Lactobacillus and Bifidobacterium species are the most widely used probiotics and their health effects have been reviewedReference Salminen1–Reference Ouwehand, Bianchi Salvatori, Fonden, Mogensen, Salminen and Sellars3. Probiotics generally do not colonise the gut permanently because the exogenous bacteria are outcompeted by the endogenous microbiota, which is better adapted to the prevailing conditions in the gastrointestinal tractReference Ouwehand, Nurminen, Mäkivuokko and Rautonen4. Prebiotics (typically non-digestible carbohydrates) may give a competitive advantage to the live-fed probiotic bacteria in the gastrointestinal tract and may also have direct effects on the resident microbial community in the large intestineReference Gibson and Roberfroid5.
Non-digestible carbohydrates can modify both the composition and metabolism of the intestinal microbiota and thereby the immunological responses of the gutReference Gibson and Roberfroid5, Reference Swanson, Grieshop, Flickinger, Bauer, Healy, Dawson, Merchen and Fahey6. Thus far, the most studied prebiotics are fructo-oligosaccharidesReference Gibson, Beatty, Wang and Cummings7–Reference Kruse, Kleessen and Blaut10. In the present study two different types of approved food-grade non-digestible carbohydrates were included in the trial: partially fermentable polydextrose (PDX) (Litesse® Ultra) and completely fermentable galacto-oligosaccharide (GOS)-containing syrup (Elix'or). Litesse® Ultra is a randomly bonded polymer of dextrose containing minor amounts of sorbitol (6 %) and citric acid. The GOS syrups typically contain a mixture of oligosaccharides (60 %) and lactose (20 %), glucose (19 %) and galactose (1 %). Previous studies have shown that both PDX and GOS can improve bowel function in humans but GOS, however, increases gastrointestinal symptoms such as flatulence and abdominal pain at lower dosages than PDXReference Flood, Auerbach and Craig11, Reference Teuri, Korpela, Saxelin, Montonen and Salminen12. Intake of PDX more than 50 g/d may cause diarrhoeaReference Flood, Auerbach and Craig11.
In studies of intestinal microbiota and its health implications it is a major challenge to determine the usefulness of different models. Both conventional and human-faecal-microbiota-inoculated rats have been used as models for studying the effects of prebiotics on the human gut microbes (for example, Meslin et al. Reference Meslin, Andrieux, Sakata, Beaumatin, Bensaada, Popot, Szylit and Durand13). The microbial enzyme profiles of conventional rats and rats inoculated with the human microbiota are quite similar to that of human faecesReference Mallett, Bearne, Rowland, Farthing, Cole and Fuller14 but the faecal concentrations of indigenous bifidobacteria (BIF) and lactobacilli in rats and humans are quite differentReference Wang, Brown, Khaled, Mahoney, Evans and Conway15–Reference Langendijk, Schut, Jansen, Raangs, Kamphuis, Wilkinson and Welling17. The main anatomical difference is that the microbial fermentation compartment in the rat is the caecum whereas in humans fermentation occurs mainly in the proximal half of the colonReference Stevens, Hume, Stevens and Hume18. Other differences include the lack of gastric juice in the proximal part of a rat's stomach, which also enables bacterial fermentation. Although the rat model is used for the prediction of dietary fibre fermentation in humansReference Nyman, Asp, Cummings and Wiggins19, it may also have disadvantages arising from the differences between rats and humans in the handling of low-digestible, high-fibre feedReference Stevens, Argenzio and Roberts20. The aim of the present study was to investigate the effects of two different prebiotic candidates on survival of live lactic acid bacteria (LAB) and BIF.
Materials and methods
Animal model
Rats and experimental design
Male Wistar rats (weight 71–101 g) were obtained from the Biocenter of the University of Helsinki (Finland) and housed in a Scantainer-system of sixteen cages with a controlled temperature (27 ± 2°C), a 12 h light–12 h dark cycle, and had constant access to food and tap water. The rats were weighed once per week and the weight gain of each rat was calculated. All the experimental animals were anaesthetised with CO2 and killed by cervical dislocation at the end of the trial. The trial protocol was approved by the ethical committee of the University of Helsinki (Finland).
A completely randomised design was used whereby forty rats were randomly allocated to four dietary groups in such a way that each diet was given to ten rats divided into three cages. Thereafter the actual data on each diet were collected and pooled samples of three rats per diet group (i.e. one rat per cage) were analysed for faecal bacterial population giving three observations on each diet. In each diet group the microbial population is supposed to differ more between the cages than within the cages.
Dietary treatments
The rats, all of which were approximately aged 4 weeks on arrival, were fed a basal low-fibre diet (a calculated dietary fibre content of 1 %, w/wReference Rastas, Seppänen, Knuts, Karvetti and Varo21) for the first week. This basal diet was prepared using the same protocol as with the study of Peuranen et al. Reference Peuranen, Tiihonen, Apajalahti, Kettunen, Saarinen and Rautonen22 and stored at − 20°C until used. The basal diet contained (%, w/w): potatoes, 38·5; minced meat, 23·3; eggs, 20·4; wheat bread, 8·1; sugar, 8·1; butter, 1·6. The ingredients were minced, mixed and baked in a steam oven at 200°C for 2–3 h. The mixture was allowed to cool to room temperature and the following ingredients were mixed (%, w/w) in the diet: vitamin mixture (Diet 1324; Altromin International, Lage, Germany), 0·87; cholesterol (Sigma, St Louis USA), 0·377; salt (NaCl), 2·0.
After 1 week's adaptation the rats were fed on the experimental diet for the following 2 weeks. The feeding groups were: the control group (without prebiotic or probiotic supplementation), the probiotic mixture (PRO) group (probiotics), the PDXPRO group (polydextrose and probiotics) and the GOSPRO group (galacto-oligosaccaharide and probiotics). The amount of PRO (containing Lactobacillus rhamnosus GG and LC705, Propionibacterium freudenreichii ssp. shermanii JS and Bifidobacterium breve Bb99; Valio Ltd, Helsinki, Finland) was 10Reference Bouhnik, Vahedi, Achour, Attar, Salfati, Pochart, Marteau, Flourie, Bornet and Rambaud9 colony-forming units (cfu)/d per rat. PRO, containing low-lactose and -fat cows' milk, was introduced to the PRO rats once daily during the 2-week intervention period, while the control rats received only milk. Milk (30 ml/d) was given to all rats during their daily active time to ensure that they would drink all the milk that contained PRO.
In the PDXPRO and GOSPRO groups the basal diet was enriched with either 2 % (w/w) PDX (Litesse® Ultra; Danisco Sweeteners, Redhill, Surrey, UK) or 2 % GOS (Elix'or; Borculo Domo Ingredients, Borculo, the Netherlands) respectively which is also the level of prebiotic that has also been used in trials studying degradation of prebiotics in ratsReference Djouzi, Andrieux, Pelenc, Somarriba, Popot, Paul, Monsan and Szylit23. The fibre intake recommendation for humans is 25–35 g/d or 3 g/MJ. The equivalent fibre intake recommendation for an average adult rat would be about 1·5 g/d if the energy consumption difference between a rat (maximum 0·49 MJ) and a human (10·47 MJ) is taken into account. Based on the energy value of the basal diet, the feed consumption for each rat was approximately maximally 40 g/d and the sum of fibre and prebiotic intake was approximately 1·2 g/d.
Faecal sampling
The faecal samples for microbiological analyses were collected immediately after defecation and stored at − 70°C until analysis.
Clinical studies
Human subjects and experimental design
The study was carried out as two prospective, randomised, parallel-group trials. The PDXPRO trial and the GOSPRO trial were concurrently conducted at two different sites (Fig. 1). The subjects in the study consisted of thirty-eight healthy adults aged 25–52 years; all were consumers of an omnivorous diet. In Southern Finland, the fibre content of the typical diet of the 25–52 years age group is 19·7 g /d for males and 17·2 g/d for femalesReference Männistö, Ovaskainen and Valsta24. The average fibre intake in the PDXPRO (consisting of fifteen females and five males) trial was approximately 17–18 g/d and in the GOSPRO trial (consisting of eighteen males) 19·7 g/d.
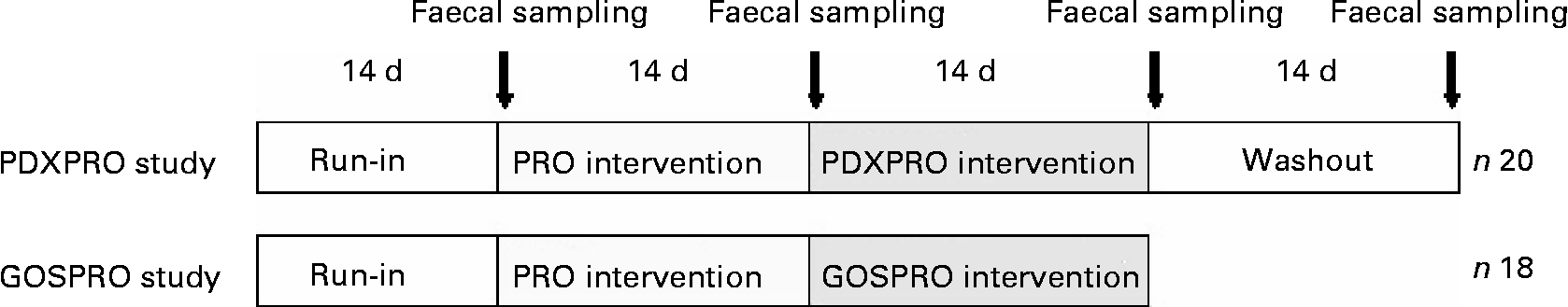
Fig. 1 Design of the human clinical studies. For details of the designs, see the Materials and methods section. Both studies started with a run-in period, followed by probiotics (PRO), then by a polydextrose + probiotics (PDXPRO) or a galacto-oligosaccharide + probiotics (GOSPRO) period. The PDXPRO trial also included a washout period.
The composition of the background diet of the subjects was not monitored or controlled although the following steps were taken. First, the use of all other products containing probiotics and/or fortified with either PDX or GOS was forbidden. Second, to reduce variation in their background diets the study subjects were all offered a regular lunch at their staff canteen. Third, none of the subjects had used antibiotics for at least 1 month before the initiation of the experiment. Fourth, during the study itself, in the case of intestinal infection or the required use of antibiotics, individuals were excluded from the trial.
Both intervention trials started with a run-in, followed by a PRO period, and then a PDXPRO or a GOSPRO period. The PDXPRO trial also included a washout period. Each of the 2-week periods ended with faecal sampling (Fig. 1). At the very onset of the study, the protocol was approved by the Foundation for Nutrition Research, Finland.
Dietary treatments
The PRO mixture (in total 2 × 10Reference Kruse, Kleessen and Blaut10 viable bacteria/d) consisted of the same strains as in the rat trial. The proportions of different probiotics and their viability in the mixture were monitored throughout the study (data not shown). The PRO mixture was supplemented with either 5 g PDX or 3·8 g GOS and dissolved in a 100 ml raspberry–blueberry juice. The daily drink was taken immediately after lunch in order to prevent the effects of an empty stomach on the probiotics. The daily intake of 4 g or more of PDX has shown to improve bowel functionReference Jie, Bang-Yao, Ming-Jie, Hai-Wei, Zu-Kang, Ting-Song and Craig25. Such amounts are also suitable for use in food products.
Faecal samples
The faecal samples for microbiological analysis were obtained in plastic jars and frozen at − 70°C within 4 h, but typically less than 2 h after defecation. Before freezing the samples the toxic effect of air onto anaerobes was minimised by packing the plastic full and airtight.
In the PDXPRO trial two spot samples were not included because the samples could not be delivered within 2 d of the end of the 2-week intervention period.
Microbiological analyses
Faecal samples were homogenised at a ratio of 1:10 in a Wilkins-Chalgren broth (Oxoid Ltd, Basingstoke, Hants, UK) in an anaerobic chamber. Ten-fold serial dilutions were prepared and 0·1 ml of each dilution was plated on an appropriate agar. Samples were analysed for total LAB on De Man–Rogosa–Sharpe (MRS) agar (LAB M; International Diagnostics Group, Bury, Lancashire, UK), for BIF on raffinose agar (RBReference Hartemink, Ko, Weenk and Rombouts26) and for propionibacteria (PAB) on yeast extract lactose agar (YEL, 30) supplemented with 1 % (w/v) β-glycerolphosphate (Merck, Darmstadt, Germany). The MRS plates were incubated at 37°C for 3 d, the RB plates at 37°C for 2 d and the YEL plates at 30°C for 7 d; all the plates were incubated anaerobically.
Statistical analysis
Statistical Analysis Software release 9·1 (SAS Institute, Inc., Cary, NC, USA) was used for the analysis. The faecal bacterial population usually shows high inter-subject variation. Therefore, bacteria were expressed in log10 cfu/g wet weight and the data were summarised as mean values and standard deviations.
To discover the effect, if any, of the diet on the BIF and LAB concentrations in the rat trial, ANOVA was carried out. If the F ratio in ANOVA for dietary effects was significant then differences in the effects between diets were tested using Tukey's multiple comparison tests.
The differences in the bacterial concentrations from the start to finish of a treatment period were compared using Student's paired t test separately for each trial. To carry out a meaningful assessment of the effects of PDXPRO or GOSPRO, the two parallel and independent trials were compared with respect to the run-in microbial counts by using a two-sample t test. The studies of PDXPRO and GOSPRO were conducted independently, thus permitting the two-sample t test. If the two groups had differed significantly at the run-in stage then further comparisons were carried out by adjusting for the run-in measurements. The analysis of covariance (ANCOVA) for comparing the two groups with respect to the concentrations of bacteria after PDXPRO and GOSPRO periods were performed by adjusting for the run-in microbial counts and using a binary variable for the groups (1 = PDXPRO, 0 = GOSPRO). This method allows pooling of the data from the two trials and their comparisons with respect to the concentrations of the bacteria after adjusting for the initial microbial counts. All analyses were performed as intention-to-treat analyses. All statistical comparisons were made using two-sided tests with the level of significance of 0·05. The only exception is in the rat trial where the small number of observations allows the use of P < 0·1 (Table 1).
Table 1 Numbers of total cultured lactic acid bacteria (LAB) and bifidobacteria (BIF) in faeces of rats and human subjects†
(Mean values and standard deviations)
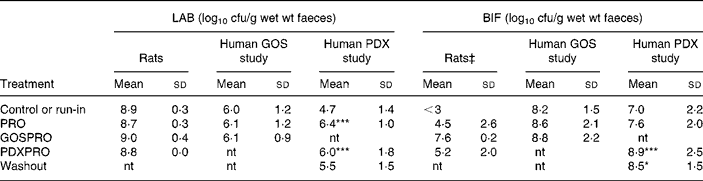
cfu, colony-forming units; GOS, galacto-oligosaccharide; PDX, polydextrose; PRO, probiotic bacteria mixture; GOSPRO, galacto-oligosaccharide and probiotics; nt, not tested; PDXPRO, polydextrose and probiotics.
Mean value was significantly different from that of the run-in treatment: *P < 0·05, ***P < 0·001 (pairwise t test within each human trial).
† Three rats per group; eighteen subjects in the GOS study and nineteen subjects in the PDX study. The detection limit was 3 log 10 cfu/g.
‡ ANOVA showed significant differences between the four treatments (P = 0·10). Further, pairwise comparisons showed a significant difference between the control or run-in treatment and the GOSPRO treatment.
Results
The quantities of live LAB, BIF and PAB were analysed from faecal samples by a plate-counting method. The indigenous run-in LAB cell numbers in rats were 8·9 (sd 0·3) log10 cfu/g wet faeces and in the human subjects ranged between 4·7 (sd 1·4) in the PDX study and 6·0 (sd 1·2) log10 cfu/g wet faeces in the GOS study. The LAB numbers in the rats were not affected by the different treatments as indicated by the ANOVA, whereas in the PDXPRO human trial, LAB numbers increased by a 100-fold already during the PRO period, from 4·7 to 6·4 log10 cfu/g (P < 0·001), and maintained this during the PDXPRO period (6·0 (sd 1·8) log10 cfu/g; P < 0·001). After the washout period the LAB numbers in the PDXPRO trial returned to the run-in concentrations. In contrast, in the GOSPRO trial, concentrations of LAB remained virtually unchanged throughout the trial (Table 1).
The BIF numbers in the rats were affected by the different treatments, as indicated by the ANOVA (P value of F test < 0·10). The baseline BIF cell numbers in the rats were below the detection limit (3 log10 cfu/g) and were significantly increased by the GOSPRO treatment (P < 0·0001). In the human subjects the initial BIF cell numbers ranged from 7·0 (sd 2·2) log10 cfu/g in the PDXPRO study, to 8·2 (sd 1·5) log10 cfu/g in the GOSPRO study. The high initial BIF amount in the GOSPRO study did not increase significantly during the treatments. In contrast to the rats, the PDXPRO treatment increased cell numbers of BIF in the human subjects (P < 0·001; Table 1). Furthermore, in the PDXPRO treatment group the BIF concentrations also remained higher during the washout period (P < 0·05) when compared with the run-in period. In the rats the increase in the BIF concentration with PDXPRO was not statistically significant (Table 1).
The ANCOVA of the BIF data showed a significant main effect of group (P = 0·0013) and the interaction between the run-in concentrations and the group (P = 0·0011). The least-square means for the two human trials for BIF, after adjusting for run-in concentrations, did not differ significantly (PDXPRO mean 8·61 (se 0·52) log10 cfu/g; GOSPRO mean 8·10 (se 0·45) log10 cfu/g; P = 0·45).
The ANCOVA of the LAB data showed no significant main effect of the group or the interaction between the run-in concentrations and the group. The least-square means for the two human trials for LAB after adjusting for run-in concentrations did not differ significantly (PDXPRO mean 5·87 (se 0·36) log10 cfu/g; GOSPRO mean 5·99 (se 0·42) log10 cfu/g; P = 0·82).
Both in rats and in the human subjects, the initial PAB cell counts were below the detection level ( < 3 log10 cfu/g). In the human subjects the supplementation of PRO containing P. freudenreichii ssp. shermanii JS increased the PAB cell numbers significantly (P < 0·001) from the initial concentration of 3 log10 cfu/g to 6 and 7 log10 cfu/g in the GOSPRO and PDXPRO studies, respectively. This occurred during the PRO supplementation (P < 0·001); the effect was not increased by either of the prebiotics (data not shown). Similarly in the rat study the PAB cell numbers increased from the initial concentration of 3 log10 cfu/g to 6–9 log10 cfu/g during the PRO supplementation (data not shown).
Discussion
The effect of different treatments on the numbers of recovered live LAB and BIF differed between the rat model and the human studies. In the rat study the baseline cell numbers of BIF were below the detection limit. Similar results have been described by Sembries et al. Reference Sembries, Dongowski, Jacobasch, Mehrländer, Will and Dietrich16 in Wistar rats, and Wang et al. Reference Wang, Brown, Khaled, Mahoney, Evans and Conway15 in Balb/c mice. The factors affecting different baseline cell numbers of BIF in humans and rats may originate from the attachment of BIF to intestinal mucosaReference Kawai, Suegar and Shimohashi27 and the bifidogenic factors in the dietReference Wang, Brown, Khaled, Mahoney, Evans and Conway15. In the present study only the GOSPRO treatment increased the cell numbers of faecal BIF in rats significantly. A corresponding increase in BIF (and LAB) with solely 5 % GOS (transgalactosylated oligosaccharide) supplementation has been previously demonstrated by Rowland & TanakaReference Rowland and Tanaka28 using germ-free rats inoculated with a human microbiota. In humans the BIF normally exist at concentrations of 6–9 log10 cfu/g and the LAB at the concentrations of 5–9 log10 cfu/g, depending of the age and physiological status of the hostReference Reuter29, Reference Hopkins, Sharp and Macfarlane30. In contrast to the rats, supplementation of the PRO with PDX increased the cell numbers of BIF in the human subjects, finally reaching a level of approximately 9 log10 cfu/g. The high BIF baseline (8·2 log10 cfu/g) in the other human trial group with GOS may explain the lack of any significant increase in the BIF counts with PRO or GOSPRO treatments; however, the final BIF concentrations were equal in the GOSPRO and PDXPRO trials. Other human studies have demonstrated an increase in faecal cell numbers of BIF after a daily intake of 2·5 g GOSReference Ito, Deguchi, Matsumoto, Kimura, Onodera and Yajima31 or 4 g PDXReference Jie, Bang-Yao, Ming-Jie, Hai-Wei, Zu-Kang, Ting-Song and Craig25.
None of the treatments in the present study had any significant effect on the total LAB cell numbers in rats, presumably due to the very high indigenous LAB cell numbers. This finding is consistent with results from a previous study where an average amount of total LAB over 8 log10 cfu/g wet weight faeces in rats had been describedReference Wang, Brown, Khaled, Mahoney, Evans and Conway15. As with the BIF, the variations in the LAB cell numbers between the human subjects were also considerable, whereas in the rats the variations were unremarkable. The difference between the LAB concentrations found in rat and human faeces may be partially explained by the differences in their digestive physiology. The proximal stomach in rats is almost free of gastric juice, which enables LAB also to survive in the upper part of the stomach. Thus, the LAB originating from different species may be adapted to different intestinal conditions, as suggested in studies with gnotobiotic rats, where the LAB isolated from the rats were dominant in the stomach whereas the LAB isolated from the human subjects were dominant in the lower gastrointestinal tractReference Kawai, Suegar and Shimohashi27.
The use of germ-free rats inoculated with a human microbiota may have greater relevance in pro- and prebiotics studies than conventional rats despite the fact that the colonisation sites of LAB and BIF isolated from humans and rats have been shown to be differentReference Kawai, Suegar and Shimohashi27. It is of note that rats also have higher energy metabolism and a different pattern of handling coarse food compared with humans. Rats fed with a diet rich in fibre and of low digestibility can utilise the microbial nutrients by eating faecal pellets originated from caecal contents, called coprophagyReference Hörnicke, Björnhag, Ruckebusch and Thivend32. Coprophagy was minimised in the present study by giving the rats easily digestible human food. Thus, conventional rats may be used as a model for studying host interactions with intestinal microbes but correlations between specific probiotic species and host responses are far more complex and require a more in-depth knowledge of interspecies differences in terms of the microbial community structure and function.
In the human clinical trials the effects of the treatments on LAB and BIF appeared to depend on the initial numbers of LAB and BIF. Total LAB numbers were not affected by the treatments if the baseline numbers were moderate or high (> 6 log10 cfu/g) whereas in individuals with a low baseline level ( < 5 log10 cfu/g) the number of LAB increased significantly during the PRO mixture feeding. Neither of the studied prebiotics increased the number of LAB. Previously it has been found that a daily intake of 15 g transgalactosylated disaccharideReference Ito, Kimura, Deguchi, Miyamori-Watabe, Yajima and Kan33 or 4 g PDXReference Jie, Bang-Yao, Ming-Jie, Hai-Wei, Zu-Kang, Ting-Song and Craig25 increased faecal lactobacilli numbers. The initial concentrations of lactobacilli in both of those studies were relatively low (for example, Jie et al. Reference Jie, Bang-Yao, Ming-Jie, Hai-Wei, Zu-Kang, Ting-Song and Craig25; 2·7 log10 cfu/g).
Unlike in the human subjects the baseline BIF cell numbers in the rats were below the detection limit and were increased by the GOSPRO intervention period. In the human subjects BIF numbers were increased by the PDXPRO period attributable to the low BIF numbers at the start. Correspondingly, the rats and the subgroup of human subjects having high LAB numbers at the start showed no effects in LAB numbers by the intervention periods. An increase in LAB numbers after PRO and PDXPRO periods was detected in the subgroup of human subjects having low initial LAB numbers. In summary, the observations on pre- and probiotics in rats cannot automatically be considered representative of humans. Our findings suggest that PDXPRO maintains high concentrations of BIF in humans. Such efficacy was demonstrated among subjects with moderate initial BIF counts. It was also shown that the comparisons between different prebiotic–probiotic combinations are not conclusive using information from independent studies. For the demonstration of the efficacy of pro- and prebiotics the parallel control or the cross-over study should be preferred over the run-in control studies. Moreover, further research is needed on protocol standardisation, for example, the allocation of participants to treatments. This may require pre-study assessment of microbial counts and the use of the counts to minimise differences between groups possible due to high variability in cell counts among participants. Standardisation may also require control of the basal diet in human subjects.
Acknowledgements
We thank Dr Seppo Peuranen and Dr Hannele Kettunen for their help with the rat trials, and Päivi Nurminen and Katja Hatakka for their help with the clinical trials. Thanks are also given to Pirkko Sirviö for preparing the products for clinical trials, Brita Mäki and Sari Kantonen for their analytical support, Akra-Numero Research and Consultancy Centre, India, and Dr Juha Apajalahti and Dr Arthur Ouwehand for their comments. We thank all the volunteers of the Danisco Sugar and Sweeteners Development Centre and Valio Ltd staff who participated in the study. The study was supported by the Finnish Funding Agency of Technology and Innovation. There are no conflicts of interests.




