Over recent years, the growing prevalence of obesity and the metabolic syndrome (MetS), as predisposing factors for noncommunicable chronic diseases, is of particular global concern, affecting both children and adults(Reference Ng, Fleming and Robinson1). A recent nationwide study provided evidence that based on National Cholesterol Education Program – Adult Treatment Panel III (NCEP-ATP III) criteria, 14·1 % of adolescents in Iran has the MetS(Reference Kelishadi, Ardalan and Gheiratmand2). Moreover, it can be argued that overweight children are at higher risk of becoming obese adults than those normal weight and consequently are more susceptible to cardiometabolic abnormalities and shorter lifespan(Reference Field, Cook and Gillman3,Reference Singh, Mulder and Twisk4,Reference Li, Pinot de Moira and Power5) . While some studies have investigated the status of childhood MetS as a risk factor for adult MetS, the clinical utility of identifying the MetS in children and its association with future cardiometabolic risk factors is a matter of debate, and some studies showed that overweight or obesity adolescent is a better predictor of cardiovascular risk factors and adult MetS in comparison with paediatric MetS(Reference Hosseinpanah, Salehpour and Asghari6,Reference Magnussen, Koskinen and Chen7) . However, it is not yet known whether childhood obesity predicts future cardiometabolic risk factors independent of adulthood BMI.
Obesity phenotypes can be regarded as an indicator of interactions between BMI and CVD risk factors. Thus, individuals can be divided into different subtypes based on their obesity phenotypes: some obese individuals are not affected by metabolic abnormalities associated with their body fat. These ‘metabolically healthy obese’ (MHO) subjects display a favourable metabolic status. On the other hand, some other individuals known as ‘metabolically unhealthy normal weight’ (MUNW) suffer from metabolic abnormalities despite their normal-weight profile.
A series of previous studies in adults have indicated that MUNW subjects carry an elevated risk of developing type 2 diabetes and cardiovascular events compared with MHO subjects(Reference Bell, Kivimaki and Hamer8,Reference Voulgari, Tentolouris and Dilaveris9,Reference Pajunen, Kotronen and Korpi-Hyovalti10) . Furthermore, a systematic review and meta-analysis of observational studies showed that MHO group compared with the MUNW and ‘metabolically unhealthy obese’ (MUO) counterparts had higher cardiovascular events(Reference Koskinen, Magnussen and Sabin11).
In spite of the large body of evidence regarding the predictive role of different phenotypes of obesity on development of CVD outcomes in adults, there is paucity of data for adolescents. However, it is important to note that there are several studies on metabolic risk factors or obesity individually in prediction of adult MetS(Reference Graversen, Sørensen and Petersen12,Reference Sachdev, Osmond and Fall13,Reference Liang, Hou and Zhao14) . In fact, existing data are limited to only one population-based study that has demonstrated positive associations between cardiovascular risk and metabolic complications in adulthood and paediatric obesity phenotypes(Reference Koskinen, Magnussen and Sabin11).
To facilitate more data emphasising the importance of obesity phenotypes in adolescents, this population-based cohort study aimed to determine the prevalence of obesity phenotypes including ‘metabolically healthy normal weight’ (MHNW), MHO, MUNW and MUO among 10- to 18-year-old adolescents and to investigate the role of obesity phenotypes in prediction of adult MetS, in the context of the Tehran Lipid and Glucose Study (TLGS), during a median follow-up of 11·3 years.
Materials and methods
Study population
In the progression of our information in the TLGS data, in the present prospective study, we investigated the role of adolescence obesity phenotypes in the prediction of adult MetS. The TLGS is an ongoing large-scale community-based programme for monitoring the trend of metabolic risk factors and developing healthy lifestyles to reduce these risk factors(Reference Azizi, Ghanbarian and Momenan15). Baseline data were collected from 15 005 participants, aged ≥3 years, under the coverage of three medical health centres residing in District No. 13 of Tehran. The participants were followed up every 3 years to update their data on demographics, lifestyle, biochemical and clinical information and anthropometric examination; the baseline survey was a cross-sectional study conducted from 1999 to 2001, and surveys 2 (2002–2005), 3 (2006–2008), 4 (2009–2011), 5 (2012–2015) and 6 (2016–2019) were prospective follow-up surveys. The cohort is still ongoing.
Of the 15 005 subjects recruited at the baseline examination of the TLGS, 3149 children and adolescents aged 11–18 years from surveys 1 and 2 were included. After exclusion of those with missing anthropometric values and biochemical data (n 149), 3000 subjects remained. After a median of 11·3 years of follow-up, 2159 subjects returned for reassessment. The design of the present study was approved by the institutional ethics committee of the Research Institute for Endocrine Sciences, affiliated to the Shahid Beheshti University of Medical Sciences. All participants signed an informed written consent form to participate.
Measurements
Anthropometric measurements were obtained by qualified healthcare professionals according to standard protocols(Reference Azizi, Ghanbarian and Momenan15); participants were measured while they were minimally clothed and without shoes. Weight was measured to the nearest 0·1 kg with digital scale (range 0·1–150 kg; Seca 707). A tape metre stadiometer was used to measure height while the subjects were against the wall with their shoulders in normal alignment. Waist circumference (WC) was measured using an unstretched tape at the end of expiration at the narrowest level between iliac crest and lowest rib, while the participants were in standing position and there was no pressure on their body surface. Height and WC were recorded with an accuracy of up to 0·1 cm. BMI was calculated as weight (kg) divided by square of height (m2).
Systolic and diastolic blood pressures were determined by a physician using standard mercury sphygmomanometer (calibrated by Iranian Institute of Standards and Industrial Researches). After the subject remained seated for 15 min, blood pressure measurement was taken two times with at least a 30-s interval from the right brachial artery at the heart level. The mean values of measurements were considered as the individual’s blood pressure.
After 12–14 h fasting, a blood sample was taken between 07.00 and 09.00 hours, based on the standard protocol. After centrifuging the collected blood sample, the serum was used for measurement and analyses of fasting plasma glucose (FPG) and lipid concentrations on the day of blood collection at the TLGS Research Laboratory, using commercially available laboratory kits (Pars Azmoon Inc.) adapted to a Selectra 2 auto analyser. Plasma glucose concentration was measured by the enzymatic colorimetric method with glucose oxidase. Both inter- and intra-assay CV were 2·2 % for FPG. For measurement of TAG, we used an enzymatic colorimetric method with glycerol phosphate oxidase; inter- and intra-assay CV for TAG were 0·6 and 1·6 %, respectively. Total cholesterol (TC) was assessed with cholesterol esterase and cholesterol oxidase using the enzymatic colorimetric method. HDL-cholesterol was assayed after precipitation of apo B containing lipoproteins with phosphotungistic acid. Inter- and intra-assay CV for both TC and HDL-cholesterol were 0·5 and 2 %, respectively. When TAG concentrations were <400 mg/dl, the Friedwald formula was used to calculate LDL-cholesterol from the serum TC, TAG and HDL-cholesterol concentrations(Reference Friedewald, Levy and Fredrickson16).
Definition
Obesity and overweight in adolescents were defined as age- and sex-specific BMI > 95th and BMI between ≥85th and <95th percentiles in Iranian populations, respectively(Reference Kelishadi, Ardalan and Gheiratmand2).
Cardiometabolic risk factors were defined as follows: abdominal obesity as WC ≥ 90th percentile for age and sex, according to national reference curves(Reference Kelishadi, Gouya and Ardalan17); hypertriacylglycerolaemia as TAG ≥ 1·24 mmol/l(Reference Cook, Weitzman and Auinger18); low HDL-cholesterol as HDL-cholesterol <1·03 mmol/l(Reference Cook, Weitzman and Auinger18); hypertension as systolic and/or diastolic blood pressure ≥90th percentile for sex, age and height according to the Heart, Lung, and Blood Institute standards(19); high FPG ≥ 5·55 mmol/l according to American Diabetes Association(Reference Genuth, Alberti and Bennett20).
At baseline, adolescents were categorised into the following four different obesity phenotype groups based on their BMI and metabolic status: MHNW: normal BMI (<85th percentile) and ≤1 the previously mentioned parameters of cardiometabolic risk factors; MHO: overweight or obese (BMI ≥ 85th percentile) and ≤1 parameters of cardiometabolic risk factors; MUNW: normal BMI (<85th percentile) and ≥2 parameters of cardiometabolic risk factors; MUO: overweight or obese (BMI ≥ 85th percentile) and ≥2 parameters of cardiometabolic risk factors.
The MetS in adults was defined according to joint interim statement criteria(Reference Alberti, Eckel and Grundy21), as having more than or equal to three of the following conditions: (1) TAG ≥ 1·69 mmol/l or drug treatment, (2) HDL-cholesterol < 1·03 mmol/l in men and <1·29 mmol/l in women or drug treatment, (3) WC ≥ 89 cm in men and WC ≥ 91 cm in women(Reference Delavari, Forouzanfar and Alikhani22), (4) systolic and/or or diastolic blood pressure ≥130/85 mmHg or drug treatment and (5) FPG ≥ 5·55 mmol/l or drug treatment.
Statistical analysis
Continuous variables with normal and skewed distributions were expressed as means and standard deviations and medians and 25–75 interquartile ranges, and differences were assessed using the t test and Mann–Whitney test, respectively. Categorical variables were reported as percentages and were compared using Pearson’s χ 2 test. In the present study, as the exact time of the MetS incident was not known, this was considered as interval-censored data. Interval censoring takes into account the event happening between two time periods. Considering alternate interval censoring approaches, results were investigated using midpoint censoring, which converts interval-censored data to the right-censored data problems. Midpoint censoring was set to the midpoint between the last negative and the most recent positive event time minus the first positive observation for the incidence of obesity and to the time span between the first and the last observation for censored subjects. End points were considered as the time of incident adult MetS, and censoring was defined as lost to follow-up or end of follow-up. Cumulative incidences of the MetS with 95 % CI were calculated as the number of new cases of the MetS over the total number of subjects in that group minus half of the censored population. The person-year method was used to obtain MetS incidence rates (IR); IR is reported as the number of cases per 10 000 person-years. Cox proportional hazard modelling was used to estimate unadjusted, age- and adult BMI-adjusted hazard ratios (HR) along with 95 % CI for baseline groups of obesity phenotypes. The proportionality assumption was verified by assessing the correlation between the Schoenfield residuals and person-days along with observing log minus log plots (considering different groups as strata variables). All proportionality assumptions were generally met, with exception of the visual assessment for the MUO phenotype; it was noticed that the two curves are much closer until almost 6 years of follow-up, but they diverge greatly after that. Therefore, we performed an extended Cox model containing two heavy-side functions with the mentioned time cut-off. The corresponding model provides two HR for any time-dependent covariate, one that is constant above the cut-off time and the other that is constant below it. All analyses were performed using IBM SPSS for Windows version 20 (SPSS) and STATA version 12 (STATA Inc.), with two-tailed P values of <0·05 being considered significant.
Results
In the present study, 2159 children and adolescents (975 boys) with a mean age of 14·6 years were included. Subjects were divided into four groups based on their obesity phenotypes at baseline: MHNW (n 1211, 56·1 %), MHO (n 177, 8·2 %), MUNW (n 461, 21·4 %) and MUO (n 309, 14·3 %). Baseline characteristics, except for age, sex, BMI and WC, between participants were followed up and those missed to follow-up were not significantly different (online Supplementary Table S1). The statistically significant differences in age, sex, BMI and WC were not clinically important.
MHO and MUNW boys and girls were older and had higher prevalence of hypertension compared with MHNW (P < 0·001). Furthermore, the prevalence of abdominal obesity was significantly higher in MHO subjects compared with those who were of normal weight (P < 0·001; Tables 1 and 2).
Table 1. Baseline characteristics of boys based on different obesity phenotypes
(Mean values and standard deviations; medians and interquartile ranges (IQ 25–75))
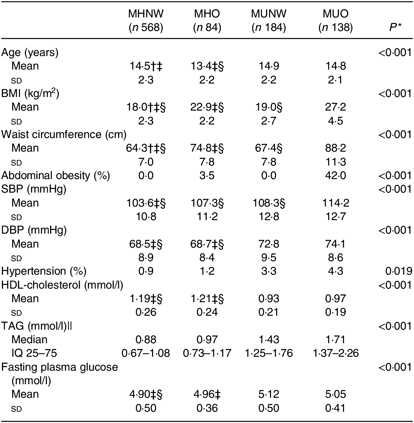
MHNW, metabolically healthy normal weight; MHO, metabolically healthy obese; MUNW, metabolically unhealthy normal weight; MUO, metabolically unhealthy obese; SBP, systolic blood pressure; DBP, diastolic blood pressure; LSD, least significant difference.
* P values are for the comparison across obesity phenotypes using ANOVA.
† Significantly different from MHO phenotype using post hoc LSD analysis test.
‡ Significantly different from MUNW phenotype using post hoc LSD analysis test.
§ Significantly different from MUO phenotype using post hoc LSD analysis test.
|| Log-transformed values were used for comparison.
Table 2. Baseline characteristics of girls based on different obesity phenotypes
(Mean values and standard deviations; medians and interquartile ranges (IQ 25–75))
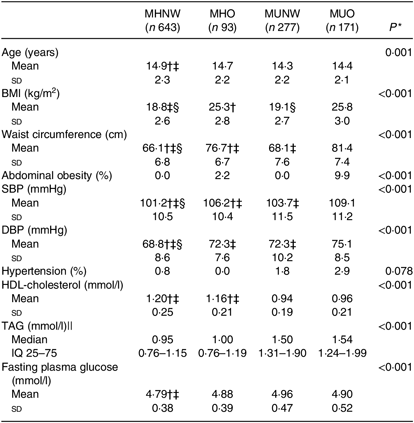
MHNW, metabolically healthy normal weight; MHO, metabolically healthy obese; MUNW, metabolically unhealthy normal weight; MUO, metabolically unhealthy obese; SBP, systolic blood pressure; DBP, diastolic blood pressure; LSD, least significant difference.
* P values are for the comparisons across obesity phenotypes using ANOVA.
† Significantly different from MHO phenotype using post hoc LSD analysis test.
‡ Significantly different from MUNW phenotype using post hoc LSD analysis test.
§ Significantly different from MUO phenotype using post hoc LSD analysis test.
|| Log-transformed values were used for comparison.
The IR of the MetS in early adulthood was 111·6 (95 % CI 98·7, 126·3) per 10 000 person-years, with higher values in boys (210·1 (95 % CI 183·0, 241·3)) compared with girls (39·7 (95 % CI 30·2, 52·1)). In boys, MHO and MUNW phenotypes predicted the MetS in adulthood compared with MHNW in the fully adjusted model, with higher HR for MUNW (2·52 v. 1·71). In both sexes, adolescents in the MUO group had a statistically significant higher HR to predict the MetS in adulthood compared with other groups. Regarding violation of proportionality assumption, based on the extended Cox model, MUO phenotype in both sexes with less than 6 years of follow-up had significant HR (3·33 in boys and 5·72 in girls) compared with the reference group after adjustment for adulthood BMI (Table 3).
Table 3. Association between different adolescent obesity phenotypes and incidence of the metabolic syndrome (MetS) in early adulthood in children and adolescents
(Incidence rates, hazard ratios and 95 % confidence intervals)
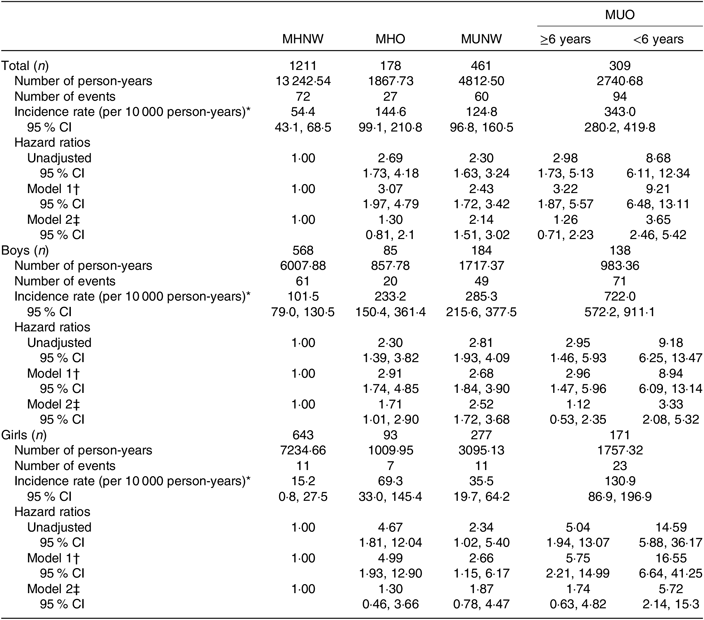
MHNW, metabolically healthy normal weight; MHO, metabolically healthy obese; MUNW, metabolically unhealthy normal weight; MUO, metabolically unhealthy obese.
* Incidence rate, number of incident MetS cases divided by person-years of follow-up.
† Model 1 is adjusted for age.
‡ Model 2 is adjusted for age and adulthood BMI.
Discussion
The present study provides evidence regarding the role of adolescent obesity phenotypes in prediction of adulthood MetS. MUNW and MHO phenotypes in boys, but not in girls, and MUO phenotype in both sexes with less than 6 years of follow-up increased the risk of adult MetS compared with MHNW, independent of adult BMI.
While some studies showed a positive correlation between higher childhood BMI and developing the MetS as adults later in life(Reference Magnussen, Koskinen and Chen7,Reference Graversen, Sørensen and Petersen12,Reference Sachdev, Osmond and Fall13,Reference Liang, Hou and Zhao14) , the predictive value of childhood BMI for developing adult MetS is a controversial issue. Lyold et al. in their systematic review reported that childhood obesity was associated with increased risk of adult MetS without adjustment of adult BMI; however, after adjustment for adult BMI, the association was ameliorated or inversed(Reference Lloyd, Langley-Evans and McMullen23). In an analysis of four prospective cohort studies, adolescents who had an increased BMI and remained obese or overweight as adults had an elevated risk of developing type 2 diabetes, hypertension, dyslipidaemia and carotid artery atherosclerosis(Reference Juonala, Magnussen and Berenson24). The role of childhood MetS in prediction of adult MetS is also unclear. While some studies provided evidence for tracking of the MetS from childhood into adulthood(Reference Juhola, Magnussen and Viikari25), the debate about the clinical utility of identifying the MetS in children and its association with future cardiometabolic risk factors continues(Reference Hosseinpanah, Asghari and Barzin26).
Obesity phenotypes, in other words, an interaction between BMI and CVD risk factors, have been used in a series of previous studies conducted in adults. Roberson et al. showed in their systematic review that the MHO obesity phenotype is an emerging phenotype with higher CVD risk compared with healthy normal-weight phenotypes(Reference Roberson, Aneni and Maziak27); however, to the best of our knowledge, only one study has examined the predictive role of adolescent obesity phenotypes in the development of adult MetS prospectively(Reference Koskinen, Magnussen and Sabin11), and there is still conflict as to whether children with MHO and MUNW phenotypes are more susceptible to future cardiometabolic risk factors(Reference Li, Chen and Srinivasan28).
In our study, the MHO phenotype in boys was associated with increased risk of adult MetS; however, in girls, after adjustment for adult BMI, this association disappeared. In the young Finns study, before adjustment for BMI, adolescents with MUNW, MHO and MUO phenotypes had increased risk of adulthood MetS; however, after adjustment for adult BMI, only the MUNW and MUO groups showed significant association with development of adult MetS(Reference Koskinen, Magnussen and Sabin11). The discrepancy between findings of the Koskinen et al. study, compared with ours may be explained by (1) the baseline older age of participants who had passed unstable stage of puberty, (2) longer follow-up led to older age for outcome assessment, (3) different definitions of ‘healthy metabolic status’ in adolescents which was ‘not having any components of the MetS’ and last but not the least (4) not using WC in the definition of adolescent MetS.
It is important to note that in our study despite the positive association between the MHO phenotype and adult MetS in boys, the strength of association was weaker than that of the MUNW one (1·71 v. 2·52), highlighting the importance of metabolic abnormalities compared with BMI in predicting adult MetS. In the present study, the IR (per 10 000 person-years) of the MetS was found to be significantly lower in girls compared with boys (39·7 v. 210·1 per 10 000 person-years), similar to the previously reported findings(Reference Pan and Pratt29,Reference Ahmadi, Gharipour and Nouri30) . This lower prevalence might be due to easier access of boys to junk food and eating more out of home. Moreover, it has been found that among boys TV watching >2 h/d, leisure time computer working >2 h/d and screen time >4 h/d were dramatically higher compared with girls(Reference Ahmadi, Gharipour and Nouri30). Similarly, a study among US adolescents aged 12–19 years from the National Health and Nutrition Examination Survey (1999–2002), using the Healthy Eating Index to assess the overall picture of diet quality showed that girls had better scores for fruits, vegetables, saturated fat, cholesterol and Na compared with boys(Reference Pan and Pratt29). Higher body fat and lower muscle mass, as a result of diverse sexual maturation, and body image and physical fitness in girls might be another reason(Reference Lim, Hwang and Cheon31,Reference Kirchengast and Marosi32,Reference Voelker, Reel and Greenleaf33) .
An unexpected finding, in both sexes, after adjusting for adult BMI, the risk of developing adult MetS in MUO phenotype was observed only in adolescents followed less than 6 years. It seems that in those individuals who had more follow-up time, the contribution of adult BMI would be more prominent than in those with less follow-up time, that is, those with <6 years of follow-up time had more adolescent metabolic characteristics than those with longer follow-up time. In fact, majority of adolescents with obesity in the category of <6 years of follow-up remained obese (60·9 %) compared with those having >6 years (35·7 %) at the end of study (data not shown). The reason for this finding might be the instability of MetS components in children and adolescents and occurrence of physiological insulin resistance in adolescents(Reference Steinberger, Daniels and Eckel34). An increased risk of adulthood MetS in adolescents with unhealthy metabolic characteristics, regardless of obesity status, was reported in a prospective longitudinal study with 25–30 years of follow-up(Reference Morrison, Friedman and Wang35), a finding illustrating the importance of adolescents’ unhealthy metabolic status, despite having normal weight. We can hypothesise that a considerable proportion of MUO subjects with more follow-up time achieved favourable BMI at the end of study. Accordingly, we have previously reported that adolescent overweight or obesity did not predict early adult MetS independent of adult BMI(Reference Hosseinpanah, Asghari and Barzin26).
Different cardiometabolic risk factors have different impacts on the incidence of adult MetS, for example, high WC/low HDL-cholesterol was the strongest predictor of adults MetS(Reference Hosseinpanah, Salehpour and Asghari6). However, as the present study was conducted on the subgroups of obesity phenotypes, we lacked sufficient power to determine the cardiometabolic risk factors that have higher impact on development of adult MetS.
Of the several limitations in the present study, we did not have data regarding puberty, nutrition intake and physical activity of participants. Furthermore, serum insulin was not measured to calculate insulin resistance as metabolic risk factor; however, its predictive value for developing the MetS is a controversial subject due to passing the pubertal status and instability of insulin concentration. Strengths of the present study deserve comments as well. Having the prospective nature of the study and its length of follow-up are main strengths. Nation-based definitions and cut-off points were used in the present study. Data and measures were obtained by trained technicians in order to reduce subjective errors and no self-report measure was used. Another important advantage of the present study is the use of WC, which indicates more accurately the actual adiposity mass of body as a metabolic risk factor compared with BMI used previously in several studies.
In conclusion, our study evidently is the first in the Middle East North Africa region that prospectively investigated the role of adolescent obesity phenotypes in prediction of the MetS in adulthood, independent of adult BMI. The present study gives deeper insight on the interaction of childhood excess weight and unfavourable cardiometabolic profiles, tracking BMI from childhood to adulthood. Obviously obesity in adolescents is associated with major concern regarding the development of the MetS in early adulthood; however, from a practical point of view, the lack of obesity in adolescents does not protect them from development of the MetS in the future (especially boys). This finding highlights the importance of prevention, screening and early control of metabolic abnormalities in adolescents even in the absence of obesity.
Acknowledgements
The authors express appreciation to the participants of the Tehran Lipid and Glucose Study for their enthusiastic support, and the staff of the Tehran Lipid and Glucose Study Unit of the Research Institute for Endocrine Sciences for their valuable help. We would like to acknowledge Ms. Niloofar Shiva for critical editing of English grammar and syntax of the manuscript.
This work was funded by a grant from the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
G. A. and F. H. conceptualised and designed the study, drafted the initial manuscript and reviewed and revised the manuscript. S. S. and S. H. collected data, carried out the initial analyses and reviewed and revised the manuscript. F. A. designed the data collection instruments, coordinated and supervised data collection and critically reviewed the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
The authors declare no conflicts of interest.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0007114519002344






