INTRODUCTION
The varicella zoster virus (VZV) produces two distinct diseases, namely, varicella (chickenpox) and herpes zoster (HZ). After causing varicella, VZV remains dormant in the sensory ganglia and reactivates later in life and leads to HZ. The cause of reactivation is not understood clearly, although it has been associated with a decrease in cell-mediated immunity [Reference Arvin1]. Before the introduction of the varicella vaccine, there were an estimated 4 million varicella cases in the USA annually. More than 90% of these cases involved individuals aged <15 years, with the highest age-specific incidence in children aged 5–9 years [Reference Davis, Patel and Gebremariam2, Reference Nguyen, Jumaan and Seward3]. A similar situation was observed in Europe, with the highest age-specific incidence in children aged <9 years [Reference Bonanni4]. Varicella rarely occurs in individuals aged >40 years because >99·6% of adults have VZV-specific antibodies [Reference Kilgore5].
Establishing and/or enhancing varicella and HZ surveillance has been essential in assessing the impact of widespread varicella vaccination. The introduction and the widespread use of varicella vaccine has led to a 75–80% decrease in incidence, hospitalization rates, and even mortality associated with varicella in selected US surveillance sites from 1995 to 2000 [Reference Davis, Patel and Gebremariam2, Reference Nguyen, Jumaan and Seward3, Reference Marin, Meissner and Seward6, Reference Watson7]. The biggest decline was observed in children aged 1–4 years [Reference Seward8]. On the other hand, there are concerns regarding an increase in the incidence of HZ in adults because of the decreased circulation of wild-type viruses brought about by the varicella vaccination programme. Presumably, exposure to varicella reduces the risk of reactivation by boosting specific immunity to the virus [Reference Edmunds and Brisson9, Reference Levin10]. Several epidemiological studies suggested that exposure to varicella is greater in adults living with children, and that this exposure is highly protective against HZ [Reference Thomas, Wheeler and Hall11]. Although this hypothesis is further supported by mathematical modelling [Reference Gao12, Reference Karhunen13], so far, results from epidemiological studies do not suggest an increase in risk [Reference Hambleton14–Reference Thomas and Hall17]. Therefore, the monitoring of the long-term impacts of varicella vaccinations on HZ incidence is crucial.
The varicella vaccine was introduced onto the Taiwanese market in July 1997. From 1998, Taipei City has included the varicella vaccine in its free paediatric vaccination programme for children aged >12 months. Taichung City/County followed this protocol in 1999. In children aged 12–18 months, a 39–52% range in vaccination rate was observed in 2000–2003 in these two areas. The nationwide free varicella immunization programme for children aged 12–18 months was not implemented until 2004 when the vaccination rate increased to 82·93% in Taipei City and in Taichung City/County. No catch-up vaccination programme has been performed in Taiwan. Other areas in Taiwan did not provide free varicella vaccinations in their public vaccination programme until 2004. However, people could purchase private varicella vaccination. From 2000 to 2008, a gradual increase in vaccination rate was also observed, from <10% before 2003 to >90% after 2006 (Taiwan CDC, personal communication). A significant decrease in varicella incidence in children aged 3–6 years has been consistently observed in areas implementing the free vaccination policy [Reference Lin18–Reference Lian21].
Establishing a reliable system to monitor the epidemiological pattern of varicella and HZ in Taiwan is important, especially when it had been suggested that the surveillance of national notifiable diseases was under-reported [Reference Tan22]. The National Health Insurance (NHI) database has been valuable for evaluating different kinds of health problems, including HZ [Reference Wen, Tsai and Chung23, Reference Chen24], but the secular trends of HZ in Taiwan are unknown. The current study aims to investigate the incidence trend and the epidemiological characteristics of varicella and HZ from 2000 to 2008, the period when varicella vaccination rates increased gradually in Taiwan.
MATERIALS AND METHODS
Data collection
Taiwan started its compulsory NHI system in 1995. The prevalence of health insurance coverage soon increased from 56% in 1995 to over 95% after 2000. Since 2007, 98% of the 23 million population in Taiwan have been covered by health insurance. Inasmuch as clinics and hospitals had to register all treatments to apply for payment of expenses, the accuracy of the databank is exytremely reliable, especially after 1995, when medical records of more than 95% of Taiwan's hospitals contracted with NHI were included in the database [Reference Wen, Tsai and Chung23]. Before our analysis, the temporal consistency between date of birth, dates of diagnosis, and clinical visits of each outpatient were checked to ensure data accuracy. The classification of the location of patients was based on the county/city of one's family registry. Varicella vaccination rate was based on official data registry of the nationwide survey conducted by the Changhua County Public Health Bureau and the Centers for Disease Control in Taiwan (Taiwan CDC).
Definitions
About 240 000 residents of Taiwan who were enrolled in NHI in 2005 were randomly selected by the National Health Research Institute (NHRI). Their outpatient healthcare records were analysed in this study. The sample comprised of almost 1/100 of Taiwan's population of more than 23 million people, and was re-sampled by NHRI to confirm its structural resemblance to the general population with respect to gender and age. The data included diagnosis records of the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes related to varicella (052) and HZ (053) of each person in 2000–2008. A minor number of records of some individuals did not have complete 8-year data because they were uninsured for certain periods. For example, those who were born from 2005 onwards, or those who died before 2008. Demographic variables, like age, gender, date of diagnosis, and residency, were also itemized. All cases in the database were counted only once. Because some cases of post-herpetic neuralgia may last for several months or years, each case in the database was counted only once regardless of the duration and number of times such a case sought medical care during the study period from 2000 to 2008, as the reoccurrence of HZ cannot be differentiated from post-herpetic neuralgia and others. Usually the incidence of diseases including hypertension, diabetes mellitus, chronic nephritis, chronic bronchitis, chronic heart disease, treatment with cancer drug therapy, chronic obstructive pulmonary disease, bronchiectasis, and cerebrovascular diseases, varies with age and time-period. Moreover, it is correlated with HZ incidence in the elderly because of reduced immunity caused by the diseases. Therefore, these diseases are considered as ‘underlying diseases’ (UD). Incidence of UD was incorporated as a confounder in the model.
Statistical analysis
The age-standardized incidence rates (ASIRs) of varicella and HZ, as well as the corresponding 95% confidence interval (CI), were calculated based on the age distribution of the world standard population in 2000 as proscribed by the World Health Organization. Each area is considered as the sole subject, with its incidence being repeatedly measured in each year, the non-parametric Page's trend test was then applied to examine the monotony of the trends. The χ2 test was used to compare the incidence in Taipei City and Taichung City/County (Taipei/Taichung hereafter) with other areas in Taiwan. Control variables were adjusted.
Poisson regression was used to estimate age, gender, and period effects, with the assumption that the number of HZ cases follows a Poisson distribution. The incidence rates manifested a multiplicative function of the included model parameters, making the logarithm of the rates an additive function of the parameters. The Poisson regression model is given as
where a i is the ith age group, i=1~3 (i.e. 50–59, 60–69, 70–79 years), t j is the jth time-period, j=1~4 (i.e. 2000–2001, 2002–2003, 2004–2005, 2006–2008), s k is the gender (i.e. k=1 for men and 0 for women), r ijk is the effect of UD in relation to age and gender, s k*t j is the interaction effect between gender and time-period, d ijk is the number of HZ cases, and p ijk is the population within stratum ijk (i.e. age i, period j, gender k). In addition, E(·) denotes expectation. To account for the different spans of periods, the number of years in each period was used as the weight in regression. Model parameters were interpreted as the log of the rate ratio or the relative risk (RR) adjusted for other factors. In this study, the 50–59 years age group (with higher HZ incidence occurring in people >50 years) and the 2004–2005 period (the start of the implementation of the national varicella vaccination programme) were used as reference categories. Parameter estimates with 95% CIs were obtained using the maximum-likelihood method. Goodness-of-fit test was performed to evaluate data fitting into the Poisson regression. Modelling was performed using the genmod procedure of SAS version 9.1 (SAS Institute Inc., USA).
RESULTS
Descriptive epidemiology
Table 1 shows the crude rates of varicella and HZ in Taiwan from 2000 to 2008 and the comparison between Taipei/Taichung and other areas in Taiwan. The crude rate of varicella shows a decreasing trend from 2000 to 2008 (8·29 and 2·23 cases/1000 person-years, respectively), whereas the rate of HZ fluctuated in 2000–2001 and then gradually increased from 2002 to 2008 (5·35 and 6·89 cases/1000 person-years, respectively). The increase of the incidence in HZ before the implementation of nationwide free paediatric varicella vaccination in 2004 was also noted (Table 1). Crude incidence rates of HZ increased to 5·35 cases/1000 person-years in 2002 compared to 4·45 cases/1000 person-years in 2000. In particular, Taipei/Taichung area which had implemented free vaccination since 1999 (i.e. 5 years earlier), had a higher incidence of HZ than the rest of Taiwan after 2003 (P<0·03 by Wilcoxon's signed rank test) (Table 1). Furthermore, the average incidence rate of varicella in Taipei/Taichung was significantly lower (4·60 cases/1000 person-years) than that in other areas in Taiwan (6·92 cases/1000 person-years) in 2000–2003. After the implementation of the nationwide varicella immunization program in 2004, a significantly lower average of the crude incidence rate of varicella was also observed in Taipei/Taichung (2·56 cases/1000 person-years) compared to that in other areas in Taiwan (4·08 cases/1000 person-years) in 2004–2008. In particular, the incidence rate of varicella in Taipei/Taichung was lower than that in Taiwan. The gap in the incidence rate of varicella between Taipei/Taichung and the other areas gradually diminished after 2007.
Table 1. Unadjusted Incidence rates of varicella and herpes zoster (HZ) in Taiwan, Taipei/Taichung, and other areas, 2000–2008
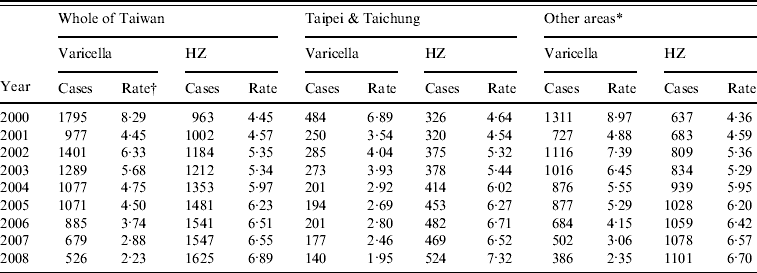
* Other areas=all the counties in Taiwan except Taipei City, Taichung City/County, and the outside island counties like Penghu and Kinmen.
† Rate=unadjusted incidence rate (per 1000) of the indicated diseases.
Table 2 shows the distribution of gender, age, and UD in the population from 2000 to 2008. Females comprised half of the population and remained constant. However, females with HZ reactivation increased by about two-thirds from 2000 (4·82 cases/1000 person-years) to 2008 (cases/1000 person-years 7·45). The proportion of the population aged >60 years also increased gradually (10·81% and 15·93% in 2000 and 2008, respectively), along with an increased proportion in HZ reactivation (11·45 and 15·46 in 2000 and 2008, respectively) and prevalence of UD (65·03% and 78·20% in 2000 and 2008, respectively).
Table 2. Incidence of herpes zoster (HZ) in females and the >60 years age group, and the corresponding prevalence rates of underlying diseases in Taiwan, 2000–2008
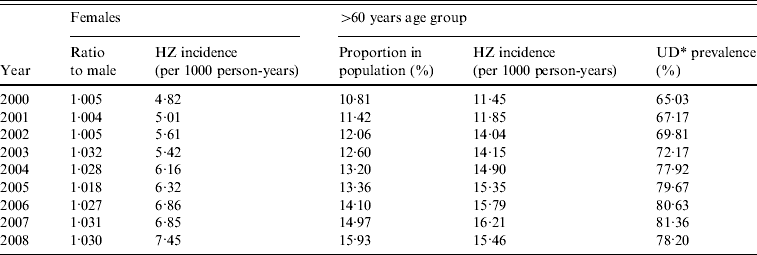
* UD, Underlying diseases including hypertension, diabetes mellitus, chronic nephritis, chronic bronchitis, chronic heart disease, treatment with cancer drug therapy, chronic obstructive pulmonary disease, bronchiectasis, and cerebrovascular diseases.
ASIRs of varicella and HZ
The ASIRs of varicella and HZ in Taiwan in 2000–2008 are shown in Figure 1. Using 2004, the year for implementing nationwide free vaccination as the watershed, we observed that the incidence of varicella fluctuated in 2000–2003 at a range of 9·48 to 7·08 cases/1000 person-years, and then steadily decreased from 2004 (6·19 cases) to 2008 (3·09 cases), a much more obvious drop (50%) than that of the 2000–2003 period. In contrast, the ASIR of HZ in Taiwan gradually increased over time, with an average of 4·81 cases/1000 person-years in 2000–2003 and 5·67 cases/1000 person-years in 2004–2008. A low but gradual increase in the vaccination rate was observed from 2000 to 2003, which was followed by an abrupt increase to 80% in 2004 through the implementation of a free paediatric varicella vaccination programme in Taiwan. From 2005 to 2008, the vaccination rate increased to nearly 94% (Fig. 1).

Fig. 1. Age-standardized incidence rates and 95% confidence interval of varicella and herpes zoster (HZ) as well as the varicella vaccination rate in children reaching the age of 18 months in Taiwan, 2000–2008.
Age-specific incidence rate of varicella and HZ
Figure 2 shows the age-specific incidence rates of varicella in Taiwan from 2000 to 2008. Children in the 0–4 and 5–9 years age groups had the highest incidence rate of all age groups. Children aged 0–4 years, the target population for receiving free vaccination, had an average incidence of 32·42/1000 person-years during 2000–2003, which decreased to 14·41/1000 person-years during 2004–2008, a decline of 56%. In contrast, there was a 2-year lag for a similar decreasing trend for the 5–9 years age group. Adults aged >20 years, who presented the lowest incidence rates, experienced the lowest decrease (29%) during the same time span (1·48 and 1·05 cases/1000 person-years in 2000–2003 and 2004–2008, respectively).

Fig. 2. Age-specific incidence rates of varicella in Taiwan, 2000–2008.
Figure 3 shows the age-specific annual incidence rates of HZ from 2000 to 2008. The age-specific annual incidence rates of HZ increased with age, and those aged >70 years had the highest risk of HZ reactivation. In particular, HZ incidence for the 50–59 and 60–69 years age groups significantly increased with time from 2000 (8·05 and 10·46 cases/1000 person-years, respectively) to 2008 (12·13 and 14·92 cases/1000 person-years, respectively). A similar trend was also observed for the 70–79 years age group, with an increase in incidence from 2000 (12·48 cases/1000 person-years) to 2007 (17·24 cases/1000 person-years). However, there was a slight decrease in 2008. In addition, the 10–49 years age group manifested relatively stable HZ incidence rates in 2000–2008. The rates in children aged 0–9 years, the age group that most frequently received the varicella vaccine, were the lowest in all age groups, with an average of 1·53 cases/1000 person-years for most years in 2000–2008. Its two lowest rates occurred in 2006 and 2008 (1·41 and 1·12 cases/1000 person-years, respectively). Note that the fluctuation for those aged ⩾80 years is due to the small number of cases.
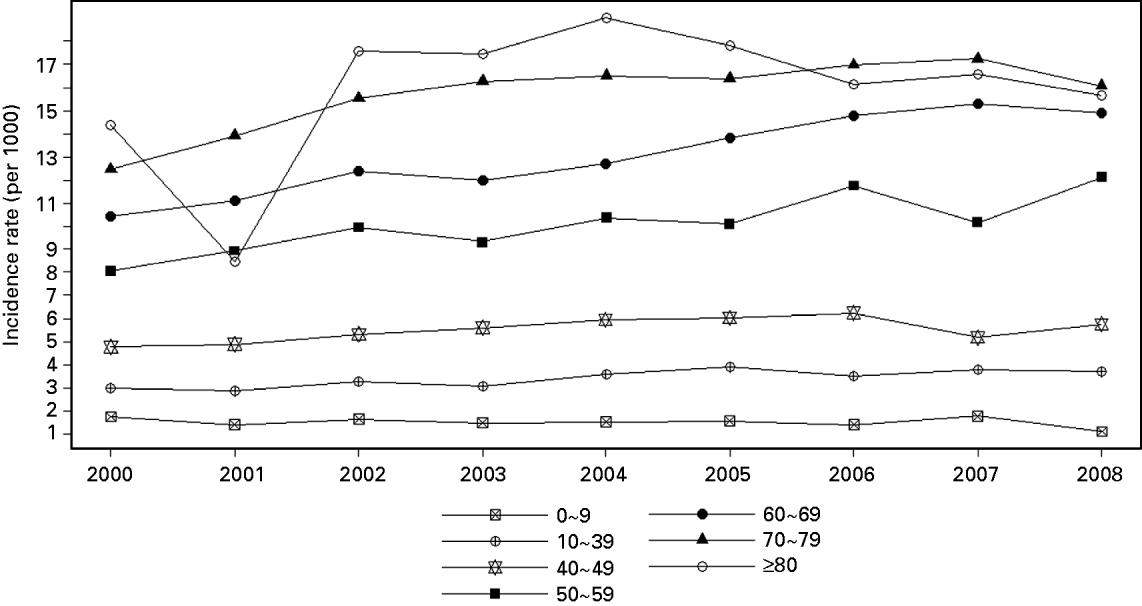
Fig. 3. Age-specific incidence rates of herpes zoster in Taiwan, 2000–2008.
Poisson regression
To examine potential factors affecting the increase in the incidence of HZ over time, the Poisson regression model was used. Based on univariate analysis, as shown on the left of Table 3, males have a lower likelihood of HZ reactivation compared to females (RR 0·87, 95% CI 0·82–0·91). The 70–79 and 60–69 years age groups have greater risks of HZ reactivation compared to those aged 50–59 years (RR 1·55, 95% CI 1·45–1·65 and RR 1·28, 95% CI 1·21–1·36, respectively). Period effect is also significant as incidence increases with time. Using 2004–2005 as reference year for the start of the nationwide free varicella vaccination programme, there was an estimated 8% increase in the incidence rate in 2006–2008 (RR 1·08, 95% CI 1·01–1·16). Conversely, 2000–2001 showed an 18% decrease in incidence rate compared to the reference year (RR 0·82, 95% CI 0·72–0·90). The prevalence of UD increased HZ reactivation risks as opposed to being without UD (RR 1·02, 95% CI 1·01–1·02).
Table 3. Crude and adjusted rate ratios of herpes zoster in Taiwan from univariate and multivariate Poisson regression analysis
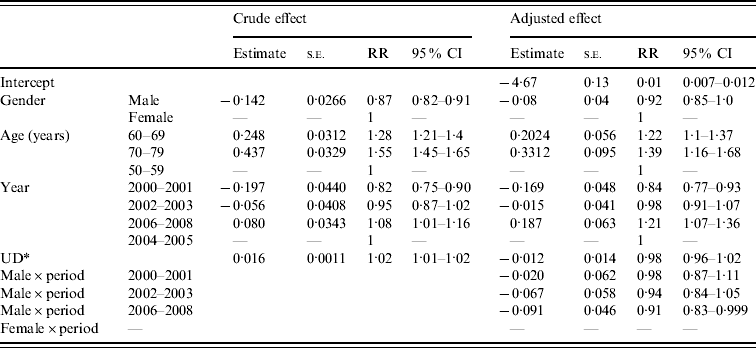
CI, Confidence interval; RR, rate ratio; s.e., standard error.
* UD, underlying diseases including hypertension, diabetes mellitus, chronic nephritis, chronic bronchitis, chronic heart disease, treatment with cancer drug therapy, chronic obstructive pulmonary disease, bronchiectasis, and cerebrovascular diseases.
After implementing the different confounding variables mentioned above into the multivariate Poisson regression models, the estimates of the different periods were affected by gender. Therefore, the interaction between different periods and gender was incorporated into the model (see right portion of Table 3). After adjusting gender, age, UD, and the interaction between gender and period, 2006–2008 presented a 20% excess in incidence rate of HZ reactivation compared to the 2004–2005 reference period (RR 1·21, 95% CI 1·07–1·36). Notably, the interaction between gender and the 2006–2008 period was significant as the incidence rate in males was 9% lower than that in females during 2006–2008 (RR 0·91, 95% CI 0·83–0·999).
DISCUSSION
In this large longitudinal study we examined the incidences of varicella and HZ before and after the implementation of nationwide free paediatric varicella vaccination in Taiwan. We observed that the incidence rates of varicella decreased by 65% in children, while in contrast the ASIR of HZ gradually increased over time, from an average of 4·81 cases/1000 person-years in 2000–2003 to 5·67 cases/1000 person-years in 2004–2008. After controlling gender, UD, and ageing effect, there was a 20% increase in incidence rate of HZ in 2006–2008 compared to 2004–2005. This increase in HZ incidence should not come from the case ascertainment because the same method was applied for the database. This report demonstrated an increase in trend of HZ in Taiwan in 2000–2008, even before the national free varicella vaccination programme was implemented in 2004. Therefore, the widespread vaccination of children is only one of several possible explanations for the increase in the incidence rate of HZ. In some countries, varicella vaccination programmes either have not been instituted or have been abandoned because of concerns on potential increases in HZ incidence after routine varicella vaccinations [Reference Bonanni4, Reference Pinot de Moira and Nardone25]. Long-term follow-up studies of HZ incidence are needed to understand the impact of mass varicella vaccination programmes in Taiwan and in other countries.
Published studies have reported various unadjusted incidence rates of HZ, ranging between 2·15 and 4·97 cases/1000 person-years, during the pre-vaccination era in the USA, The Netherlands, England, Australia, and Taiwan [16, 26–32]. Such data can be used as baseline for comparison of the incidence rates for post-varicella vaccinations. In the present study, the unadjusted rates of HZ in Taiwan from 2000 to 2003, the quadrennium before the implementation of universal vaccination, ranged between 4·45 and 5·34 cases/1000 person-years and are slightly higher than those in other countries with published rates.
Several studies have documented increases in zoster incidence that started before the use of varicella vaccine [Reference Jardine, Conaty and Vally33, Reference Nardone34]. The present study also reveals that before the implementation of nationwide varicella vaccination in 2004, there was already a moderate increase in HZ incidence, from 4·45 in 2000 to 5·34/1000 person-years in 2003, while during the same period the nationwide vaccine coverage rate, although quite low, also slowly increased. After 2004, the vaccine coverage rate soon reached >85%, while the HZ incidence rate maintained a steady increase. Exposure to varicella boosts specific immunity to the virus (exogenous boosting). The unknown mechanism of endogenous boosting through intermittent reactivation of the virus might also have influenced the increase in HZ incidence before the vaccination era. As no catch-up vaccination has been performed in Taiwan, there may be sufficient circulation of varicella virus to boost immunity of older people. Further, this epidemiological phenomenon could have been influenced by other factors independent of the wide usage of varicella vaccine, such as an increase in visits to healthcare for HZ treatment, particularly the residents of Taipei/Taichung Metropolitan City. The establishment of an age-specific seroprevalence rate and force of infection will help elucidate the impact of VZV vaccination on HZ in the future [Reference Nardone34].
Many factors (e.g. gender) that might influence the risk of HZ have been reported previously [Reference Thomas, Wheeler and Hall11, Reference Thomas and Hall17, Reference Brisson35]. In our study, after controlling the three most important confounding effects (i.e. age, gender, UD), the HZ incidence rate increased in 2006–2008, the post-era of the implementation of the free varicella vaccine policy. This period presented values that were significantly higher than those of 2004–2005. Further, a higher HZ incidence rate in females than in males was observed in 2006–2008. However, the difference between both genders in 2000–2001 and 2002–2003 was insignificant. An association between decreased risk of HZ and a family history of exposure to varicella cases, or children affected by such, has been proposed [Reference Garnett and Grenfell36, Reference Garnett and Grenfell37]. Females, especially those aged >60 years, are usually the main caregivers of children in families in Taiwan. Based on data generated from this study, females had a significantly higher HZ incidence rate than males, particularly in 2006–2008, 3 years after the implementation of the nationwide varicella vaccination programme.
The possible reason for the increase in HZ incidence rate in 2006–2008 may be partially attributed to the high varicella vaccination coverage rate in Taiwan. Mathematical modelling has predicted that high varicella vaccination coverage (e.g. 90%) prevents a substantial number of inpatient days due to of varicella, but also generates extra inpatient days because of zoster. Paradoxically, less effective vaccines or vaccination programmes could effectively reduce the overall morbidity of varicella and HZ by allowing the virus to circulate more frequently. Consequently, this might lead to a smaller shift in the age of infection and to a minimized increase in zoster cases [Reference Goldman38]. Increasing vaccination levels is speculated to reduce exogenous exposures to wild-type VZV. Children appear to have poorer cell-mediated response following primary infection, which predisposes them to early reactivation [Reference Brisson35]. Since 2004–2005, the vaccination coverage rate in Taiwan has reached >90%. The more effective vaccination is in the prevention of varicella, the larger will be the increase in zoster incidence [Reference Edmunds and Brisson39]. However, other factors that affect increase in HZ incidence, such as increase in the accessibility to healthcare systems after implementation of the national health insurance system or a change in host immunity, cannot be completely ruled out from this population-based study design.
Nevertheless, the present study has several limitations. First, automated medical-encounter data were used, which led to an underestimation of incidence rates. Such underestimations should remain constant over time [Reference Tseng, Tan and Chang20]. Moreover, varicella rates in the present study were based only on cases in which persons sought healthcare in person. This number is lower than those obtained from studies based on self-report. However, it is possible that the health-seeking behaviour for the treatment of varicella remained the same during the 8-year study period; hence, the observed trend should not be affected by under-ascertainment [Reference Wen, Tsai and Chung23]. Second, reliance on ICD-CM-9 codes for clinical diagnoses at discharge on claims made to Taiwan NHI without reviewing the detailed clinical charts may result in misclassification of varicella and HZ cases. We did not know whether the cases were diagnosed based on clinical features or microbiologically confirmed. Therefore, it is difficult to judge whether the patients were admitted due to HZ. Data from the USA suggest that the positive predictive value of a zoster primary code is substantially greater than primary and secondary codes [Reference Jackson, Reynolds and Harpaz40]. Although the predictive value for the diagnosis of varicella has decreased over time, such misclassification might overestimate the incidence of varicella and minimize the impact of vaccination. Finally, the population in the present study may not be representative of those who do not have health insurance (<3% in Taiwan), although it is presumed that this should not affect the trends observed during the study period.
ACKNOWLEDGEMENTS
This study was based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by National Health Research Institutes (Registered number 97 113). The interpretation and conclusions contained herein do not represent those of Bureau of National Health Insurance, Department of Health or National Health Research Institutes. The study was partially supported by National Science Council of Taiwan (NSC99-2314-B018001-MY3).
DECLARATION OF INTEREST
None.








