed as having new-onset schizophrenia (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] 295) between January 1, 2000, and December 31, 2013, were included in the schizophrenia cohort (n = 9243). To recruit only patients who were first diagnosed with schizophrenia, we excluded patients with a previous diagnosis of schizophrenia before 2000 (n = 2771). The enrollment date was considered the index date. We also excluded patients with a previous history of dementia (ICD-9-CM 290, 294.1 ∼ 294.2, and 331) that was diagnosed before the first diagnosis of schizophrenia (n = 231), those of unspecified sex (n = 16), and those younger than 18 years (n = 185). The final schizophrenia cohort consisted of 6040 patients with newly diagnosed schizophrenia.
Our control cohort was selected from the remaining patients during the same period (January 1, 2000 to December 31, 2013). Using similar exclusion criteria as in the study cohort, patients with any diagnosis of schizophrenia before 2000 (n = 2118) and patients who had been diagnosed with any mental disorder (n = 5082; ICD-9-CM 290 ∼ 319) were also excluded to ensure that no psychiatric patients were included in the control group. We further excluded patients who had previously been diagnosed with dementia (n = 207). Patients on antipsychotic medications (n = 621) were excluded from the control cohort to ensure that no one with a psychotic disorder was included. Similarly, patients of an unspecified sex (n = 27) or younger than 18 years (n = 176,442) were also excluded. From the remaining eligible subjects, we selected a control cohort that contained four times the number of patients with schizophrenia, propensity score-matched by sex, age, and index year Reference Caliendo and Kopeinig[22]. The first time the patient in the control cohort sought medical consultation during 2000 ∼ 2013 was considered the index date. Schizophrenia and dementia must have been diagnosed at least twice for consecutive outpatients or once for inpatient medical records for validation. The reason for the requirement for two consecutive diagnoses from outpatient medical records was to minimize the possibility of recruiting patients who were erroneously coded in a single outpatient visit. Discharge diagnoses are highly reliable; therefore, a single record was sufficient. Finally, our study included 24,160 control patients. A flow chart of the study selection process is provided in Fig. 1.
2.3. Study endpoint and covariates
The date of any form of dementia diagnosis (ICD-9-CM 290, 294.1 ∼ 294.2, and 331) made for the first time during the follow-up (from enrollment to December 31, 2013) or the end of the study (December 31, 2013) was considered the study endpoint. In our study, types of dementia other than Alzheimer’s and vascular dementia were categorized as unspecified dementia, such as frontotemporal dementia (FTD), Parkinson’s dementia, and dementia with Lewy body (DLB) or dementia of unknown etiology. Sociodemographic variables consisted of sex, age, income-related insurance premium, hospital type, season, and urbanization level. Potential baseline dementia-related clinical factors were also assessed as medical comorbidities, including cardiovascular disease (ICD-9-CM 430 ∼ 438), hypertension (ICD-9-CM 401 ∼ 405), diabetes mellitus (ICD-9-CM 250), hypercholesterolemia (ICD-9-CM 272), chronic respiratory disease (ICD-9-CM 491 ∼ 494, 496, 415.0, 416.8, and 416.9), traumatic brain injury (ICD-9-CM 800 ∼ 804, 850 ∼ 854, and 959), alcohol-related disorders (ICD-9-CM 291, 303, 305.0, 357.5, 425.5, 535.3, 571.0, and 571.1 ∼ 571.3), and Parkinson’s disease (ICD-9-CM 332) 1 year before enrollment Reference Aarsland, Zaccai and Brayne[23–Reference Wolf, Ecke, Bettin, Dietrich and Gertz47]. Medications in medical claims during the entire follow-up period considered in the analysis were diuretics, renin-angiotensin system inhibitors (RASIs), calcium channel blockers, beta blockers, insulin, oral antidiabetics, statins, first-generation antipsychotics (FGAs), and second-generation antipsychotics (SGAs). The reason for considering these medications during the entire follow-up period was to adjust for drug effects on the development of dementia. In order to achieve full diagnostic validity, diagnoses of dementia and any medical comorbidity had to be specified at least twice for consecutive outpatients or once for inpatient medical records.

Fig. 1. Flowchart of study sample selection.
2.4. Statistical analysis
For between-group comparisons, an independent t-test was used for continuous variables, and Pearson χ2 test for nominal variables. To estimate the cumulative incidence of dementia risk in the two cohorts, a survival analysis was performed using the Kaplan-Meier method, with significance based on the log-rank test. A Cox proportional regression model was used to investigate the crude and adjusted hazard ratio (HR; aHR) with the 95% confidence interval (CI) of developing any type of dementia before and after adjusting for demographic data, medical comorbidities, and medications. Fine and Gray's competing risk analysis was used to determine the risk of dementia, as death can act as a competing risk factor for dementia Reference Li and Luo[48]. Stratified analyses were conducted to compare the effects of age, sex, medical comorbidities, and medications on the development of dementia. Sensitivity tests were also performed to validate the findings after excluding the first year of observation and the first 3 years of observation. All data processing and statistical analyses were performed using SPSS, vers. 22 (IBM, Armonk, NY, USA).
3. Results
In total, 6040 cases of schizophrenia and 24,160 matched control cases were selected from the NHIRD during the defined follow-up period. The main age group at onset was 18 ∼ 49 years and comprised 76.8% of the schizophrenia and control cohorts. The mean follow-up duration of the schizophrenia cohort was 9.4 ± 11.8 years and that of the control cohort was 9.5 ± 10.1 years (p = 0.005). Men comprised 56.7% of the schizophrenia and control cohorts. Patients with schizophrenia had a significantly higher prevalence of medical comorbidities than did control patients, including cardiovascular disease (p = 0.002), diabetes mellitus, hypertension, hypercholesterolemia, traumatic brain injury, chronic respiratory disease, alcohol-related disorders (all p < 0.001), and Parkinson’s disease (p = 0.045). Detailed data of relevant baseline characteristics of the schizophrenia and control groups are shown in Table 1.
Table 1 Baseline characteristics of the schizophrenic and comparison cohorts.

Abbreviations: NTD, New Taiwan Dollar; RASIs, renin-angiotensin system inhibitors; CCB, calcium channel blocker; FGAs, first-generation antipsychotics; SGAs, second-generation antipsychotics; SD, standard deviation.
p value: * <0.05, ** <0.01, *** <0.001.
The aHR for dementia is presented in Table 2. After adjusting for potential confounders, our data demonstrated that the newly diagnosed schizophrenia cohort was associated with a 1.80-fold risk of dementia (aHR = 1.80; 95% CI = 1.36 ∼ 2.21; p < 0.001). Other significant factors were an age of 50 ∼ 64 years (aHR = 1.42; 95% CI = 1.10 ∼ 1.84, p < 0.001), an age of ≥ 65 years (aHR = 4.95; 95% CI = 4.10 ∼ 5.97, p < 0.001), cardiovascular disease (aHR = 5.26; 95% CI = 4.50 ∼ 6.72; p < 0.001), hypertension (aHR = 1.83; 95% CI = 1.77 ∼ 2.04; p = 0.002), traumatic head injury (aHR = 1.35; 95% CI = 1.24 ∼ 1.78; p < 0.001), chronic lung diseases (aHR = 1.64; 95% CI = 1.13 ∼ 2.56; p < 0.001), alcohol-related disorders (aHR = 3.67; 95% CI = 2.68 ∼ 4.92; p < 0.001), and Parkinson’s disease (aHR = 1.72; 95% CI = 1.25 ∼ 2.40; p < 0.001). Patients who were treated with diuretics (aHR = 1.20; 95% CI = 1.09 ∼ 4.72; p = 0.037) and RASIs (aHR = 1.39; 95% CI = 1.03 ∼ 2.97; p = 0.045) were also associated with a higher risk of dementia.
Table 2 Crude and adjusted hazard ratios (HRs) for dementia using Cox proportional hazard regression and Fine and Gray's competing risk model.
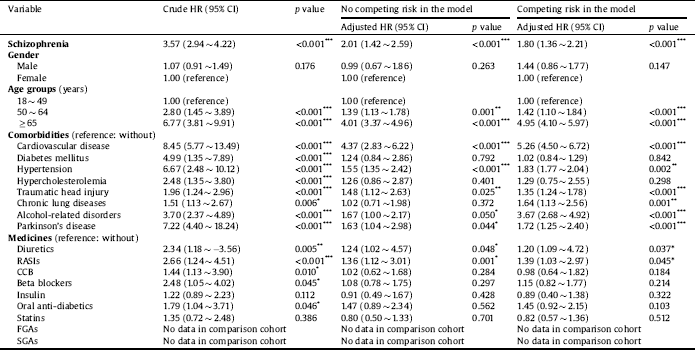
Abbreviations: CI, confidence interval; Adjusted HR, adjusted variables listed in Table 1; RASIs, renin-angiotensin system inhibitors; CCB, calcium channel blocker; FGAs, first-generation antipsychotics; SGAs, second-generation antipsychotics.
p value: * <0.05, ** <0.01, *** <0.001.
Table 3 shows the incidence of all-cause dementia in the schizophrenia and control cohorts. During the observation period, 867 patients (incidence rate: 15.33 per 1000 person-years) in the schizophrenia cohort and 370 patients (incidence rate: 1.62 per 1000 person-years) in the control cohort developed dementia. The incidence of developing all-cause dementia was significantly higher in the schizophrenia cohort than in the control cohort (p < 0.001). In stratified analyses, men aged ≥ 65 years (aHR = 2.37; 95% CI = 1.95 ∼ 3.81; p < 0.001) had a higher risk of dementia than did younger male age groups. Similarly, women aged ≥ 65 years (aHR = 4.30; 95% CI = 2.45 ∼ 6.97; p < 0.001) had a higher risk of dementia than younger female age groups. With regard to dementia subtypes, the incidence of Alzheimer’s [dementia/disease?] was significantly higher in the schizophrenia cohort than in the control cohort (1.03 vs. 0.39 per 1000 person-years, p < 0.001). Similarly, the incidence of vascular dementia was also significantly higher in the schizophrenia cohort than in the control cohort (1.20 vs. 0.41 per 1000 person-years, p < 0.001). Patients with schizophrenia had the highest risk of developing Alzheimer’s [dementia/disease?] among dementia subtypes (aHR = 2.10; 95% CI = 1.88 ∼ 3.86; p < 0.001), followed by vascular dementia (aHR = 1.67; 95% CI = 1.27 ∼ 2.12; p < 0.001), and unspecified dementia (aHR = 1.30; 95% CI = 1.04 ∼ 2.01; p < 0.001).
Table 3 Comparing incidences of dementia in the comparison and schizophrenic cohorts using a Cox proportional hazard regression and Fine and Gray's competing risk model, stratified analyses for sex and age groups.
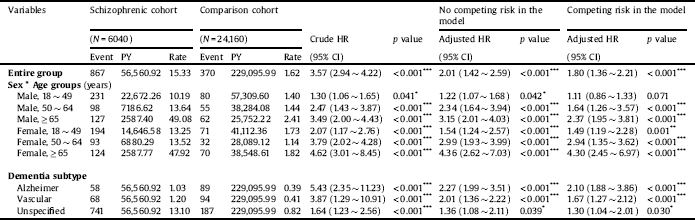
Abbreviations: PY, person-years; HR, hazard ratio; CI, confidence interval; Rate, incidence rate per 1000 PY; Adjusted HR, adjusted variables listed in Footnote Table 1.
Rate, per 1000 person-years.
p value: * <0.05, ** <0.01, *** <0.001.
Table 4 points out the interactive effects of comorbidities, medications, and schizophrenia on outcomes. A statistically increased risk was observed for patients diagnosed with both schizophrenia and cardiovascular disease (aHR = 4.27; 95% CI = 3.64 ∼ 4.99; interaction p < 0.001). Interactions of diabetes mellitus (aHR = 3.64; 95% CI = 3.13 ∼ 4.25; interaction p = 0.011), hypertension (aHR = 3.95; 95% CI = 2.71 ∼ 5.76; interaction p = 0.026), traumatic head injury (aHR = 4.73; 95% CI = 1.60 ∼ 13.95; interaction p < 0.001), chronic lung diseases (aHR = 3.40; 95% CI = 2.96 ∼ 3.94; interaction p = 0.001), and alcohol-related disorders (aHR = 3.76; 95% CI = 1.99 ∼ 7.13; interaction p < 0.001) were also associated with an increased risk of dementia.
Table 4 Comparing incidences of dementia in the comparison and schizophrenic cohorts using a Cox proportional hazard regression and Fine and Gray's competing risk model.
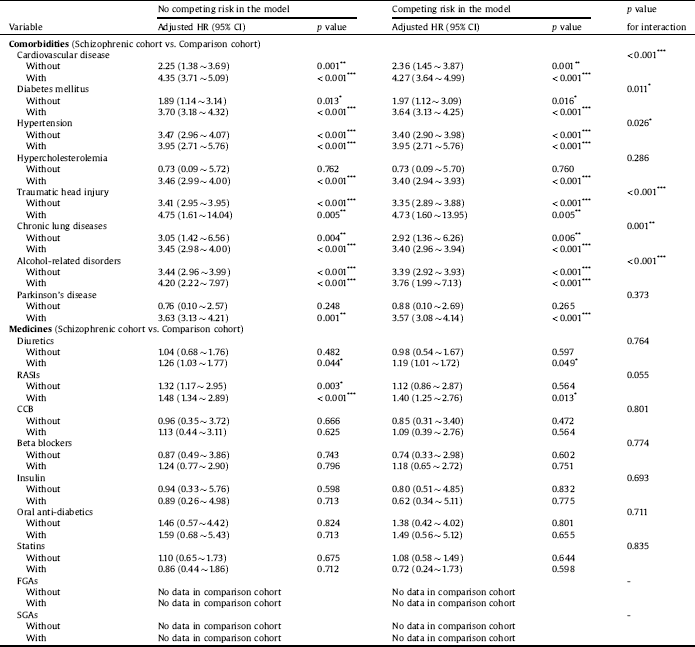
Abbreviations: HR, hazard ratio; CI, confidence interval; Adjusted HR, adjusted variables listed in Table 1. RASIs, renin-angiotensin system inhibitors; CCB, calcium channel blocker; FGAs, first-generation antipsychotics; SGAs, second-generation antipsychotics.
p value: * <0.05, ** <0.01, *** <0.001.
Stratified analyses among antipsychotic drugs demonstrated lower risks of developing all-cause dementia in patients with schizophrenia treated with FGAs (aHR = 0.80; 95% CI = 0.56 ∼ 0.95; p = 0.044) and SGAs (aHR = 0.24; 95% CI = 0.11 ∼ 0.60; p < 0.001) than in those with no antipsychotic treatment (Table 5). Sensitivity analyses after excluding the first year and the first 3 years of observation also provided significant findings (Table 6). The Kaplan-Meier survival analysis, shown in Fig. 2, demonstrated a significantly higher cumulative incidence risk of dementia in the schizophrenia cohort than in the control cohort (log-rank test; p < 0.001).
Table 5 Adjusted hazard ratios (HRs) for dementia among the schizophrenia cohort using a Cox proportional hazard regression and Fine and Gray's competing risk model, stratified analyses for medical comorbidities and medicines.

Abbreviations: CI, confidence interval; Adjusted HR, adjusted variables listed in Table 1. RASIs, renin-angiotensin system inhibitors; CCB, calcium channel blocker; FGAs, first-generation antipsychotics; SGAs, second-generation antipsychotics.
p value: * <0.05, ** <0.01, *** <0.001.
Table 6 Sensitivity analyses for the risk of developing dementia between the schizophrenic and comparison cohorts using a Cox proportional hazard regression and Fine and Gray's competing risk model.
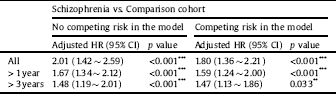
Abbreviations: HR, hazard ratio; CI, confidence interval; Adjusted HR, adjusted variables listed in Table 1.
p value: * <0.05, ** <0.01, *** <0.001.
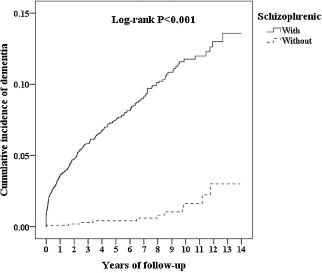
Fig. 2. Comparison of Kaplan-Meier survival analyses for the cumulative incidence of dementia between the two study cohorts.
4. Discussion
Our results supported the study hypothesis that patients with newly diagnosed schizophrenia would have a significantly increased risk of dementia in later life. The overall incidence rate of dementia was 15.33 per 1000 person-years in the schizophrenia cohort, which was significantly higher than that in the control cohort (p < 0.001). The risk and incidence of dementia were also the highest in patients aged ≥ 65 years with schizophrenia, which is in concordance with longitudinal studies of patients with schizophrenia, in which individuals aged ≥ 65 years demonstrated considerable cognitive decline or development of dementia more frequently than expected Reference Harvey, Silverman, Mohs, Parrella, White and Powchik[18, Reference Friedman, Harvey, Coleman, Moriarty, Bowie and Parrella49]. With regard to dementia subtypes, patients with schizophrenia had the highest risk of developing Alzheimer’s [dementia/disease?]. Notably, FGA or SGA treatment was significantly associated with a lower risk of developing all-cause dementia in later life. In addition, our study revealed that cerebrovascular disease, hypertension, traumatic head injury, chronic lung diseases, alcohol-related disorders, and Parkinson’s disease were significantly associated with the risk of dementia.
The mechanisms underlying schizophrenia and dementia remain unknown.
Several plausible explanations exist for the increased risk of dementia in patients with schizophrenia. First, recent evidence has converged on mitochondrial dysfunction and oxidative stress as potential pathogenic mechanisms in schizophrenia, with findings of both cerebral and peripheral perturbations in these systems Reference Rosenfeld, Brenner-Lavie, Ari, Kavushansky and Ben-Shachar[50]. Second, oxidative stress on cellular constituents, such as DNA and RNA, may play critical roles in cellular aging Reference Finkel and Holbrook[51] and even neurodegeneration Reference Nunomura, Hofer, Moreira, Castellani, Smith and Perry[52]. Third, genotoxic stress from oxidation activates DNA damage signaling pathways and accelerates telomere erosion, thereby increasing the risk of cellular senescence or apoptosis, which are known to be key events in the aging process Reference Sahin and DePinho[53]. Hence, there is evidence that mitochondrial dysfunction and oxidative stress may be specifically involved in the cognitive deficits of schizophrenia Reference Jablensky, Angelicheva, Donohoe, Cruickshank, Azmanov and Morris[54].
Alzheimer’s dementia is the most common type of dementia, accounting for 60% ∼ 80% of all such cases Reference Mayeux and Stern[55, Reference Purohit, Perl, Haroutunian, Powchik, Davidson and Davis56]. In contrast to the aforementioned studies, our study did not find this phenomenon. Instead, unspecified dementia was dominant, which supported the viewpoint of a study by Korner et al. (2009) pointing to the possibility that people with late-onset and very-late-onset schizophrenia have an increased risk of developing a different type of dementia other than Alzheimer’s disease Reference Korner, Lopez, Lauritzen, Andersen and Kessing[13]. One study examined the presence of senile plaques and neurofibrillary tangles in the post-mortem brains of patients with schizophrenia and cognitive impairment and compared them with brains of normal controls. However, none of the brains from the schizophrenia group demonstrated sufficient senile plaques and neurofibrillary tangles to make a diagnosis of Alzheimer’s disease Reference Purohit, Perl, Haroutunian, Powchik, Davidson and Davis[57]. Evidence from the aforementioned studies implies that most people with schizophrenia and cognitive impairment do not have Alzheimer’s disease pathology. Some possible reasons may explain this discrepant result. First, neuropsychiatric symptoms (NPSs) of dementia are noncognitive or behavioral and psychiatric symptoms of dementia and include disturbances of mood, perception, and behavior associated with neurodegenerative diseases Reference Lyketsos, Carrillo, Ryan, Khachaturian, Trzepacz and Amatniek[58]. NPSs are present in the prodromal or mild cognitive impairment (MCI) stages of dementia Reference Rosenberg, Mielke, Appleby, Oh, Geda and Lyketsos[59]. On the other hand, mild behavioral impairment (MBI) was proposed as a diagnostic construct to identify patients with an increased risk of developing dementia, but who may or may not have cognitive symptoms. MBI was first proposed by Taragano et al. Reference Taragano, Allegri, Krupitzki, Sarasola, Serrano and Lon[60] to identify FTD patients earlier in the course of their illness, and the basis of MBI criteria is the assumption that neurodegeneration can present with personality changes, as well as behavioral or other psychiatric symptoms in advance of cognitive impairment. The proposed MBI criteria allow for, but do not require, the concurrent presence of MCI. MBI and MCI are both prodromes to dementia Reference Ismail, Agüera-Ortiz, Brodaty, Cieslak, Cummings and Fischer[61]. FTD is a leading cause of pre-senile dementia with an earlier age of onset than other dementias Reference Ratnavalli, Brayne, Dawson and Hodges[62]. Similarly to schizophrenia, FTD involves the frontal and temporal lobes Reference Neary, Snowden and Mann[63, Reference Neary, Snowden, Gustafson, Passant, Stuss and Black64] and shares many characteristic neuropsychiatric disturbances including behavior and personality change, loss of empathy and insight, emotional blunting, apathy, and executive dysfunction Reference Neary, Snowden, Gustafson, Passant, Stuss and Black[64, Reference McKhann, Albert, Grossman, Miller, Dickson and Trojanowski65] with schizophrenia. People with young-onset FTD may initially present with psychosis and be misdiagnosed with a psychiatric disorder such as schizophrenia Reference Chan, Stolwyk, Neath, Kelso, Walterfang and Mocellin[66]. Therefore, dementia may be underestimated, while schizophrenia may be overestimated. Dementia might have presented atypically as prodromal dementia declaring itself with psychotic symptoms Reference Donaghy, O'brien and Thomas[67, Reference Velakoulis, Walterfang, Mocellin, Pantelis and McLean68] or have been undiagnosed by clinicians reluctant to make a diagnosis in the context of schizophrenia. In other words, late-life onset of psychiatric symptoms that are not described in schizophrenia may be early manifestations of prodromal dementia. Therefore, both dementia and schizophrenia may be underestimated. Third, the major age group of our schizophrenia cohort was 18 ∼ 49 years with a maximum 14 years of follow-up; thus, the average highest age at follow-up was far below 65 years. As the onset of schizophrenia generally begins in early adulthood Reference Flaum and Schultz[6] and dementia begins in late-life Reference van der Flier and Scheltens[1], the observation period is still too short from early-onset schizophrenia to the diagnosis of dementia despite a maximum 14-year follow-up and the fact that established early-onset schizophrenia patients younger than 18 years were excluded. Although common in FTD, NPSs can be an early manifestation of other dementias. In the original Taragano study, patients with NPSs as presenting complaints in advance of cognitive impairment were followed for 3 years. At the end point, 36% had FTD, 28% had Alzheimer’s [dementia/disease?], 18% had vascular dementia, and 18% had other types of dementia Reference Taragano, Allegri, Krupitzki, Sarasola, Serrano and Lon[60], suggesting any type of dementia can first present only behavioral symptoms such as MBI with or without cognitive impairment Reference Taragano, Allegri, Krupitzki, Sarasola, Serrano and Lon[60].
Other factors associated with an increased risk of dementia identified in the present study were consistent with those described in previous reports, including age, cardiovascular disease Reference Moroney, Bagiella, Desmond, Paik, Stern and Tatemichi[32–Reference Moroney, Bagiella, Tatemichi, Paik, Stern and Desmond34, Reference Snowdon, Greiner, Mortimer, Riley, Greiner and Markesbery40, Reference Wolf, Ecke, Bettin, Dietrich and Gertz47], hypertension Reference Kivipelto, Helkala, Laakso, Hänninen, Hallikainen and Alhainen[30, Reference Swan, DeCarli, Miller, Reed, Wolf and Jack69–Reference Whitmer, Sidney, Selby, Johnston and Yaffe71], traumatic head injury (25, 26), chronic lung diseases Reference Liao, Lin, Chang, Tu and Kao[72, Reference Liao, Ho, Ko and Li73], alcohol related disorders Reference Langballe, Ask, Holmen, Stordal, Saltvedt and Selbæk[74] and Parkinson’s disease Reference Huang, Wu, Lin, Lin and Kao[75, Reference Pagonabarraga and Kulisevsky76]. A number of significant interaction between covariate conditions and schizophrenia were noted to heighten risk of dementia. The highest interactive effect with schizophrenia on the risk of dementia shown in our study were cardiovascular disease and traumatic head injury as key potential confounders, which was about 4 to 5 times risk compared with the comparison cohort.
In the present study, we found that the risk of dementia was the highest at an age of 65 years or more. Kraepelin (1971) first conceptualized schizophrenia as a disorder of progressive cognitive decline, and the cognitive changes with aging in schizophrenia are also consistent with the hypothesis that schizophrenia is associated with accelerated aging Reference Kirkpatrick, Messias, Harvey, Fernandez-Egea and Bowie[77]. Cognitive changes observed in schizophrenia have considerable overlap with those abilities that are vulnerable to the substantial changes seen in normal aging. However, cognitive decline in schizophrenia may occur in a shorter time than that taken by normal aging-related changes. The mechanisms underlying the cognitive changes affected by schizophrenia and normal aging and those that are prone to deterioration with aging and schizophrenia remain unclear, and further research is needed. Otherwise, high medical morbidity in schizophrenia may be attributed to an environment in which unhealthy and high-risk behaviors are prevalent, such as smoking, substance abuse, lack of exercise, and poor diet Reference Brown, Birtwistle, Roe and Thompson[78]. Such unhealthy lifestyles and risky behaviors may place patients with schizophrenia in a dementia-prone condition. Moreover, SGAs have been the mainstream treatment for schizophrenia, but these also may have serious metabolic side effects with respect to the incidence of treatment-related cardiovascular disease and hypertension Reference Nasrallah[79]. In addition, SGAs may have a negative impact on cognition Reference Nielsen, Levander, Kjaersdam Telléus, Jensen, Østergaard Christensen and Leucht[80], which may promote a general lack of awareness with respect to situations wherein traumatic head injury might occur. Notably, unlike previous studies, our study demonstrated that SGAs may be associated with a lower risk of developing all-cause dementia than that without any antipsychotic treatment in stratified analyses. With regard to effects of SGAs on cognition, SGAs, also referred to as “atypical” antipsychotics, are characterized by serotonin 5-HT2A and dopamine D2 antagonism. There is a general consensus that SGAs possess fewer side effects on the motor system and even cognitive benefits Reference Green[81, Reference Brenner, Böker and Genner82], which is supported by our findings. In contrast, FGAs, also known as “typical” antipsychotics, were first brought to wide clinical use in 1950s, and all FGAs possess dopamine D2 receptor antagonism effects. In addition to reducing positive symptoms of schizophrenia (e.g., delusions, hallucinations, and disorganization) as SGAs, FGAs can produce more adverse effects on the motor system than SGAs Reference Kapur, Zipursky, Jones, Remington and Houle[83–Reference Reilly, Harris, Keshavan and Sweeney86]. With regard to the effects of FGAs on cognition, the general consensus is that FGAs do not improve cognition, and instead, can have specific adverse cognitive effects Reference Spohn and Strauss[87–Reference Bilder, Turkel, Lipschutz-Broch and Lieberman89], which contradicts our findings. However, recent evidence suggests that D2 receptor blockade reduced tau phosphorylation in both a C. elegans model of tauopathy Reference McCormick, Wheeler, Guthrie, Liachko and Kraemer[90] and in a tau mouse model of Alzheimer’s disease Reference Koppel, Jimenez, Adrien, Greenwald, Marambaud and Cinamon[91]. These results suggest dopamine D2 receptors holds promise as a potential neuroprotective strategy for targeting tau aggregation and neurotoxicity. Nearly all antipsychotics are dopamine D2 antagonists and may reduce the neurofibrillary tangle production via inhibition of tau phosphorylation in vivo, which may be the mechanism responsible for reduced dementia risk in those with schizophrenia who were exposed. It is hard to draw a conclusion regarding whether FGAs or SGAs have positive effects on cognition based on aforementioned research because there are many residual confounders that should be considered when interpreting cognitive changes reported in patients with schizophrenia following antipsychotic treatment. For example, relative dosing of antipsychotics, randomized double blind or open-label studies, duration of trial, the choice of the neurocognitive test batteries, the baseline pharmacological status, multiple study arms with random assignment to pharmacological condition, and dosing strategies Reference Harvey and Keefe[92, Reference Hill, Bishop, Palumbo and Sweeney93] should be taken into account. In addition, every antipsychotic agent has its unique properties and needs comparing with the others. However, our study only divided antipsychotics into FGAs or SGAs instead of individual antipsychotic agents. Further clinical research is warranted to investigate antipsychotics’ impact of this reduction on tau neuropathology and determine whether D2 receptor antagonism may be a viable therapeutic approach for the prevention of tau pathology in mammalian brain.
The strengths of our study included the wide range of the population and adjustment for demographic characteristics, potential medical comorbidities and prescribed medicines. Additional strengths include that the study results were derived from a long observation period, with complete follow-up, our ability to recruit patients with an initial diagnosis of schizophrenia, and the presence of matched controls. Therefore, selection bias and loss to follow-up cannot explain our findings.
Although our findings are compelling, our results should be viewed in light of several limitations. First, the incidence of dementia may have been underestimated because only those who sought medical consultation and help were included. However, to enhance diagnostic validity, all diagnoses in our study were provided at least twice in outpatient medical records and once in inpatient medical records. In addition, diagnoses in our study were more reliable than those derived by proxy, e.g., via questionnaires and self-reported symptoms Reference Chou, Lin, Lin, Tsai, Cheng and Chuo[94]. Although the NHIRD does not include laboratory data, the quality of diagnosis is monitored by the Department of Health and Welfare: random samples of a fixed percentage of claims from every hospital are obtained annually to verify the validity and quality of diagnoses. Fines for fraud are 100 times the amount of the claim charged to the NHI Bureau to minimize the possibility of inappropriate diagnoses Reference Lin, Chen, Chou and Chung[95]. Second, some information, such as schizophrenia severity, family history, educational level, environmental factors, and lifestyles, was not available in the NHIRD. In addition, laboratory data were not available in the NHIRD, such as body mass index. Therefore, we could not postulate the validity of the diagnosis of obesity. However, obesity is both related to schizophrenia and dementia Reference Kiliaan, Arnoldussen and Gustafson[96, Reference Manu, Dima, Shulman, Vancampfort, De Hert and Correll97]. Without these data, we could not investigate their influence on the risk of dementia. Third, our study included only patients with onset of schizophrenia after the age of 18 years, which may impede generalizability of the results for patients with schizophrenia who are diagnosed before the age of 18 years.
5. Conclusions
To the best of our knowledge, this study is the first to use the NHIRD to investigate the risk of dementia in patients with schizophrenia. Schizophrenia was associated with an increased risk of dementia in later life. Further research is required to clarify the underlying common pathophysiology between schizophrenia and dementia and to investigate whether antipsychotic therapy can mitigate this risk.











Comments
No Comments have been published for this article.