Implications
Strategies to control worms without using synthetic chemical drugs are increasingly needed because of the development of resistance to chemical drugs in pathogens. In addition, in organic farming (OF) systems, the use of chemical drugs to control worms is usually avoided. In ruminants, the concept of nutraceuticals (plants that combine both nutritive value and beneficial effects on health) has emerged as an alternative strategy to control nematodes (Hoste et al., Reference Hoste, Torres-Acosta, Quijada, Chan-Perez, Dakheel, Kommuru, Mueller-Harvey and Terrill2015), particularly through the use of forages that contain tannins, such as sainfoin. The interest of such an option in rabbit has remained unexplored. Furthermore, local alternatives to alfalfa meal for rabbit feeding are in great demand worldwide.
This article presents an original scientific study aimed at evaluating the nutritive value of dehydrated sainfoin for the growing rabbit. In the context of organic rabbit farming, it also provides results on the possible anthelmintic effects of condensed tannins (CTs) in experimentally infected rabbits.
Introduction
Health management is one of the main issues for rabbit breeders in France. The use of antibiotics, coccidiostatics and anthelmintic drugs to control diseases in rabbit meat production is increasingly questioned by consumers due to the possible harmful effects of chemical residues from drugs in the environment. In addition, the demand for products from rabbits bred according to OF specifications is increasing (Martin et al., Reference Martin, Duprat, Goby, Theau, Roinsard, Descombes, Legendre and Gidenne2016). Organic specifications require that rabbits have a link to the soil. Consequently, in addition to coccidian infections, rabbits bred in OF systems are more exposed to the free-living stages of helminths and, particularly, to gastrointestinal nematodes (GINs). On the other hand, OF rules severely restrict the use of synthetic chemical drugs to control pathogens. Therefore, the need to explore non-chemical alternative strategies to control parasites in rabbits is now a priority. One of the potential alternative strategies, inspired by research performed in small ruminants, is the use of CT-containing plants (in particular, legumes) as nutraceuticals (Hoste et al., Reference Hoste, Torres-Acosta, Quijada, Chan-Perez, Dakheel, Kommuru, Mueller-Harvey and Terrill2015). Moreover, alternatives to the use of alfalfa meal as a combined source of fibre and proteins for growing rabbits are being explored in many countries today in order to reduce importations.
Sainfoin (Onobrychis viciifolia) has been widely explored as a model of a tannin-containing legume used as a nutraceutical in small ruminants. It is also a good candidate for rabbit feed as it contains high ADF and ADL levels associated with a high level of protein. This legume was historically used to feed rabbits but, to our knowledge, studies dealing with the potential effect of sainfoin as a nutraceutical in rabbit are still lacking.
Trichostrongylus colubriformis is a common nematode, a parasite of small ruminants, which could be experimentally used to infect the European rabbit (Oryctolagus cuniculus) (Audebert et al., Reference Audebert, Cassone, Kerboeuf and Durette-Desset2003). It has been considered as a model of the specific nematode of lagomorphs, Trichostrongylus retortaeformis, as well as an experimental model to examine the host–nematode interactions in the case of infections with Trichostrongylus sp. (Hoste et al., Reference Hoste, Jackson, Athanasiadou, Thamsborg and Hoskin1988). Sainfoin and its contained CTs could disturb the life cycle of T. colubriformis, as previously shown in vitro and in vivo in ruminants (Paolini et al., Reference Paolini, Frayssines, De La Farge, Dorchies and Hoste2003 and Reference Paolini, Fouraste and Hoste2004).
Thus, our study aimed (i) at measuring the nutritive value of sainfoin for the growing rabbit by analysing the effect of alfalfa replacement by sainfoin in a pelleted balanced diet on digestion and performance, and (ii) at determining the effect of sainfoin intake during an experimental infection of rabbits with T. colubriformis.
Material and methods
Animals and experimental design
The experiment was carried out within the framework of the Regional Ethical Committee on Animal Experimentation of the Midi-Pyrénées (France) at ENVT, and concerned 64 rabbits of the INRA 1777 strain, from weaning (28 days old) to slaughter (73 days). Rabbits used in this experiment were obtained from specific pathogen-free (SPF, including coccidia) mothers, and bred from birth to weaning on a coccidian-free farm. During the trial, rabbits were no longer maintained in SPF conditions but, instead, in a specific high hygiene breeding room adapted for complete cleaning. In order to test the effect of infection and the effect of sainfoin incorporation, 64 rabbits were randomly divided into four groups according to a bifactorial design (2×2), and the groups were balanced according to weight at weaning and litter origin. The four groups, composed of 16 rabbits each, consisted of infected control (IC), infected sainfoin (IS), non-infected control and non-infected sainfoin (NIS). Two rabbits were housed per cage. According to the experimental group, the rabbits were fed either a pelleted experimental control diet (alfalfa base; Table 1) or a sainfoin diet in which 40% of the control diet (except minerals) were substituted with dehydrated pellets of sainfoin (Perly variety) provided by MG2Mix-Multifolia (Chateaubourg, France). Consequently, we were able to calculate the nutritive value of the dehydrated sainfoin – namely, the digestible energy and protein content (DE and DP) (Villamide et al., Reference Villamide, Maertens, Cervera, Perez and Xiccato2001) – by comparison with the control. Diets were formulated to have similar values in crude ash, CP, NDF and DE, and to cover the requirements for the growing rabbit (Gidenne et al., Reference Gidenne and Ruckebusch2015). Both diets were free of any chemical anthelminthic or coccidiostatic drug. Animals were fed ad libitum throughout the experiment. The weight and health status of animals were controlled on a weekly basis, as was their feed intake.
Table 1 Ingredients and chemical composition of the experimental diets

1 values calculated from tabulated data.
2 % Equivalent tannic acid; for sainfoin, total tannins are equal to condensed tannins.
3 Protein precipitation activities (cm²/g).
Digestibility trial and chemical analyses
For the digestibility trial, faeces were collected according to the ‘European’ Reference method (Perez et al., Reference Perez, Lebas, Gidenne, Maertens, Xiccato, Parigi-Bini, Dalle Zotte, Cossu, Carazzolo, Villamide, Carabaño, Fraga, Ramos, Cervera, Blas, Falcao, Cunha and Bengala Freire1995), between 60 and 64 days of age (corresponding to D21 and D25 post-infection (DPI), D0 being the day of experimental infection with T colubriformis). Rabbits were allowed a 4-week period of adaptation to the experimental diet and nematode challenge before total faecal collection. A device was designed to collect the total amount of faeces excreted by the two rabbits per cage (without urine contamination). Rabbit growth and feed consumption per cage (i.e. for two rabbits) were controlled for the 5 days of the digestibility trial.
Dry matter (DM) contents of the feed were determined at 103°C for 24 h and ash at 550°C for 5 h. Fibrous fractions (NDF, ADF and ADL) were carried out according to the sequential method of Van Soest et al. (Reference Van Soest, Robertson and Lewis1991), nitrogen was measured according to the Dumas combustion method and energy according to adiabatic combustion. We calculated hemicelluloses as NDF-ADF.
Tannins were analysed by the method of Folin–Ciocalteu (Makkar, Reference Makkar2003; Inovalys Laboratory, Nantes, France). The biological activity of tannins was assessed by the method of radial diffusion using bovine serum albumin (BSA) medium (Hagerman and Butler, Reference Hagerman and Butler1978). As sainfoin contains no hydrolysable tannins (Marais et al., Reference Marais, Mueller-Harvey, Brandt and Ferreira2000), the amount of CTs was considered to be equal to the total tannins
Parasitological techniques
Four days after weaning, rabbits in groups IC and IS were orally infected with 2125 infective, third-stage larvae (L3) of T. colubriformis. This date was counted as D0 of infection. The larvae were collected from one donor sheep in the Hellenic Agriculture Organisation (Veterinary Research Institute of Thessaloniki, Greece), sent to France and maintained for 8 weeks at 4°C before being used to infect the rabbits. Faecal samples were collected weekly at the beginning of the experiment and twice a week after 21 days DPI. The faecal worm egg counts (FECs, expressed as eggs per gram (EPG) of fresh faeces) were assessed on each cage sample based on the modified McMaster technique. The total faecal excretion was weighed once a week to determine the total faecal worm egg output. Faecal DM was determined during the digestibility trial. On DPI 39, 75 g of faeces of each cage from the two infected groups were stored for 10 days at 23°C in humid conditions in order to allow egg hatching and to calculate the rate of larval development. The larvae were extracted from the faeces with a Baermann apparatus for 6 h and then counted. The number of larvae was extrapolated to the total amount of cultured faeces and divided by the number of total faecal eggs in order to calculate the egg-hatching rate. Finally, the rabbits were euthanized on DPI 45. The small intestines were individually collected and identified, as well as faecal samples in the distal colon. The small intestines were then opened and washed to collect digesta on a 100-µm sieve. The adult worms were counted for each rabbit based on a 10% aliquot method, and males and females were identified. The survival rate (% of the initial dose of larvae) was estimated by dividing the number of total adult worms by the given number of infective larvae. After cleaning the worms with lactophenol, the number of eggs contained in utero (female fecundity) was also determined on 10 female worms/rabbit by direct counting under microscopic examination.
Statistical analysis
All data were analysed using R software. Normality was checked with the Shapiro–Wilk test or the D’Agostino–Pearson test in the presence of ex aequo. An ANOVA on repeated measurements was performed on FEC values after being log (n+1)-transformed. Wilcoxon and Kruskal–Wallis tests were performed on FEC values when normality could not be obtained by logarithmic transformation. Faecal egg count at slaughter, the number of adult worms in the intestine and fecundity values were analysed using T-tests, and animal performances were analysed using a two-way ANOVA. Ratios were analysed using a χ 2 test. As T. colubriformis is not a usual parasite of rabbit and due to some difficulties during individual infection, 10 rabbits (out of 32) were negative with regard to infection (five in each infected group) and were thus excluded from the statistical analysis.
Results
Feed intake and growth performances
There were no significant interactions between the diet and infection effects, regardless of the parameter analysed. On average, a rabbit in the sainfoin groups ingested 110 g of feed/day, corresponding to 1.8 g of tannins (tannic acid equivalent (TA eq.)) per day, whereas a rabbit from the control groups ingested 105 g of feed, corresponding to 0.9 g of TA eq. per day; Table 2). The feed intake was not significantly affected by the infection. Among the four groups and during the whole experiment (6 weeks), the rabbits did not show any sign of degraded health status (no signs of diarrhoea). Accordingly, from weaning to slaughter, there was no mortality or morbidity, even in the infected groups. Disregarding the infection status, the live weight and growth were similar between all groups, although the growth tended (P=0.06; Table 2) to be 3% lower in the S groups (IS, NIS). The feed intake was 5% higher in the sainfoin groups compared with the control groups (+5.2 g DM/day; P<0.01). Accordingly, the feed conversion ratio was 10% higher for the sainfoin groups compared with the control groups (3.2 v. 2.9; P<0.001).
Table 2 Effect of infection and diet on growth performance in growing rabbits
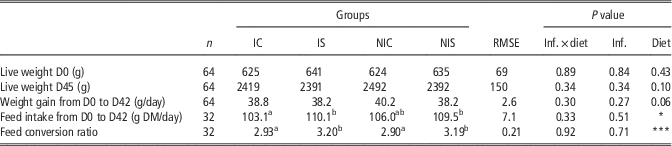
IC=infected control diet; IS=infected sainfoin diet; NIC=non-infected control diet; NIS=non-infected sainfoin diet; RMSE=root mean square of the error that applies to the statistical model; Inf.=infection; DM=dry matter.
a,bValues within a row with different superscripts differ significantly at P<0.05.*P<0.05, ***P<0.01.
Digestibility and nutritive value of sainfoin
The digestibility of organic matter averaged 65.8% in accordance with classical values recorded for such a diet. There was no impact either of the diet or of the infection on organic or energy digestibility (Table 3). Conversely, the digestive efficiency of protein was reduced by six units (%) in both ‘sainfoin groups’ (NIS and IS) compared with the control groups (P<0.001). Digestibility coefficients for hemicelluloses were reduced by 10% in infected animals compared with non-infected ones (−5 points; P<0.05).
Table 3 Effect of infection and diet on the digestibility coefficient in growing rabbits

IC=infected control diet; IS=infected sainfoin diet; NIC=non-infected control diet; NIS=non-infected sainfoin diet; RMSE=root mean square of the error that applies to the statistical model; Inf.=infection.
a,bValues within a row with different superscripts differ significantly at P<0.05, according to a one-way ANOVA.
1 Calculated by the difference between C and S diets.*P<0.05, ***P<0.01.
Pelleted dehydrated sainfoin was substituted at the level of 39.6% for alfalfa in the two sainfoin diet groups. Based on the results of the non-infected group, the DE concentration of the dehydrated sainfoin was calculated as 11.2 MJ/kg as fed, and DPs as 110 g/kg as fed in the raw material. These values were calculated with no significant difference in the infected groups (Table 3).
Measurements of the parasitological effects
The kinetics of FEC (Figure 1) indicated that the infection was successful in 22 out of 32 rabbits. The mean values of FEC (excluding the zero values) ranged between 100 and 400 EPG, and the variability between samples was high (±200 EPG). The results of the ANOVA on repeated measurements did not show any overall difference in EPG between the two infected groups (IC v. IS). A difference was only observed on DPI 32 when FEC in the IC group was significantly lower than in the IS group (P=0.026; Figure 1). Considering the whole experimental period, no difference was observed between the IC and IS groups (on average, 258 EPG; P=0.57). On DPI 45, the mean egg counts from individual faeces collected directly in the distal colon were reduced by more than 30% in the IS group compared with the IC group, but the difference was not significant (P=0.20). The same observations (lack of difference) applied to the total faecal egg output (20 558 eggs; P=0.25), and on FEC expressed in EPG of faecal DM (168 eggs; P=0.75).

Figure 1 Comparison of means of faecal egg counts on eggs per gram (EPG) in infected groups fed with control diet – IC (black) – or sainfoin diet – IS (grey). Error bars indicate standard errors. *P<0.05.
Table 4 Effect of diet on daily excretion of faecal eggs, egg hatching, survival rate, sex ratio and fecundity of Trichostrongylus colubriformis

IC=infected control diet; IS=infected sainfoin diet; RMSE=root mean square of the error that applies to the statistical model.
1 Some rabbits were not correctly infested (five for each group) and were thus excluded from the statistical analysis.
2 Percentage of gastrointestinal nematode eggs in faeces hatching and developing into L3 larvae after 10 days of incubation.
After slaughter (DPI 45), the number of adult T. colubriformis recovered in the small intestine (972) did not differ between the IC and IS groups (P=0.50; Table 4). Overall, the survival rate of the infective larvae of T. colubriformis was higher than 40% in the 10-week-old experimentally infected rabbits (the rabbits that were negative with regard to infection were excluded). The number of eggs in utero per female worm (13.8) was similar in both groups (P=0.83). In contrast, the rate of eggs hatched in the sainfoin-fed group tended to be lower than for the control-fed group (58.3% v. 85.2%; P=0.08).
Throughout the whole experiment, no coccidian oocysts were detected using a modified McMaster technique for all groups, nor were any other propagules detected in samples of non-infected groups.
Discussion
The aim of our study was to evaluate the nutritive value of dehydrated sainfoin and its potential use as a nutraceutical for rabbits. We will first discuss the results obtained on the nutritive value of sainfoin and the effects on digestion and performance for the growing rabbit by analysing the effect of alfalfa replacement by this legume in a pelleted balanced diet. We will then discuss the effects of sainfoin intake on different parameters that characterise experimental infection of rabbits with T. colubriformis.
Sainfoin as an alternative to alfalfa in rabbit feed
Effect of sainfoin dietary incorporation on digestion and performance
The higher feed intake observed with the sainfoin diet suggested a high palatability of this product for growing rabbits. This higher intake could be related to the twofold higher lignin concentration of the S diet (8%) when compared with the control. The lower digestion of proteins in the sainfoin group (−6 points compared with control values) could be explained by the effect of tannins in the small intestine, although this has never been quantified for the growing rabbit. It is suspected that proteins are bound with tannins in the range of intestinal pH and might therefore be less available for digestive processes. Accordingly, the DP intake tended to be less in the two sainfoin groups than in the two control groups (−7.9 g DP/day/rabbit; P=0.07). This could explain that rabbits fed with the control diet tended to grow faster (+3.3%; P=0.06) even though the DE content of sainfoin was higher. Moreover, consideration must be given to the fibre intake of the rabbits and, particularly, to a twofold higher lignin intake for S groups compared with control ones (9.2 v. 4.5 g ADL/day). As shown by Perez et al. (Reference Perez, Gidenne, Lebas, Caudron, Arveux, Bourdillon, Duperray and Messager1994), a high lignin intake could explain the poorer feed conversion ratio observed in the NIS and IS rabbits even though their DE intake was higher than in the C and IC groups.
Nutritive value of sainfoin
The nutritive value observed for the dehydrated and pelleted sainfoin was high, as the DE content reached 12.97 MJ/kg DM and 128 g of DP/kg DM. For a sainfoin hay (incorporated into a pelleted diet) containing a high NDF level (55%), Fernández-Carmona et al. (Reference Fernández-Carmona, Cervera and Blas1996) reported a 35% lower level of DE (8.2 MJ/kg DM) and a 50% lower DP content (62 g/kg DM) measured in 4 to 5-month-old rabbits (3 to 4 kg live weight (LW)). Similarly, Liu et al. (Reference Liu, Zhang, Chang, Pen and Xu1992) reported a DE of 8.5 MJ/kg DM for a sainfoin hay fed to adult angora rabbits. This considerable difference in nutritive values between hay and dehydrated sainfoin could be explained by several factors such as the delay in harvesting time or variations in plant physiological stages. Moreover, dehydrating legumes led to a better conservation of nutritive compounds when compared with conversion to hay by reducing the loss of foliage.
The high-fibre and high-DE concentration of dehydrated sainfoin pellets indicated that this raw material is a valuable source of fibre and of energy, comparable with dehydrated alfalfa. In fact, compared with dehydrated alfalfa, sainfoin had a 40% higher DE content (11.2 v. 7.6 MJ/kg as fed) and a similar DP content (110 v. 113 g DP/kg). Therefore, on the basis of the results of our study, dehydrated sainfoin seems to be an excellent trade-off feedstuff to formulate balanced diets for growing rabbits, as it provides large quantities of lignins that are essential for digestive health in rabbits (Gidenne et al., Reference Gidenne and Ruckebusch2015), whereas its DE and DP contents remain high. Further studies with more moderate incorporation of dehydrated sainfoin (around 20%) will aim at confirming our initial results.
Sainfoin a potential nutraceutical to control gastrointestinal nematodes in rabbits
T. colubriformis infections of young weaned rabbits as a model
Wild rabbits are natural carriers of GINs. In OF, grazing rabbits that are potentially exposed to the faeces of wild rabbits can therefore become infected. Among the main GIN species, Graphidium strigosum and Trichostrongylus sp. could impact their condition and health (Schoeb et al., Reference Schoeb, Cartner, Baker, Gerrity and Baker2007). Consequently, there is the need for an experimental model to study the interactions between GINs and rabbits in order to test alternatives to chemical drugs in OF systems. Regarding the difficulty to obtain T. retortaeformis larvae and the availability of T. colubriformis larvae, T. colubriformis was used. Experimental infection of young weaned rabbits with T. colubriformis has been previously demonstrated (Williams and Palmer, Reference Williams and Palmer1964; Hoste et al., Reference Hoste, Jackson, Athanasiadou, Thamsborg and Hoskin1988). Despite the fact that T. colubriformis has the potential to naturally infect rabbits, it remains occasional (Saulai and Cabaret, Reference Saulai and Cabaret1998), with T. retortaeformis being the specific Trichostrongylus species found in rabbits (Audebert et al., Reference Audebert, Cassone, Kerboeuf and Durette-Desset2003). Therefore, in experimental infections in rabbits, the biological traits of T. colubriformis can present some variations compared with infections in the usual hosts (small ruminants; Audebert et al., Reference Audebert, Cassone, Kerboeuf and Durette-Desset2003). For example, the mean number of eggs per utero in female worms appeared to be reduced when hosted in rabbits: 14 in our study and 24 eggs per utero in sheep. Despite these limits, T. colubriformis infections in young weaned rabbits make it possible to explore sainfoin as a nutraceutical in OF systems, and more widely the opportunity to use rabbits infected with T. colubriformis as a model to explore several aspects of the effects of sainfoin and other tannins containing resources on GINs.
Effect of condensed tannins on T. colubriformis
Condensed tannins form stable bindings with proteins in a range of pH from 4 to 7 (Jones and Mangan, Reference Jones and Mangan1977). pH values in the stomach of young rabbits ranged from 1.5 to 2.0, with a maximum of 3.5 in the fundus (in the presence of soft faeces) at the beginning of the day (Blas and Gidenne, Reference Blas and Gidenne2010). These values seem to be too acidic to enable the formation of stable complexes of CT-proteins in the stomach. pH values are higher in the small intestine: from 6.4 to 7.2; in the caecum: 6.0; and in the colon: 6.5. Therefore, it is more likely that CTs might bind with proteins in the intestines. Exsheatment of T. colubriformis infective larvae occurs in the compartment anterior (the stomach) to the compartment of adult establishment (the small intestine). Due to low pH values in the rabbit stomach, it is hypothesised that the exsheatment could not be impaired by the formation of complex CT-larval proteins, explaining similar recovery rates in sainfoin v. control groups.
Min et al. (Reference Min, Solaiman, Shange and Eun2004) suggested that CTs could reduce helminth fecundity rather than survival of worms in the digestive tract. In sheep, the required amount of tannin in the diet necessary to trigger anthelminthic activity in ruminants has been evaluated at approximately 30 to 40 g of CTs/kg DM (3% to 4%; Hoste et al., Reference Hoste, Kerboeuf and Parodi2006). In our sainfoin diet, the level of CT attained was only 16.4 g/kg DM, and it might have been insufficient to impair larvae establishment and/or worm fecundity. However, at the end of the experiment (at slaughter) and when feed intake was higher, we observed that FEC values were higher by 30% in the control group compared with the sainfoin group.
The levels of CTs in the sainfoin diet appeared to have been sufficient to affect the development of eggs into larvae, as demonstrated by our results. In horses fed with a diet containing 3.6% of CTs/kg DM, Collas et al. (Reference Collas, Salle, Dumont, Cabaret, Cortet, Martin-Rosset, Wimel and Fleurance2015) reported similar effects. The FECs were not reduced but the rate of larval development was. The mode of action of CTs on worm eggs could be either direct or indirect. Molan et al. (1999) suggested that the direct effect could be linked to the exposure of eggs to CTs during their development, as CTs are not absorbed from the digestive tract and remain in the faeces. However, the hypothesis of an indirect effect of CTs based on antibacterial activity could be also raised. As shown by Wang (Reference Wang1970), Escherichia coli is the suitable bacteria for feeding the free-living stage (L1 and L2) of T. colubriformis and for favouring their development into L3 larvae. Min et al. (Reference Min, Pomroy, Hart and Sahlu2007) determined a dose-dependent activity of tannins to reduce a faecal population of E. coli in steers, with an intermediate effect at 0.75% and a maximum effect at 1.5% (corresponding to 7.5 and 15 g DM of tannins/day). Methanogens bacteria could be also inhibited by CTs of pine bark in goats (Min et al., Reference Min, Pinchak, Anderson and Callaway2014). Dalle Zotte and Cossu (Reference Dalle Zotte and Cossu2009) also reported bactericidal activity on E. coli in the caecum of rabbits supplied with 1% or 3% of quebracho tannin extract. More largely, the use of tannin extract seems to reduce the rabbit mortality and morbidity in an enteropathy infected environment (Maertens and Struklec, Reference Maertens and Struklec2006). In the current experiment, the level of tannins in the sainfoin diet (1.65%) compared with the control diet (0.95%) could be responsible for the decrease in the E. coli and other bacteria population and could therefore indirectly explain the reduced development of eggs into larvae. However, the tannins are widespread in plants, thus the difference in tannin content of the control and sainfoin-contained diet was rather small.
Besides, as the sainfoin also contains lectins (such concanavalin A), we also hypothesise that these molecules might have an impact on T. colubriformis infection, as shown in sheep (Rios de Alvarez et al., Reference Rios de Alvarez, Greer, Jackson, Athanasiadou, Kyriazakis and Huntley2010). This should be further studied in the rabbit.
Effect of infection
Musongong et al. (Reference Musongong, Chiejina, Fakae and Ikeme2004) described a dose-dependent effect on BW in T. colubriformis-infected rabbits. The infection with a medium dose of infective larvae of T. colubriformis (3000 L3/kg LW0.75) in our experiment did not result in any significant difference in BW or growth between animals infected or not. Purvis and Sewell (Reference Purvis and Sewell1971) also indicated that the number of eggs excreted by rabbits was higher when it resulted from an infective dose of larvae obtained from rabbits than from the same dose of larvae obtained from sheep. Purvis and Sewell (Reference Purvis and Sewell1971) also indicated that egg excretion is higher at the 3rd or the 7th generation of rabbits. Therefore, in future experiments, it might be worth using infective larvae of T. colubriformis directly obtained from 2nd-generation rabbits in order to maximise the possible difference in egg excretion, or to use nematodes adapted to rabbits.
The resilience of the infected animals could be due to higher feed consumption. In our experiment, infected animals do not show a higher feed consumption than non-infected ones. However, feed was designed to cover all the rabbits’ needs. We therefore considered that the nutritional level was optimal. In such conditions, it would have been difficult to make any improvement based on nutrition, as suggested by Hoste et al. (Reference Hoste, Torres-Acosta, Sandoval-Castro, Mueller-Harvey, Sotiraki, Louvandini, Thamsborg and Terrill2016). Moreover, at weaning, our rabbits were free of the major pathogens (enteropathogenic E. coli) and free of coccidia. This could also explain the lack of impact of the infection on growth and intake.
However, the infection with T. colubriformis impaired the digestion of hemicelluloses that are considered to be highly digested by the rabbit (when compared with cellulose). The main hemicelluloses of dicotyledonous plants (both alfalfa and sainfoin) are xyloglucans. A consequence of gastrointestinal parasitism is the possible negative interaction for fibre digestion, possibly explained by changes both of microbiota and of the biochemical environment of the gut induced by parasites (Hoste, Reference Hoste2001). T. colubriformis develop in the proximal part of the small intestine in rabbits (Hoste et al., Reference Hoste, Jackson, Athanasiadou, Thamsborg and Hoskin1988). In contrast, the digestion of fibres predominantly occurs in the caecum. However, as there is also some activity of xylanases in the small intestine (De Blas et al., Reference De Blas, Garcia and Carabaño1999), the digestion of hemicelluloses may begin in the small intestine (Gidenne and Ruckebusch, Reference Gidenne, Lebas, Savietto, Dorchies, Duperray, Davoust and Fortun-Lamothe1989). Hence, the presence of T. colubriformis in the small intestine might be responsible to some extent for a lower bacterial hemicellulase activity.
Pelleting process and biological activity of tannins
The biological activity on BSA of the sainfoin diet was lower than expected by 21%, based on the results of the raw material (10.6 v. 13.5 cm²/g). On the basis of our results, it seems that the protein precipitation activity of tannins contained in sainfoin was reduced by the pelleting process. However, Terrill et al. (Reference Terrill, Mosjidis, Moore, Shaik, Miller, Burke, Muir and Wolfe2007) found that the pelleting of Sericea lespedeza (another tannin-containing legume) did not impact the anthelmintic properties and even improved them. Nevertheless, in Terrill et al. (Reference Terrill, Mosjidis, Moore, Shaik, Miller, Burke, Muir and Wolfe2007), the temperature did not exceed 70°C during the process. To prepare the rabbit feed, the pellets were processed at 80°C to 85°C. Therefore, either a threshold between the improvement and the deterioration of CT activity may exist between 70°C and 80°C, or Sericea lespedeza and sainfoin tannins do not respond in the same way to heating. In further studies, it would be interesting to take the influence of overheating in the pelleting process on the biological activity of tannins into account, and to determine a maximum temperature to maximise anthelmintic properties.
To conclude, on the basis of this first study, dehydrated sainfoin pellets seem to be an interesting feedstuff for growing rabbits and could constitute a real alternative to dehydrated alfalfa as its DE content is higher than alfalfa (11.12 MJ/kg as fed), whereas its DP content is similar to alfalfa (110 g/kg as fed). It is also an excellent source of fibre as well as a considerable supply of lignins. Moreover, sainfoin could reduce the development of T. colubriformis eggs into larvae through the activity of CTs in faeces, therefore limiting environmental contamination.
As recently revealed by Saratsis et al. (Reference Saratsis, Voutzourakis, Theodosiou, Stefanakis and Sotiraki2016) in lambs, condensed sainfoin tannins may also have an effect on coccidia. This will have to be examined in growing rabbits as coccidiosis is a major concern in this species. Moreover, trials conducted on an experimental organic rabbit farm are being analysed to assess, in situ under natural infections on pasture, the impact of grazing fresh sainfoin. These different studies will help to improve our understanding of the effects of sainfoin as a nutraceutical in rabbit production.
Acknowledgements
This work was funded by the INRA metaprogram GISA (PROF project) and INRA-AgriBio4 (CuniPat project). The authors thank the MG2Mix (Chateaubourg, France) and Multifolia (Viapres Le Petit, France) companies for providing the dehydrated sainfoin pellets, the UE INRA PECTOUL for providing rabbits (E. Balmisse and V. Helies) and experimental diets (M. Moulis), and Dr S. Sotiraki from the Veterinary Research Institute of Thessaloniki (HAO, Greece) for providing the infective larvae of T. colubriformis. The authors thank all of the colleagues involved in data collection and analysis, especially C. Lacassagne (INRA, ToxAlim) and C. Bannelier (INRA, GenPhySE).







