INTRODUCTION
The present gold standard for the treatment of cutaneous leishmaniasis (CL) is pentavalent antimonials either sodium stibogluconate (Pentostam) manufactured by GlaxoSmithKline, or meglumine antimoniate (Glucantime) manufactured by Aventis. These drugs are quite toxic, and associated with severe side-effects including myalgia and arthralgia, fever, headaches and toxicity to the cardiovascular and hematologic systems and to organs such as the kidneys and the pancreas, though less toxic than the first antimonial introduced in the 1930's as urea stilbamine. They are given by injection and usually administered either intramuscularly or intravenousy for 3 weeks or intralesionally for 7 or more weeks. Other treatments for CL including oral miltefosine has debilitating side-effects and require weeks as do antifungal drugs, local liquid nitrogen and paromomycin ointments. None of these, however, usually have the cure rate of either the pentavalent antimonials or heat therapy. Amphiteracin in liposomes is useful for visceral leishmaniasis, but is not used in CL because of expense. That is why the successful introduction of radiofrequency-induced heat therapy (RFHT) using a Thermomed 1.8 instrument (Thermosurgery Technologies, Inc. Phoenix, AZ), administered in a single application, with minimal toxic side-effects is so important for the treatment of CL.
REVIEW OF STUDIES
de Silviera and Brenner (Reference da Silveira and Brener1950), first used heat treatment based on reports that CL patients treated with trivalent arsenic and other toxic products improved when they became febrile. They injected one patient with CL and one with mucocutaneous leismaniasis (MCL) with the bacteria H aemophilus ducreyi. Twenty days later both MCL and CL lesions had closed.
Berman and Neva (Reference Berman and Neva1981), studied the effect of heat on Leishmania in human macrophages and found that L. tropica multiplies faster at 35 °C than at 37 °C and were completely eliminated at 39 °C, whereas L. donovani grew equally well at 35 and 37 °C and only 40% were eliminated at 39 °C.
Sacks et al. (Reference Sacks, Barral and Neva1983), compared the thermosensitivity of New and Old World Leishmania. All eight CL strains grew optimally at 35 °C. Three of the four New World strains were completely destroyed at 39 °C, whereas all L. tropica strains survived and grew well at 39 °C, concluding that L. tropica may be less responsive to heat therapy than New World strains. They were not considering the use in the future of the ThermoMed instrument, which one applies 50 °C of heat.
Neva et al. (Reference Neva, Petersen, Corsey, Bogaert and Martinez1984) treated three patients with diffuse CL with heated water 39–41 °C circulating through a pad around the lesions for cumulative total of 20 h over several days. They improved as documented by biopsy and culture.
Ordinary CL was not affected by this treatment.
Junaid (Reference Junaid1986) carried out the treatment of CL in Baghdad, Iraq, using an apparatus that produced infrared rays that raised the temperature over the lesion to 55 °C for about 5 min. Of 178 patients, only 16 needed re-treatment after 3 weeks. He further noted that treatment of only one lesion ‘provokes an immune response in patients’ that causes all the other lesions to disappear in 5–6 weeks. This systemic response was also observed in a subsequent study by Lobo et al. (Reference Lobo, Soares, Correria, der Freitas, Oliveira, Nakatani, Netto, Badaro and David2006) described below.
Aram and Leibovici (Reference Aram and Leibovici1987), used ultrasound-induced hyperthermia at 42 °C for treatment of CL, delivered 2–3× a week for 10–15 treatments to 28 lesions in 18 patients aged 4–57 years. Twenty-two of the 28 lesions (78.5%) showed compete resolution in 5–10 weeks. One patient with two lesions failed to respond. Others only responded partially or treatment had to be stopped because of headache as the lesions were on the face.
Navin et al. (Reference Navin, Arana, Arana, de M´erida, Castillo and Pozuelos1990) using an early model of ThermoMed carried out a placebo-controlled clinical trial of meglumine antimonate (Glucantime) vs. localized RFHT of CL in Guatemala. Sixty-six Guatemalans with parasitology proven CL were divided into three equal groups, 1st, Glucantime, 859 mg antimony daily given IM for 15 days, 2nd, localized RFHT at 50 °C for 30 s, three treatments at 7 day intervals, and 3rd, a placebo. Of the 53 isolates identified, 40 were L.(V) braziliensis and 13 were L. mexicana. Thirteen weeks after treatment patients with completely healed lesions and negative parasitology were with Glucantime 73% (16), Heat Rx 73% (16) and Placebo 27% (9). The cure rate of patients with L.(V) braziliensis was: Glucantime 79% (11 of 14), Heat 64% (9 of 14) and Placebo 0% (0 of 11).
Levine (Reference Levine1992) reported a case of a Sudanese patient with multiple lesions caused by L. tropica who was cured 6 months after receiving RFHT, a single treatment at 50 °C for 30 s.
Velasco-Castrejon et al. (Reference Velasco-Castrejon, Walton, Rivas-Sanchez, Garcia, Lazaro, Hobart, Roldan, Floriani-Verdugo, Munguia-Saldana and Berzaluce1997) carried out a feasibility trial for an endemic area, where the organism was L. mexicana. 201 patients with ages ranging from 2 to 75 years, 63% males, 37% female were treated with a single application on an anesthetized lesion at 50 °C for 30 s using a ThermoMed instrument. At 4 weeks, 95% of 122 patients available for evaluation were totally healed and at 8 weeks 90% of 191 were totally healed.
Lobo et al. (Reference Lobo, Soares, Correria, der Freitas, Oliveira, Nakatani, Netto, Badaro and David2006) carried out a random study on 37 patients with CL that showed that RFHT elicited a systemic cytokine response similar to that of treatment with Glucantime. The RFHT group had 17 patients, the Glucantime 20, with no significant difference in the gender of patients or ulcer size. RFHT was applied at 50 °C for 30 s to anesthetized lesions using a ThermoMed Model 1.8. (Fig. 1), which had received 510 (K) clearance from the Food and Drug Administration for treatment of CL. Glucantime was given 20 mg kg−1 for 20 days. At 28 days, the RFHT group received Glucantime for 20 days since the organism was L.V. braziliensis, which can cause MCL, and we did not know whether there would be a systemic effect of the heat therapy needed to prevent MCL. Biopsies were taken at 0, 14 and 28 days, and lymphocyte proliferation, CD4/CD8 characterization and cytokine assayed at those times. There was a significant drop in the levels of interferon (IFN)-γ, IL-5 and tumour necrosis factor (TNF)-α at 28 days compared with day zero P < 0.01 (Fig. 2). There was no statistical difference, however, between the two therapy groups. Further, there was no significant difference in the healing of the lesions by 28 days: 71% in the RFHT group and 89% in the Glucantime group. In two patients with lesions on each extremity, the lesion not treated by RFHT was also healed, further indicating a systemic effect (Fig. 3). A possible mechanism for this systemic effect is that the RFHT causes a secondary burn killing and disrupting most of the parasites, and this is followed by an influx of inflammatory cells including lymphocytes and macrophages, which could lead to an immune response similar to introducing a vaccine. During this study, about a dozen patients resistant to Glucantime, having received 60–100 days of this therapy without healing, responded well to RFHT.

Fig. 1. Thermomed™ 1.8. It is shown heating up on it way to 50 °C. The three different sized applicators are shown, the thinner one for thin skin, such as on the face, the largest when deeper penetration is needed on the extremities. From David JR, personal photos.
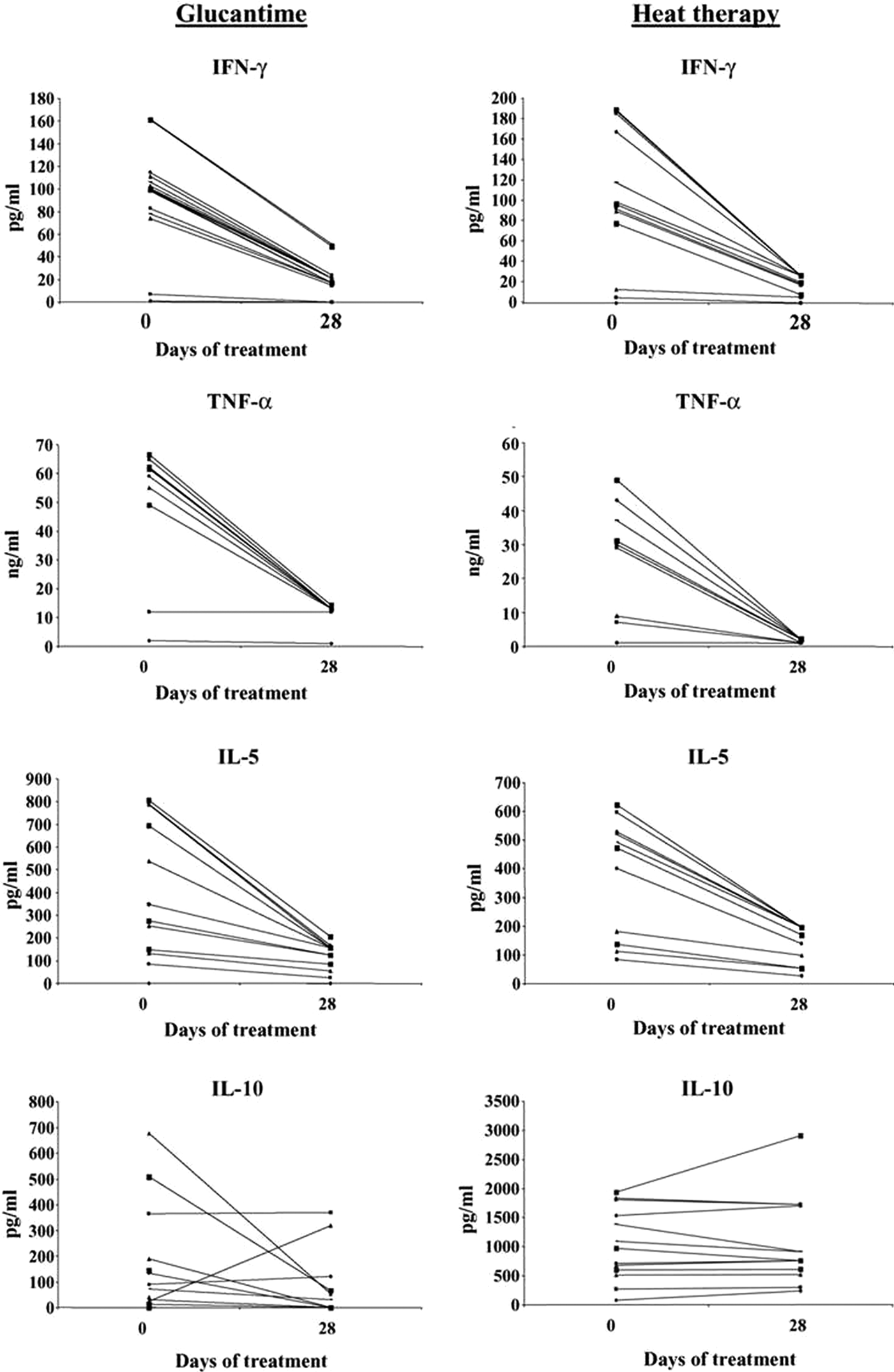
Fig. 2. The cytokine response of peripheral blood mononuclear cells (PMBC) of patients with Glucantime or heat therapy. Levels of IFN-γ, TNF-α, IL-5 and IL-10 in the supernatants of PMBC stimulated with leishmanial antigen before day O and 28 days after heat therapy or Glucantime treatment. From Lobo et al. (Reference Lobo, Soares, Correria, der Freitas, Oliveira, Nakatani, Netto, Badaro and David2006).

Fig. 3. Systemic effect of heat treatment on cutaneous leishmaniasis (CL) lesions. Two CL lesions of a patient included in the heat treatment therapy group. Heat treated CL lesion after day 0 (A) and 28 days (B). Contralateral untreated lesion at day 0 (C) and 28 (D). From Lobo et al. as Fig. 2.
Reithinger et al. (Reference Reithinger, Mohsen, Wahid, Bismullah, Quinnell, Davies, Kolaczinki and David2005) determined the efficacy of thermotherapy to treat CL caused by L. tropica in Kabul, Afghanistan in a randomized controlled trial. There were three groups: Group 1 – Single application of RFHT for 50 °C for 30 s on anesthetized lesion using a ThermoMed Model 1.8; Group 2 – Intralesional sodium stibogluconate (SSG) 2–7 ml once a week for 5 weeks; Group 3 – Intramuscular SSG, 20 mg kg−1 for 21 days. A total of 401 patients older than 5 years with a single lesion were included in the study. Parasitological diagnosis was carried out by dermal scrapings or biopsy, Giemsa stained and examined under a microscope. A subset of 60 samples in each group were tested by polymerase chain reaction (PCR) to confirm L. tropica. Patients were followed to measure size of lesion and note side-effects during 1, 4, 8, 16, 24 weeks post-therapy (wpt). Cure was complete re-epithelialization by 100 days post-therapy (dpt) and no relapse by 24 wpt. Failure was incomplete re-epithelialization by 100 dpt, or relapse by 24 wpt. The cure rate after 100 days was RFHT 69% (75/108) patients, Intralesional 75% (70/93) and Intramuscular 45% (26/58). (Fig. 4). Healing time after start of treatment was RFHT 53 days, Intralesional 75 days and Intramuscular >100 days. Between the RFHT and the Intralesional group, no statistical association was shown between age, sex, body weight, lesion size, lesion location or lesion duration and trial outcome. No statistical difference was observed between cure by RFHT and Intralesional therapy. The study showed that a single treatment of RFHT was as effective as the administration of intralesional SSG and more effective than intramuscular SSG in the treatment of CL due to L. tropica. The time to cure was shorter with RFHT than with SSG in a Kaplan–Meier analysis.
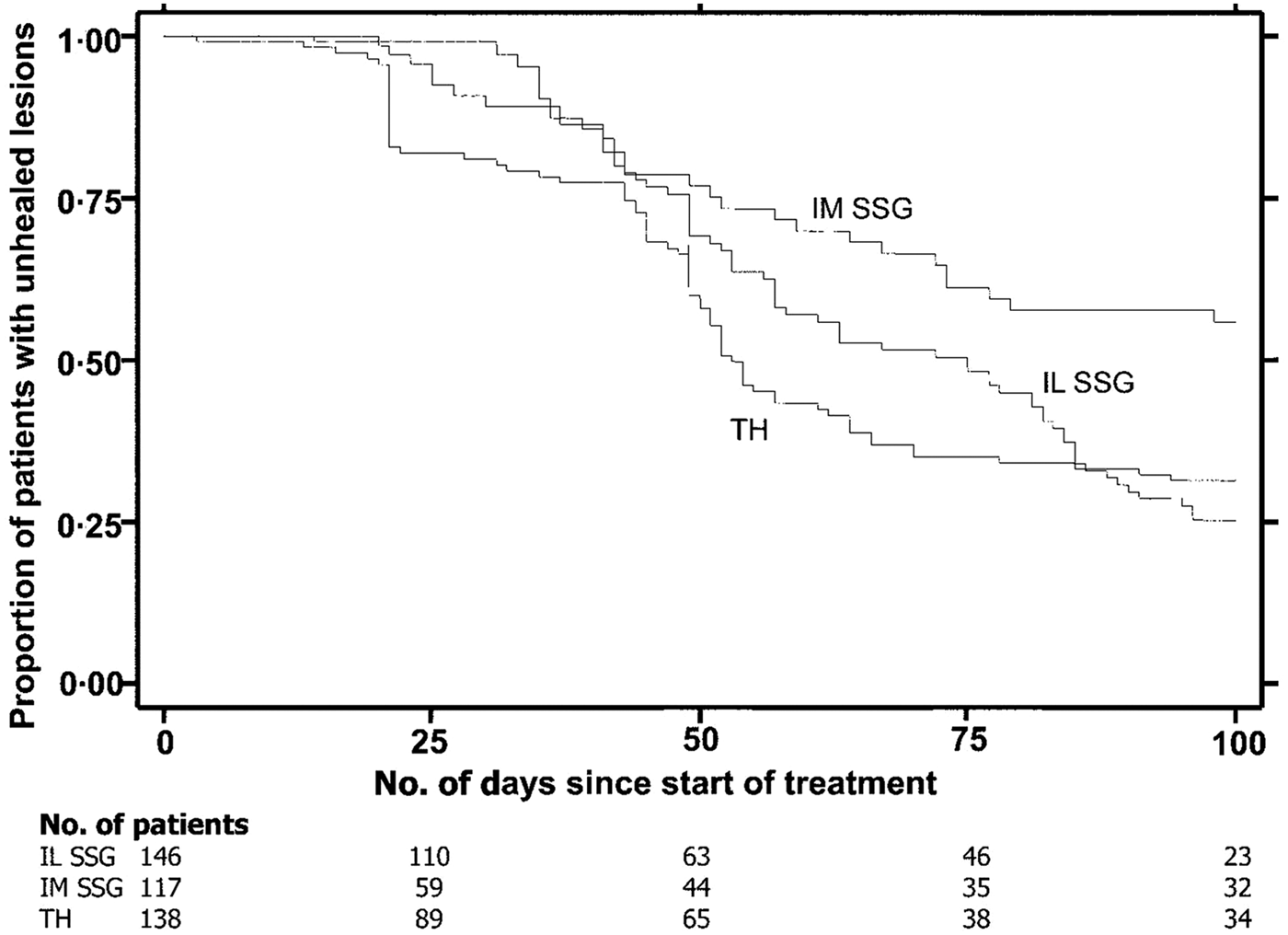
Fig. 4. Survival analysis of time to healing of cutaneous leishmaniasl lesion, with data on number of patients enrolled in the trial at baseline and 4 other time points. IL, intralesional; IM, intramuscular; SSG, sodium stilbogluconate; TH, thermotherapy. From Reithinger et al. (Reference Reithinger, Mohsen, Wahid, Bismullah, Quinnell, Davies, Kolaczinki and David2005).
Willard et al. (Reference Willard, Jeffcoat, Benson and Walsh2005) reported on CL in soldiers from Fort Campbell, Kentucky returning from Operation Iraqui Freedom. Leishmania major was the main species. Twenty-six soldiers (24 men, two women), were treated with ThermoMed. There was one failure. Eleven patients had one lesion, seven had two lesions, five had three lesions, one had four lesions and two had five lesions. They concluded that all soldiers expressed general satisfaction with the RFHT and all dermatology providers deemed the treatment safe, well tolerated and effective. Among the treated lesions, including those on dark skinned persons, no exaggerated scarring or colour changes occurred beyond those previously described in CL.
Aronson et al (Reference Aronson, Wortmann, Byrne, Howard, Bernstein, Marovich, Polhemus, Yoon, Hummer, Gasser, Oster and Benson2010) reported a randomized controlled trial of RFHT vs intravenous SSG for the treatment of L. major in military personnel who had been infected in Iraq or Kuwait. There were 27 patients in each group. Diagnosis of Leishmania species was by PCR and isoenzyme analysis. RFHT was administered on an anesthetized lesion at 50 °C for 30 s and compared with SSG 20 mg kg−1 day−1 administered IV for 10 days, infused over 10–50 min for a total of ten doses. Patients randomized to RFHT received oral antibiotics for secondary bacterial infections of their lesions prior to treatment. The results can be seen on (Fig. 5), showing that the cure rates were similar. Side-effects for the RFHT group were the result of the expected secondary burn, which were statistically significant compared to SSG such as oozing and blistering, whereas side-effects for SSG were statistically significant compared with RFHT. These side-effects were gastrointestinal symptoms, abdominal discomfort or pain, and musculoskeletal and nervous system symptoms. The conclusion of the trial was that skin lesions due to L. major treated by heat delivered by the ThermoMed device healed at a similar rate and with less associated systemic toxicity than lesions treated with intravenous SSG.
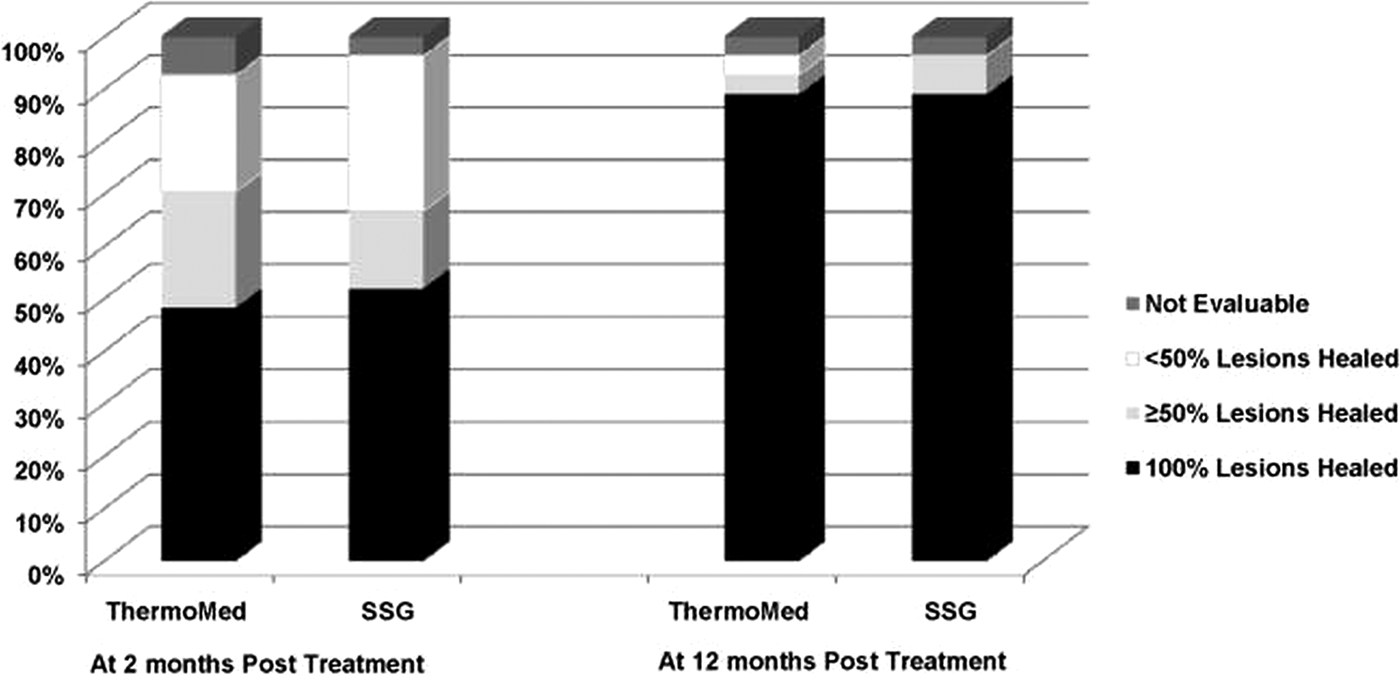
Fig. 5. Consensus treatment efficacy at two and twelve months follow-up. From Aronson et al. (Reference Aronson, Wortmann, Byrne, Howard, Bernstein, Marovich, Polhemus, Yoon, Hummer, Gasser, Oster and Benson2010).
Prasad et al. (Reference Prasad, Ghiya, Bumb, Kaushal, Saboskar, Lezama-Davila, Salotra and Satoskar2011) reported on thermotherapy on a 34-year-old truck driver with HIV infection. He also had 6-month-old red plaques on his hand, which were biopsied and showed L. tropica by restriction-fragment length polymerization PCR. HIV/AIDS was confirmed by ELISA HIV TRO-DOT and HIV COMB. His viral load was 145 000 and his CD4+T cell count was 180 µL−1. The HIV infection was treated with SSG intralesionally, 0.5 ml (100 g L) twice a week for 6 weeks. There was no improvement of the CL at 24 weeks despite the rise of CD4 + T cells to 240 µL−1. He was then treated with RFHT using a ThermoMED 1.8 instrument and showed complete healing in 12 weeks. In addition to this patient, RFHT was also effective in a 28-year-old HIV-infected man with CL who did not responded to SSG and subsequent rifampicin for 3 months. Both patients have remained free of CL for a year after treatment. The authors conclude that RFHT should be considered as the first-line treatment for CL in HIV-infected patients.
López et al. (Reference López, Robayo, Vargas and Vélez2012) reported Thermotherapy as an alternative to the treatment of American CL in five military health centres in Columbia. There were 292 volunteers with parasitology diagnosed CL by Giemsa-stained smears and PCR were randomly divided into two groups: 149 receiving a single application of thermotherapy at 50 °C for 30 s on the anesthetized lesion and 134 getting Glugantime 20 Sb5 −1kg−1 day−1 IM for 20 days.
The healing response in the Glugantime group among patients with L. (V) panamensis and L. (V) braziliensis was 72 and 65%, respectively. In the thermotherapy group, the healing response in the L.(V) panamensis and L.(V) braziliensis patients was 58 and 53%, respectively. The treatment with Glucantime was statistically superior to thermotherapy for treatment of CL in Columbia; however, Glucantime treatment was associated with severe side-effects including myalgia and arthralgia, fever, headaches and toxicity to organs such as the kidney, the pancreas and the hematologic and cardiovascular systems. These side-effects were not associated with thermotherapy, which only caused local pain 4 days after the initial treatment. Considering the side-effects and increase in costs and treatment adherence problems with Glucantime, they consider that thermotherapy should be the first line of treatment for CL. Thermotherapy is also a valid alternative in patients with renal, hepatic and cardiac illnesses who cannot receive Glucantime.
Bumb et al. (Reference Bumb, Prasad, Khandelwal, Aara, Mehta, Ghiya, Salotra, Wei, Peters and Satoskar2013) reported on the long-term efficacy of a single dose of RFHT vs intralesional antimonials for CL in India. One hundred patients were randomly assigned. Fifty received RFHT at 50 °C for 30–60 s on the anesthetized lesion using a ThermoMed 1.8 instrument. All these patients received oral non-steroidal anti-inflammatory drugs and topical antibacterial cream for 5 days. The other 50 received intralesional injections of SSG 50 mg cm−2 of lesion, twice a week for seven injections. Patients that had secondary bacterial infections of the lesions were treated with systemic or topical antibiotics without anti-leishmanial activity. Lesions were monitored every 15 days for 4 months and then at 5, 6, 9, 12 and 18 months post-treatment. Lesions were healed in 47 of the 50 patients (94%) in the RFHT group and 46 of the 50 patients (92%) in the SSG group at week 12. At 6 months post-treatment cure rates in the RFHT and SSG groups were 98 and 94%, respectively. Age, sex and lesion size had no effect on cure rates. No relapse of infection was recorded in cured patients in either group up to 12–18 months after initiating treatment (Fig. 6). Skin biopsies of cured lesions in eight out of eight from the RFHT group and three out of three from SSG group at 12 months showed minimal fibrosis and were negative for Leishmania by PCR test. Further, RFHT induced less scaring compared with SSG (Fig. 7). They concluded that a single application of RFHT is safe, cosmetically acceptable and effective inducing long-term cure for CL. An editorial by Bumb and Satoskar was printed in 2011.

Fig. 6. Efficacy of radiofrequency-induced heat therapy (RFHT) vs intralesional sodium stilbogluconate (SSG) in the treatment of cutaneous leishmaniasis (CL). (A) Survival analysis of time to heal after heat therapy (dotted blue) or intralesional SSG- injection (straight black). (B) A 79 year-old man with a lesion on the face/nose and was administered RFHT under local anesthesia. (C) The same patient 6 months post-treatment showing compete healing of the lesion with fine scarring. (D–F) Histopathology of the arm lesion from another patient prior to RFHT (D) and after 6 months (E) and 12 months (F) post-treatment. Original magnification: ×40. From Bumb et al. British Journal of Dermatology 2006; 168(5) 1114–1119.

Fig. 7. Scarring associated with radiofrequency-induced heat therapy (RFHT) is less than with intralesional sodium stilbogluconate (SSG). (A) A cured lesion from a patient treated with RFHT; (B) a cured lesion from a patient treated with intralesional SSG injections. Lesions were located on the upper extremity in both patients. Note that RFHT results in fine scarring and minimal pigmentation compared with intralesional SSG. From Bumb et al. as in Fig. 6.
Ahuja et al. (Reference Ahuja, Bumb, Mehta, Prasad, Tanwar and Satoskar2012) reported the successful treatment of canine CL using RFHT in two pet dogs using a single application on the anesthetized lesion from a ThermoMed 1.8 instrument. They found that RFHT induced complete clinical cure, lesions healed within 45 days and the dogs remained disease free for the last 16 months. This is the first report that showed that RFHT worked in canine CL
Safi et al. (Reference Safi, Davis, Nadir, Hamid, Robert and Case2012). This was a control study of patients with CL in Kabul, Afghanistan caused by L. tropica. Three hundred and eighty-two patients were randomly assigned to a single localized treatment of heat therapy or 5 days of intralesional injection of Glucatime. The cure rate of the heat therapy group was 82.5% compared with 74% in the Glucantime group. The authors concluded that heat therapy was more effective than 5 days intralesional injection of Glucantime, and that heat therapy was more cost-effective, with fewer side-effects, of shorter duration and with better patient compliance than intralesional Glucatime.
Reveiz et al. (Reference Reveiz, Maia-Elkhoury, Nicholls, Romero and Yadon2013) reported a systemic review update, using meta-analysis of interventions for American cutaneous and MCL. They included, however, only one paper on RFHT, the Lopez et al. (Reference López, Robayo, Vargas and Vélez2012) paper described above. They mention that the rational for administrating local treatment such as RFHT is that the risk of developing the mucosal form is low, and not necessarily prevented by systemic treatment; further, localized treatments are better tolerated and have less frequent and severe adverse effects as compared with systemic treatments.
Valencia et al. (Reference Valencia, Miller, Witzig, Boggild and Llanos-Cuentas2013) reported on a low-cost thermotherapy for CL in Peru. They used a low-technology device named Hand-held Exothermic Crystallization Thermotherapy for CL (HECT-CL), a sodium acetate heating pad, invented by R. Witzig, which delivers a safe, reliable and renewable conduction heat. Twenty-five patients with parasitologically confirmed CL by Giemsa staining, PCR, cysteine protenase C gene, and heat-shock-protein 70, used to distinguish L. (V) braziliensis and L. (V) guyanensis from each other and from non L. (V) braziliensis and guyanensis parasites. Twenty-five patients completed the study, 16 males and nine females. The initial temperature was 50–54 °C and applied to each lesion, some without anesthesia, for 3 min repeated daily for 7 days, and followed for 6 months by direct observation. Overall definite cure was 60%. Thirteen patients meeting minimally significant exclusion criteria had a cure rate of 68%, and of these 75% had experienced CL relapse after prior antimonial treatment.
Lopez et al. (Reference Lopez, Cruz, Godody, Robledo and Velez2013) reported that RFHT was effective and safer than miltefosine for CL in Columbia using a controlled open randomized phase III clinical trial with patients from the Columbian army. A total of 145 patients received 50 mg of miltefosine three times a day for 28 days. A total of 149 patients received a single application of RFHT to the lesions using a ThermoMed instrument at 50 °C for 30 s. Both groups were comparable with respect to sociodemographic, clinical and parasitological characteristics. In 56% of the patients, the Leishmania species was isolated and identified. In 81 patients treated with miltefosine in whom 30 were L.(V) panamensis and 51 were L. (V) braziliensis. In 83 patients treated with RFHT, 24 were L. (V) panamensis and 59 were L. (V) braziliensis. No significant difference was found for treatment efficacy between the species responsible for infection. For the miltefosine group definitive cure at 6 months follow-up was 59%, failure rate was 26% and lost to follow-up was 14%. For RFHT, the definitive cure at 6 months was 59%, failure rate 33% and lost to follow-up 9%. Midway through treatment, miltefosine was associated with greater occurrence of headache, vomiting, nausea and anorexia, and at the end of treatment with greater frequency of myalgia, arthralgia, headache, vomiting and anorexia. RFHT was associated with pain at the lesion site following application of treatment. In Columbia, the authors do not think it is necessary to treat all patients infected with the Viannia species with systemic drugs because the incidence of MCL is <0.5%, and systemic treatment does not guarantee the absence of MCL.
Agrawal et al. (Reference Agrawal, Khandelwal, Bumb, Oghumu, Salotra and Satoskar2014) reported on paediatric CL in an endemic region of India situated in the Thar Desert in Rajasthan State. A total of 151 patients with 217 lesions were reported in the study period. The ages were from 0.25 to 5 years, with 41.7% of case between 2 and 4 years. The face was the most common site involved, the lesions being plaque-type or papulonodular. Smears were positive for Leishmania in 70% of 130 cases. Parasites were identified in 13 randomly selected cases as L. tropica by PCR.
Fifty-eight patients received intralesional SSG, RFHT was used in nine patients, 50 received oral rifampicin, three received oral dapsone, 11 received both dapsone and rifampicin, and 12 patients received both intralesional SSG and oral rifampicin. Nine patients were lost to follow-up. Complete cure of the lesions was obtained within an average period of 9.7 weeks with a range of 4–20 weeks. Skin slits from 80 of 84 completely cured cases that were positive at the time of diagnosis were negative in all cases. Complete cure was seen in 125 (82.78%) of patients at 20 weeks. Complete cure for SSG patients was 84.4%, for RFHT it was 91.8%, for oral rifampicin it was 82%, for oral dapsone it was 66.67%, and for the combination of dapsone and rifampicin it was 90.1%. All 12 patients receiving a combination of intralesional SSG and oral rifampicin were cured. Thus all methods were effective. RFHT is a good alternative because of its minimal systemic toxicity, and only a single treatment session is required.
Shah et al. (Reference Shah, Memon, Auwj-e-Shamim, Baqi and Witzig2014) reported in low-cost thermotherapy of CL in Sindh, Pakistan using the HECT-CL method described in the paper by Valencia et al. (Reference Valencia, Miller, Witzig, Boggild and Llanos-Cuentas2013), administered for 3 min for 7 days, Twenty-three patients could be evaluated for full treatment. By the final 180-day evaluation, 19 (83%) had been cured and the application was well tolerated with no side-effects.
Lakhal-Naouar et al. (Reference Lakhal-Naouar, Slike, Aronson and Marovich2015) reported on the immunologic healing response in CL treatment with localized heat using RFHT or systemic antimonial therapy. They studied the peripheral blood immune cells in a cohort of 54 cutaneous L. major subjects treated with SSG and RFHT. Multiparameter flow cytometry, proliferative assays and cytokine production was analysed in order to investigate the differences in immune response before and after treatment. Healing CL led to a significant decline of circulating T and NKT (natural killer T)-like cells, with an expansion in natural killer (NK) cells regardless of the treatment modality. There was a decrease in antigen-specific CD4+T cell proliferation seen with CD8+T cell depletion. In the healing and healed state, fewer circulating regulatory T cells were seen as well as reduced IFN-γ production and an overall contraction in poly-functional CD4+T cells. The authors conclude that healing of CL alters the circulating lymphocyte populations and subsets of T, NK and NK-like cells. The immunologic healing, through either local or systemic treatment, culminates in similar changes in frequency, quality and antigen-specific responsiveness with immunomodulation possible by a CD8+ T cell-dependent mechanism.
Cardona-Arias et al. (Reference Cardona-Arias, Vélez and López-Carvajal2015) reported on the efficacy of treating leishmaniasis using a meta-analysis of controlled clinical trials in 12 databases also see Cardona-Arias et al. (Reference Cardona-Arias, López-Carvajal, Tamayo-Plata and Vélez2018). The results included 622 patients who underwent thermotherapy, with an efficacy of 73% [confidence intervals (CI) = 69.6–78.7%], and 667 patients who underwent systemic therapy with an efficacy of 70.6% (95% CI = 67.1%. 1–74.1%) (see Fig. 8). Heterogeneity between studies, good sensitivity for the combined methods, and no publication bias were observed. The relative risk of the treatment was 1.02 (95% CI = 0.91–1.15), showing that the effectiveness of thermotherapy is equal to that of pentavalent antimonial drugs. They conclude that due to efficacy, greater safety and lower cost, thermotherapy should be the first treatment option for CL in areas where the prevalence of the mucocutaneous form is low and in patients with contraindications for systemic therapy, such as kidney, liver and heart disease, as well as in pregnant women, infants and patients with immunodeficiency virus infections and acquired immunodeficiency syndromes.
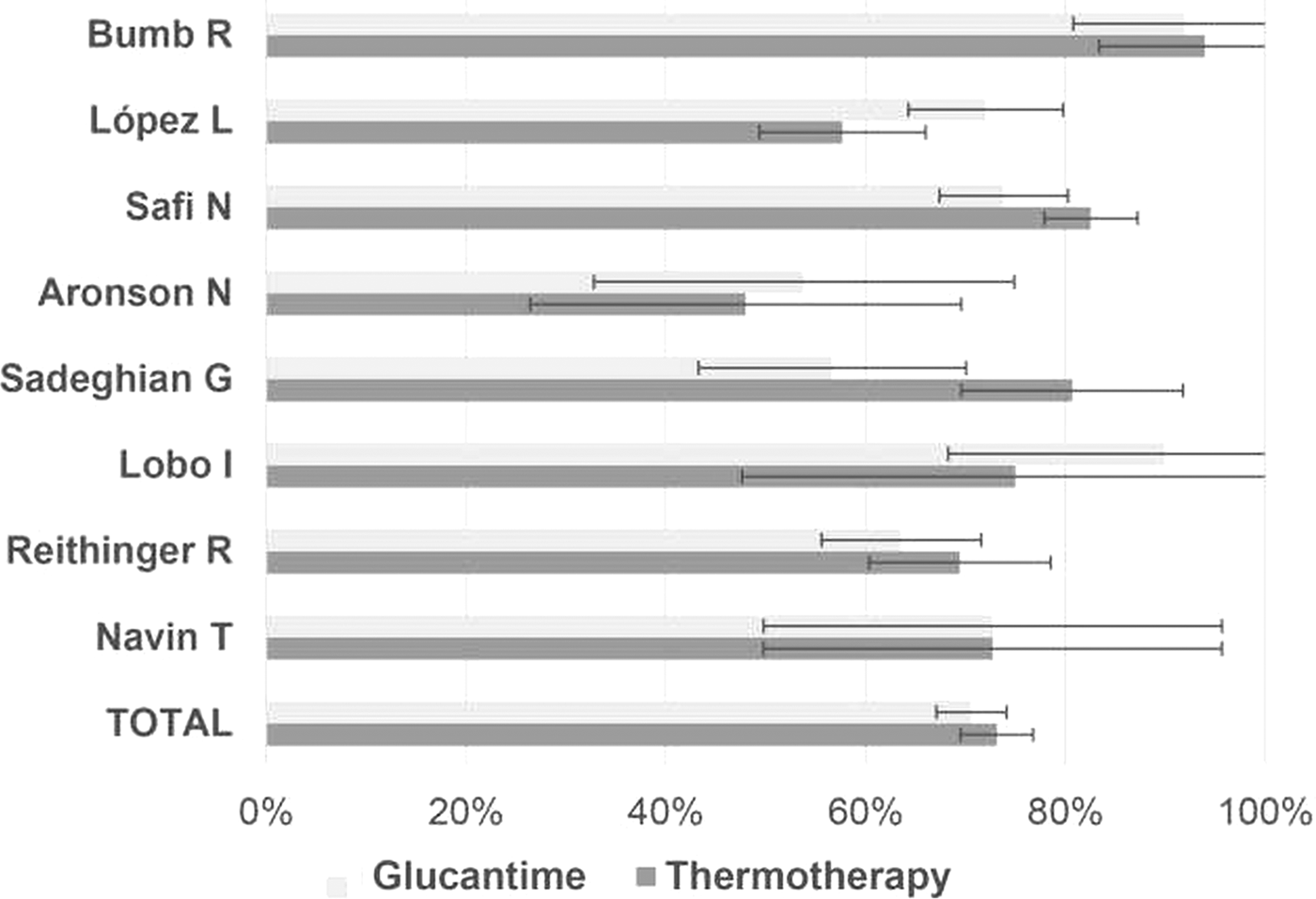
Fig. 8. Efficacy of Glucantime and thermotherapy for the treatment of cutaneous leishmaniasis (proportion with 95% confidential intervals). From Cardona-Arias et al. (Reference Cardona-Arias, Vélez and López-Carvajal2015).
Refai et al. (Reference Refai, Madarasingha, Sumanasena, Weerasingha, De Silva, Fernandopulle, Satoskar and Karunaweera2017), reported the Efficacy, Safety and Cost-Effectiveness of RFHT in the treatment of L. donavani-induced CL in a randomized controlled clinical trial in Sri Lanka. They mention that the standard treatment is the multiple painful doses of intralesional SSG, and as treatment failures were increasingly reported, the investigation of an alternative treatment was needed. A single-blinded non-inferiority randomized control trial was conducted. Laboratory confirmation of the diagnosis was made based on microscopy and culture of lesions before enrolment in the study. The thermotherapy group, containing 98 patients with single lesions, received a single session of RFHT given at 50 °C for 30 s using ThermoMed 1.8. The control group of 115 patients received 1–3 ml SSG intralesionally weekly until cure or up to ten doses. Patients were followed every 2 weeks. Cost of treatment was assessed using a scenario building technique. Cure rates at 8 weeks was 44.5% for RFHT, 28% for SSG, at 10 weeks was 56.5% for RFHT, 40.8% for SSG; and at 12 weeks 65.9% for RFHT, 59.4% for SGG, with no major adverse effects. Cure rates for RFHT were significant higher than SSG at 8 weeks (P = 0.009) and 10 weeks (P = 0.03) but comparable after. Cost for RFHT was seven times less (USD = 1.54/patient) than SSG (USD = 11.09/patient). From this trial, they conclude that a single application of RFHT is safe, cost-effective and convenient compared with intralesional SSG in the treatment of L. donovani CL, and thus RFHT should be considered with multiple benefits to the patient and the national healthcare system.
DISCUSSION
The ThermoMed™ device remains the most supported by randomized clinical trials and is WHO recommended as an alternative therapy for all American CL species. WHO, Control of Leishmaniasis, (2010). It has been approved by the FDA for use for Cutaneous Leishmaniasis (FDA 510 K number K021117). It is used by the WHO and Center for Disease Control and Prevention. Cost of treatment with antimonials is between US$100 to 200 per patient depending on the country. The ThemoMed™ now costs US$6500, so in a country where antimonial cost US$100, the instrument will have paid for itself after 65 patients, and if $200, after 33 patients.
Pentavalent antimonials Glucantime and SSG are quite toxic and associated with severe side-effects including myalgia and arthralgia, fever, headaches and toxicity to the cardiovascular and haematologic systems and to organs such as the kidneys and the pancreas when given systemically. There are less side-effects with intralesional injections, but this is almost always done without anaesthetics and is very painful and also required several weeks of administration.
The ability to obtain a cure with just one application of treatment is especially useful in rural areas where compliance is often poor as it may be difficult for patients to be seen regularly by medical professionals administering the drugs. The observation that RFHT gives a systemic response, as measured by the cytokine response reported by Lobo et al. (Reference Lobo, Soares, Correria, der Freitas, Oliveira, Nakatani, Netto, Badaro and David2006) and Lakhal-Naouar et al. (Reference Lakhal-Naouar, Slike, Aronson and Marovich2015) and by changes in the lymphocytes reported by the latter, are similar to the systemic administration of pentavalent antimonials, which is important in considering RFHT in areas where MCL caused by Leishmania of the Viannia species is low. The observation that treatment of one lesion may be followed by the cure of an untreated lesion on the same patient, as reported by Junaid (Reference Junaid1986), and by Lobo et al. (Reference Lobo, Soares, Correria, der Freitas, Oliveira, Nakatani, Netto, Badaro and David2006), appears to confirm the systemic response to heat therapy. This observation should be confirmed in future trials involving patents with multiple lesions, by treating half of the lesions and observing whether or not the untreated lesions cure. The mechanism for such a systemic effect is unknown. The secondary burn caused by the therapy results in the destruction of parasites releasing antigen that may stimulate the incoming lymphocytes and macrophages to produce an anti-Leishmania immune response similar to a vaccine. The burn may also induce pro-inflammatory cytokines such as TNF-α, and migration inhibitory factor known to be involved in the immunity to Leishmania parasites.
SUMMARY
The conclusion of this review is similar to the conclusion of these many clinical trials, namely that RFHT is safe, cost-effective and is at least as effective of pentavalent antimonial therapy for treatment of CL.
FINANCIAL SUPPORT
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.











