Impaired endothelial vasodilator function is an important contributor to the development of CVD(Reference Brown and Hu1), but it is not yet clear how it impacts on other biomarkers of cardiovascular health.
It has been well established that obesity and hypertension are associated with impaired NO-dependent vasodilatation(Reference Park, Charbonneau and Schiffrin2, Reference Van Gaal, Mertens and De Block3). Reduced availability of NO significantly impairs the degree of blood vessel dilatation in response to cardiovascular stressors such as exercise(Reference Tzemos, Lim and MacDonald4).
The typical cardiovascular response to aerobic exercise is an increase in heart rate (HR) and cardiac output, which elicits an increase in systolic blood pressure (SBP). Diastolic BP (DBP) either remains unchanged or decreases slightly due to vasodilatation in the exercising muscles, resulting in increased pulse pressure. Impaired endothelial function (as measured by flow-mediated dilatation (FMD)) has been associated with exaggerated BP responses to exercise(Reference Stewart, Sung and Silber5), which have been linked to an increased risk of developing future hypertension(Reference Miyai, Arita and Miyashita6–Reference Wilson, Sung and Pincombe8). This therefore suggests that individuals with impaired vasodilatation, such as those who are obese or have elevated BP, may have exaggerated BP responses to exercise, thus making them predisposed to acute risk during exercise(Reference Albert, Mittleman and Chae9).
Previous attempts to evaluate this emerging risk factor have been limited by the techniques available to measure BP responsiveness to exercise. Most studies have used graded exercise tests with single BP measurements taken at the end of each workload. The introduction of non-invasive techniques for continuous beat-to-beat monitoring of BP enables cardiovascular responses to be measured during rather than after exercise, thus offering a more physiological representation of the effects of impaired dilatation on cardiovascular function, compared with the commonly used but somewhat artificial FMD response to passive hyperaemia.
Recent studies have shown that the short-term intake of cocoa polyphenols can lower BP and improve endothelium-dependent vasodilatation(Reference Grassi, Necozione and Lippi10–Reference Taubert, Roesen and Lehmann13). The mechanism by which cocoa exerts its antihypertensive effect is yet to be determined but the effect may be mediated through enhanced endothelial function(Reference Keen, Holt and Oteiza14), with the cocoa polyphenols increasing the activity of NO synthase in endothelial cells(Reference Balzer, Heiss and Schroeter15, Reference Heiss, Dejam and Kleinbongard16), which can lead to enhanced endothelium-dependent vasodilatation(Reference Heiss, Kleinbongard and Dejam17, Reference Karim, McCormick and Kappagoda18) and improved BP. Thus, there may also be potential for cocoa flavanols to attenuate the BP increases in response to physiological stressors such as exercise.
The aim of the present study was to see whether improvements in FMD seen in overweight individuals following consumption of flavanol-rich cocoa(Reference Davison, Coates and Buckley11) can also improve their exaggerated BP responses to aerobic exercise (measured by decreased area under the curve for SBP, DBP and mean arterial pressure during submaximal exercise).
Methods
A randomised, double-blind, cross-over trial was conducted to test the acute effects of cocoa flavanols on BP responsiveness to exercise. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the University of South Australia Human Research Ethics Committee and were conducted at the Nutritional Physiology Research Centre. Written informed consent was obtained from all subjects.
Men and post-menopausal women who were overweight or obese (BMI>25 kg/m2) but otherwise healthy were recruited. Volunteers had no history of CVD, diabetes or renal disease, were not taking diabetic or BP-lowering medication and had seated clinic SBP ≤ 160 mmHg and DBP ≤ 100 mmHg. Participants were not intolerant to alkaloids (caffeine and theobromine) or dairy, currently smoking or using nicotine replacement therapy.
Volunteers visited the Centre at the same time of day on five occasions. At the first (screening) visit, they undertook an exercise test on a cycle ergometer (Monark, Model 828; Vansbro, Sweden) to determine the required workload for subsequent exercise tests. They were required to ride the cycle ergometer for 10 min at a workload eliciting a HR equivalent to 75 % of their age-predicted maximum (208–(0·7 × age (years)) × 0·75)(Reference Tanaka, Monahan and Seals19), and their electrocardiogram was monitored by a medical practitioner to confirm their suitability to continue exercising.
Volunteers were then provided with a dairy-based cocoa beverage powder which was either high (HF) or low (LF) in cocoa flavanols (refers herein to epicatechin and catechin as well as to their procyanidin oligomers up to and including decamers). Reconstituted in 200 ml water, the cocoa beverages provided a total of either 701 mg cocoa flavanols (HF: 139 mg epicatechin, 39 mg catechin and 523 mg procyanidins) or 22 mg cocoa flavanols (LF: 0 mg epicatechin, 9 mg catechin and 13 mg procyanidins). The LF and HF cocoa drinks were matched in appearance and macronutrient, micronutrient and alkaloid (caffeine and theobromine) contents, with the only exception being the cocoa flavanol content, see Table 1 for nutrient composition of products. The sachets were labelled with a three-digit numerical code, blinding both volunteers and investigators to their identity throughout the study. Empty sachets were collected to monitor compliance.
Table 1 Nutritional profile for each cocoa dose (two sachets) of cocoa product
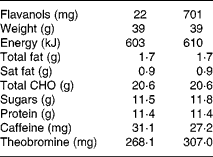
CHO, carbohydrate.
The sequence of events on each of the four visits was as follows:
(1) Volunteers fasted (except for water) for ≥ 4 h.
(2) Supplement was consumed.
(3) FMD was conducted 2 h after supplementation. Previous research has demonstrated a peak effect of cocoa flavanols on FMD at 2 h after consumption(Reference Heiss, Dejam and Kleinbongard16), which returns to baseline 6 h after consumption(Reference Balzer, Rassaf and Heiss20, Reference Heiss, Finis and Kleinbongard21). The assessment of FMD in the brachial artery was performed using two-dimensional B-mode ultrasound (LOGIQ 5; GE Medical Systems, Waukesha, WI, USA). Optimal imaging of the artery was achieved using the method of Raitakari & Celermajer(Reference Raitakari and Celermajer22). A sphygmomanometer cuff was placed about the upper forearm in line with the cubital fossa (i.e. distal to the scanned part of the artery) and inflated to suprasystolic pressure (200 mmHg) for 5 min. Images of the artery were taken before cuff inflation, 10 s before cuff release, 10 s after cuff release and then every 30 s for an additional 3 min to assess the endothelial vasodilator function response to reactive hyperaemia.
(4) Clinic BP was measured by oscillometry (SpaceLabs Model 90 217; SpaceLabs Medical, Tampa, FL, USA) while subjects were seated on the cycle ergometer before the commencement of exercise.
During the subsequent exercise test, BP and HR were measured continuously using a Finapres™ BP monitor (Ohmeda, Inc., Englewood, CO, USA) with the hand steadied in a support, which was maintained at a constant height for all occasions. The test commenced with a 5-min pre-exercise period of sitting on the cycle ergometer before a 10-min bout of exercise at a workload eliciting 75 % of the subject's age-predicted maximum HR(Reference Tanaka, Monahan and Seals19).
This protocol was repeated twice with each cocoa drink (LF or HF) in random order with a 3–7-d washout between visits, and the repeat measures for each supplement were averaged.
Diet and lifestyle requirements during the study
Volunteers were asked to consume a LF diet during the study period, specifically participants were asked to limit their intake of fruit or fruit-containing juices, apples, tea (green, black, herbal, chai, brewed or bottled), coffee or caffeinated beverages, cocoa/chocolate or cocoa/chocolate-containing products, honey, soyabeans, and soya-containing products, nuts/nut products containing nut skins and red wine. Participants were provided with written and verbal reminders to ensure compliance with this request.
Data analysis
Using the Finapres™ BP monitor, data were obtained for every heartbeat during the 15-min protocol, and were then averaged in 30-s blocks. The final 30 s of the seated BP and HR assessment were taken to be the pre-exercise HR and BP.
The changes in BP and HR during exercise were calculated by subtracting the average of each 30-s block during exercise from this pre-exercise average. These 30-s averages were used to calculate the area under the curve for the change in BP from pre-exercise values to give an integrated BP response to exercise.
Brachial artery diameter was assessed manually at each time point using the integrated digital callipers by a single observer who was blinded to the treatment group. FMD was reported as the maximum percentage change from baseline in blood vessel diameter following the cuff occlusion, as described previously(Reference Davison, Coates and Buckley11).
Two-way analysis of covariance was used to compare the effects of cocoa supplementation on both FMD and the BP response to exercise, with baseline arterial diameter and pre-exercise BP used as covariates using STATISTICA version 5.1 (StatSoft, Inc., Tulsa, OK, USA). Relationships between FMD and the BP responses to exercise were determined by linear regression analysis. P < 0·05 was taken to indicate statistical significance. All data are presented as means and standard errors unless otherwise stated.
Results
A total of twenty-one volunteers (thirteen men and eight women, age 55 (se 2·2) years, height 1·7 (se 0·02) m, weight 94·1 (se 3·5) kg, BMI 31·6 (se 0·8) kg/m2, SBP 134 (se 2) mmHg, DBP 87 (se 2) mmHg) completed the trial. Using an existing database of potential volunteers, individuals identified as having a BMI>25 kg/m2 were invited to return to perform this trial. Of the twenty-five people who were contacted, twenty-one volunteers agreed to participate. There were no withdrawals from the present study.
Flow-mediated dilatation
Fig. 1 demonstrates the FMD response to HF and LF cocoa. Two hours after consumption, the HF cocoa beverage resulted in a significantly greater FMD response than the LF cocoa beverage, P < 0·001.
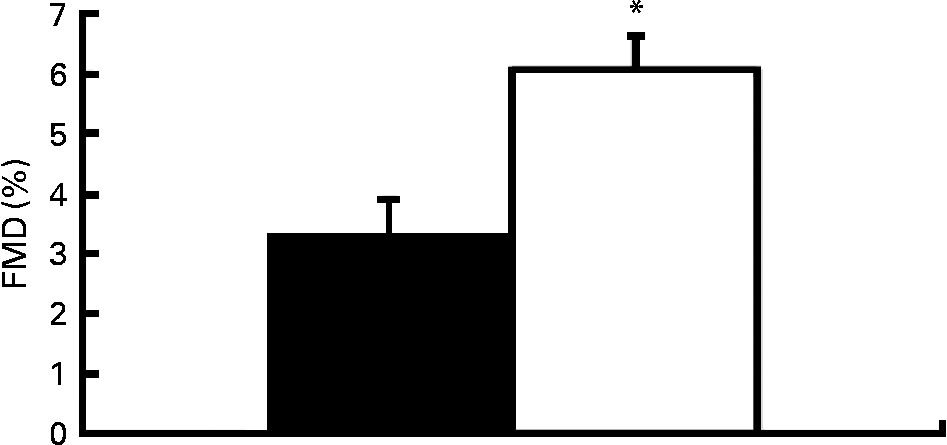
Fig. 1 Percentage of flow-mediated dilatation (FMD) following high-flavanol (HF) and low-flavanol (LF) cocoa consumption. * Mean values were significantly different between HF (□) and LF (■) (P < 0·05).
Pre-exercise blood pressure
Pre-exercise BP and HR were measured by Finapres finger plethysmography while on cycle ergometer. Data were averaged from the final 30 s of the pre-exercise period before the commencement of exercise. These readings are likely to differ from the clinic BP readings due to hydrostatic differences between the relative heights of the finger cuff and the brachial artery. There were no significant differences between HF and LF cocoa beverage consumption on the pre-exercise BP (HF: SBP/DBP 153 (se 3)/88 (se 3) mmHg, HR 79 (se 2) beats per min; LF: SBP/DBP 153 (se 4)/88 (se 2) mmHg, HR 79 (se 2) beats per min).
Responses to exercise
Both HR and BP increased in response to the cycling exercise. However, there were no significant differences in the HR response following consumption of the HF or LF cocoa beverages. On the other hand, the increases in BP were attenuated by HF cocoa consumption compared with LF cocoa consumption. Fig. 2 demonstrates the changes from pre-exercise values for the BP responses to exercise for each 30-s block over the entire 10-min exercise bout. Table 2 shows the integrated responses for BP increases during exercise. After adjusting for pre-exercise BP, the area under the curve was reduced 68 % for DBP (P = 0·03) and 14 % for mean arterial pressure (P = 0·05) following HF cocoa consumption compared with LF cocoa consumption.

Fig. 2 Blood pressure (BP) responses to exercise represented high-flavanol (○) and low-flavanol (●) cocoa consumption as change from pre-exercise values. SBP, systolic BP; MAP, mean arterial pressure; DBP, diastolic BP.
Table 2 Area under the curve for the blood pressure responses to exercise between high-flavanol and low-flavanol cocoa
(Mean values with their standard errors)
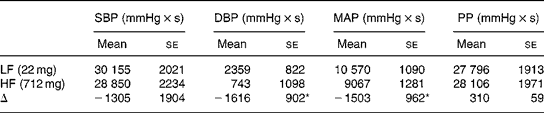
SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; LF, low flavanol; HF, high flavanol; Δ, difference between HF and LF response.
* Mean values show significant decrease in the BP response to exercise when analysed with pre-exercise BP as the covariate (P < 0·05).
Relationship between changes in flow-mediated dilatation and changes in blood pressure response to exercise
Comparison of differences between HF and LF in the FMD and BP responses to exercise revealed no significant relationships between the differences in FMD and the differences in SBP (r 0·06, P = 0·78), DBP (r 0·42, P = 0·06) or mean arterial pressure (r 0·28, P = 0·22), although the relationship between FMD and DBP approached statistical significance.
Discussion
Results of the present study confirm that consuming a single dose of HF cocoa results in a significant improvement in FMD after 2 h. Moreover, they demonstrate that acute ingestion of HF cocoa can also attenuate the BP response to exercise.
Conditions such as obesity, diabetes and hypertension are known to impair vasodilatation(Reference Park, Charbonneau and Schiffrin2, Reference Van Gaal, Mertens and De Block3), and may potentially cause an increase in DBP during exercise. In the present study, there was indeed an exercise-induced increase in DBP (Fig. 2), but the increase was attenuated by supplementation with HF cocoa.
This reduction in DBP response tended to correlate with the increase in FMD following HF consumption (P = 0·06), suggesting that this benefit may be due to improved endothelium-dependent dilatation. While some published studies have also found a relationship between exercise BP and FMD(Reference Stewart, Sung and Silber5, Reference Chang, Chung and Choi23), others have not(Reference Koller-Strametz, Matulla and Wolzt24). Green et al. (Reference Green, Walsh and Maiorana25) found no relationship between improvements in conduit vessel function (as measured by FMD) or resistance vessel function (strain gauge plethysmography of total forearm blood flow) and improvements in exercise-induced vasodilatation following exercise training. The role of NO as a mediator of exercise-induced vasodilatation is controversial. A recent review by Tzemos et al. (Reference Tzemos, Lim and MacDonald4) has concluded that there may be a role for NO in mediating exercise-induced vasodilatation, but of the studies that have examined the role of NO in exercise-induced vasodilatation(Reference Stewart, Sung and Silber5, Reference Chang, Chung and Choi23, Reference Koller-Strametz, Matulla and Wolzt24), most have reported that while NO does contribute(Reference Stewart, Sung and Silber5, Reference Chang, Chung and Choi23), there are also many other factors which mediate the vasodilatory response to exercise. Although the relationship between improvement in FMD and attenuation of the BP response to exercise was not significant, it is likely that the present study was not sufficiently powered to confirm this relationship.
The finding of an improvement in FMD following consumption of HF in the present study is consistent with a growing body of evidence indicating beneficial effects of cocoa flavanols for endothelial function(Reference Grassi, Necozione and Lippi10, Reference Davison, Coates and Buckley11, Reference Taubert, Roesen and Lehmann13, Reference Heiss, Kleinbongard and Dejam17, Reference Karim, McCormick and Kappagoda18, Reference Heiss, Finis and Kleinbongard21). The mechanism by which cocoa flavanols influence vasodilatation is yet to be clearly identified, although it appears to be via an increase in the bioavailability of NO due to increased NO production(Reference Taubert, Roesen and Lehmann13, Reference Schnorr, Brossette and Momma26, Reference Fisher, Hughes and Gerhard-Herman27). Previous research has shown that after consumption of a similar cocoa product, plasma levels of flavanols peak at approximately 2 h post-consumption. In addition, pure epicatechin consumption closely mimicked the effect of the cocoa beverage, suggesting that epicatechin may be the flavanol responsible for the improvements in vascular function(Reference Schroeter, Heiss and Balzer28); however, the present study was a proof of concept study with n 3. Therefore, further research is required to fully elucidate which flavanols in cocoa can provide the observed benefits in vascular function.
It is important to note that the consumption of flavanol-rich cocoa beverages used in the present study may not deliver the same benefits as dark chocolate consumption. In a study by Hammerstone et al., it was demonstrated that dark chocolate contains approximately 4·3 mg flavanols per g. To achieve the amount of flavanols seen in the present study (701 mg), the consumption of 163 g dark chocolate or approximately double that amount of milk chocolate would be required(Reference Hammerstone, Lazarus and Schmitz29). Given that 163 g dark chocolate provides approximately 3526 kJ and 28 g saturated fat(30), it would be preferable to deliver cocoa flavanols for health benefits in a beverage form such as that used in the present study, in which 610 kJ and 0·9 g saturated fat was delivered.
In conclusion, the results of the present study provide further support for acute consumption of cocoa flavanols to improve FMD, and they provide new evidence that cocoa flavanols can also attenuate the BP responses to exercise.
These findings suggest that the consumption of cocoa flavanols may be able to enhance muscle blood flow to allow for improved nutrient delivery and removal to exercising muscles and attenuate the BP responses to exercise, which could allow for safer and more efficient exercise performance in an at-risk population such as that included in the present study, thus placing less stress on the cardiovascular system during exercise. Furthermore, these improvements in FMD and BP response to exercise add to growing evidence that HF cocoa consumption may benefit individuals with cardiovascular risk factors.
Acknowledgements
We are grateful to Associate Professor Garry Scroop for medical supervision of volunteers and to our volunteers for giving so freely of their time. Mars, Inc. provided the cocoa drinks and financial support for the present study. All authors contributed to study design, interpretation of outcomes and preparation of the manuscript; N. M. B. and K. D. collected and analysed the data. The authors do not have any financial or personal conflicts of interest.






