Introduction
The productivity of dryland mixed crop–livestock systems in the Southern African region is distinctly restricted towards the end of the cool–dry winter; oftentimes extended to spring due to drought (Tavirimirwa et al., Reference Tavirimirwa, Manzungu, Washaya, Ncube, Ncube, Mudzengi and Mwembe2019; Mudzengi et al., Reference Mudzengi, Dahwa, Kapembeza and Muhammad2020; Lamega et al., Reference Lamega, Komainda, Hoffmann, Odhiambo, Ayisi and Isselstein2021a; Letsoalo et al., Reference Letsoalo, Samuels, Cupido, Ntombela, Finca, Foster and Knight2023; Moyo and Ravhuhali, Reference Moyo and Ravhuhali2023). Under such extended drought conditions, improved systems will demand an increase in the use of seeded forages to fill the gaps in feed (Balehegn et al., Reference Balehegn, Kebreab, Tolera, Hunt, Erickson, Crane and Adesogan2021; Paul et al., Reference Paul, Mutegi, Wironen, Wood, Peters, Nyawira, Misiko, Dutta, Zingore, Oberthür, Notenbaert and Cook2023). Therefore, integrating high-quality and quantity winter forages in the feed-base systems can improve feed availability and utilization on farms suffering from drought-related feed gaps during winter. Elsewhere (e.g. Europe, America and Australia), diverse cool-season forage legumes are of general importance for sustainable crop–livestock systems (Anil et al., Reference Anil, Park, Phipps and Miller1998; Rochon et al., Reference Rochon, Doyle, Greef, Hopkins, Molle, Sitzia, Scholefield and Smith2004; Annicchiarico et al., Reference Annicchiarico, Barrett, Brummer, Julier and Marshall2015). However, in temperate regions of e.g. Europe where cool-season forage legumes are grown, winters are mild to cold and wet. Under semi-arid Southern African climate conditions, winters are rather unfavourable with drought interspersed with hot days and cool nights.
The benefits of forage crops for livestock grazing are determined by the growth stage which is heavily influenced by photoperiodism (day length, response to light duration, quality and radiant energy) (Butler et al., Reference Butler, Evers, Hussey and Ringer2002; Iannucci et al., Reference Iannucci, Terribile and Martiniello2008). Therefore, varying photoperiods as a result of seasonal shifts, but also different sowing periods will affect the adaptation of cool-season forage legumes in drier and warmer environments. In line with this, previous studies discussed the advantage of timely sowing of cool-season annual forage legumes to increase the opportunity of sufficient herbage production for grazing under dry and warmer climates e.g. India (Singh et al., Reference Singh, Singh and Kaur2019), South Africa (Muzangwa et al., Reference Muzangwa, Chiduza and Muchaonyerwa2013). Besides different sowing dates, different cool-season annual forage species/cultivars could further alter herbage production, hence, decreasing the opportunity for sufficient herbage for grazing as reviewed by Phelan et al. (Reference Phelan, Moloney, McGeough, Humphreys, Bertilsson, O'Riordan and O'Kiely2015). Their adaptation in dry environments will also be limited by soil physico-chemical conditions, especially with respect to phosphorus availability (Phelan et al., Reference Phelan, Moloney, McGeough, Humphreys, Bertilsson, O'Riordan and O'Kiely2015).
Currently, many regions in southern Africa are faced with frequently variable summer–autumn rainfall and drier winter–spring seasons (IPCC, Reference Pörtner, Roberts, Tignor, Poloczanska, Mintenbeck, Alegría, Craig, Langsdorf, Löschke, Möller and Okem2022), altering the natural communal rangeland production which relies entirely on the summer–autumn rainfall (Tavirimirwa et al., Reference Tavirimirwa, Manzungu, Washaya, Ncube, Ncube, Mudzengi and Mwembe2019; Vetter et al., Reference Vetter, Goodall and Alcock2020; Lamega et al., Reference Lamega, Komainda, Hoffmann, Odhiambo, Ayisi and Isselstein2021a). In the past, different summer-active forage management approaches have been explored as a mitigation option for the productivity of the crop–livestock systems (Valbuena et al., Reference Valbuena, Erenstein, Tui, Abdoulaye, Claessens, Duncan, Gérard, Rufino, Teufel, van Rooyen and van Wijk2012; Balehegn et al., Reference Balehegn, Kebreab, Tolera, Hunt, Erickson, Crane and Adesogan2021; Paul et al., Reference Paul, Mutegi, Wironen, Wood, Peters, Nyawira, Misiko, Dutta, Zingore, Oberthür, Notenbaert and Cook2023). However, an evaluation of these adaptation packages in Zimbabwe (Descheemaeker et al., Reference Descheemaeker, Zijlstra, Masikati, Crespo and Homann-Kee Tui2018) or in South Africa (Pfeiffer et al., Reference Pfeiffer, Hoffmann, Scheiter, Nelson, Isselstein, Ayisi, Odhiambo and Rötter2022) showed the incapacity to fully compensate for the forage deficits during the cool–dry to wet season. Thus, autumn–winter forage production will add value to the crop–livestock systems when communal pastures are in a critical senescence state (Lamega et al., Reference Lamega, Klinck, Komainda, Odhiambo, Ayisi, Isselstein, von Maltitz, Midgley, Veitch, Brümmer, Rötter, Viehberg and Veste2024b). Nonetheless, choosing winter forage legume species that could perform at the marginal semi-arid sites in southern Africa (i.e. high temperatures, poor fertility soils) is crucial (Moyo and Ravhuhali, Reference Moyo and Ravhuhali2023).
Trifolium spp. and Vicia spp. for instance are among the most important and highly digestible forage legumes in dryland livestock-grazing systems in West Asia, North Africa or Australia (Ates et al., Reference Ates, Feindel, El Moneim and Ryan2014). Berseem or Egyptian clover (Trifolium alexandrinum L.), and hairy vetch (Vicia villosa Roth) in particular are energy-rich and widely adapted under warm climates e.g. in Iran (Balazadeh et al., Reference Balazadeh, Zamanian, Golzardi and Torkashvand2021), Egypt (Rady et al., Reference Rady, Attia, Kholif, Sallam and Vargas-Bello-Pérez2022), Pakistan (Ul-Allah et al., Reference Ul-Allah, Khan, Fricke, Buerkert and Wachendorf2015; Tufail et al., Reference Tufail, Nielsen, Southwell, Krebs, Piltz, Norton and Wynn2019) or in South Africa (Muzangwa et al., Reference Muzangwa, Chiduza and Muchaonyerwa2013). These forage legumes could, hence, be suitable as a mitigation strategy to address the forage deficits during the winter–spring periods in dry areas of southern Africa. The main objective of this paper is to provide and discuss information related to testing the suitability of Egyptian clover (T. alexandrinum L.) and hairy vetch (V. villosa Roth) as forage adaptation strategies. To evaluate their adaptation under semi-arid southern African environments, we conducted a fully irrigated field trial at two distinct sites characterized as hot and warm semi-arid over two autumn–winter seasons in the Limpopo province, South Africa. We investigated the effects of the variation between site and sowing date on the accumulated aboveground biomass for distinct harvesting dates to simulate grazing or feed flexibility. Given that the trials are fully irrigated, and that the sites are distinct in terms of soil properties and temperature dynamics, we considered variations in the accumulated aboveground biomass yields as a reason for the interaction effects between sites, and species across harvest dates. Further, we considered delayed-sown forages to induce species-specific response as a result of a combination of temperatures and daylength.
Materials and methods
Description of experimental sites and their environments
The on-station experimentation was conducted at two locations in the Limpopo Province of South Africa. Limpopo is the northernmost province of South Africa bordering internationally Zimbabwe, Botswana, Mozambique, and North West, Gauteng and Mpumalanga provinces nationally. From a biophysical point of view, the province shares many features with the neighbouring regions, thus representative of the semi-arid southern African region. The province is characterized by a varying climate from hot arid to humid subtropical. Rainy seasons in the province occur mainly during the summer (December–February) but could extend between late spring to early autumn (November–April). Meanwhile, the winter period is characterized by extended dry spells (Fig. 1). The crop–livestock management is an important farming system in the region where cropping is based on the summer rainfall and residues are used as feed for livestock in winter (Pfeiffer et al., Reference Pfeiffer, Hoffmann, Scheiter, Nelson, Isselstein, Ayisi, Odhiambo and Rötter2022; Lamega et al., Reference Lamega, Klinck, Komainda, Odhiambo, Ayisi, Isselstein, von Maltitz, Midgley, Veitch, Brümmer, Rötter, Viehberg and Veste2024b). Intercropping remains an important diversification strategy in summer among smallholder farms, while crop rotation is often limited due to limited winter rainfall (Rapholo et al., Reference Rapholo, Odhiambo, Nelson, Rötter, Ayisi, Koch and Hoffmann2019; Pfeiffer et al., Reference Pfeiffer, Hoffmann, Scheiter, Nelson, Isselstein, Ayisi, Odhiambo and Rötter2022). According to Hoffmann et al. (Reference Hoffmann, Odhiambo, Koch, Ayisi, Zhao, Soler and Rötter2018), root zone available water holding capacity limited to cropping land (0–150 cm) remains overall low and varies from 20 to 140 mm across the province. Since the winter period is generally dry (Fig. 1), cropping is limited unless supported by irrigation. Data based on remote sensing suggested that in the winter season of 2015, only 16% out of 1.6 million ha of cropland was irrigated (Cai et al., Reference Cai, Magidi, Nhamo and van Koppen2016). This is partly attributed to the underutilized irrigation schemes in the region as only 69% of the total area equipped for irrigation was irrigated (Cai et al., Reference Cai, Magidi, Nhamo and van Koppen2016).

Figure 1. Average minimum monthly temperatures (lines) and monthly precipitation sum (bars) for 2019 and 2021 compared to long-term values (1985–2020) at the warm semi-arid (black-red = Syferkuil) and hot semi-arid (grey-green = Thohoyandou) sites (top row). The bottom row indicates the monthly sum of irrigated water applied during the winter period for 2019 and 2021 at the two sites.
Two sites were selected and situated at the experimental farm of the University of Limpopo (Syferkuil in Mankweng 23°50001.5 S and 29°41 034.4 E, 1200 m above sea level), and the experimental farm of the University of Venda (in Thohoyandou 22°58049.9 S and 30°26016.8 E, 690 m above sea level). The average annual rainfall (1985–2020) at Syferkuil and Thohoyandou are 485 and 820 mm, respectively (Lam et al., Reference Lam, Rötter, Rapholo, Ayisi, Nelson, Odhiambo and Foord2023). The average minimum and maximum temperatures range from 11 to 28°C at Syferkuil, and from 17 to 30°C in Thohoyandou. Consequently, the environments refer to Syferkuil as the warm site and refer to Thohoyandou as the hot site. The soil texture at the warm site is sandy loam (clay 20%, silt 21%, sand 59%, pHwater 6.8, soil depth 100 cm) referred to as a Chromic Luvisol, whereas at the hot site, the soil texture is clayey (clay 61%, silt 18%, sand 21%, pHwater 5.5, soil depth 120 cm) and the soil type is a Rhodic Ferralsol (Rapholo et al., Reference Rapholo, Odhiambo, Nelson, Rötter, Ayisi, Koch and Hoffmann2019). Though the hot site has a higher clay content enabling good water holding capacity, the site had lower average soil water contents than the warm site in a previous study due to high potential evapotranspiration rates (Rapholo et al., Reference Rapholo, Odhiambo, Nelson, Rötter, Ayisi, Koch and Hoffmann2019). Soil organic carbon content of the topsoil (0–15 cm) ranged between 0.8 and 1.1% at the warm site and between 1.7 and 2.4% at the hot site. Plant available soil nutrient contents of P, K and Mg (mg/kg) were 11, 65 and 271 at the warm and 15, 72 and 389 at the hot sites, respectively.
Experimental design, treatments and data collection
The field trials were conducted over two winter seasons. The first season was established in 2019, while the follow-up experiment was set up in 2021 in an adjacent area. The plot size was 10 m2 (2 m × 5 m) at both sites. The experiments were set up at the onset of the cool winter season to assess the adaptation and production of the chosen forage legumes. The study used irrigation as it helps assess the yield potential of the legume species under study in the given climatic conditions. The treatments across the two experimental seasons were: crop species (vetch: V. villosa Roth: cv. ‘Dr Baumanns’ and Egyptian clover: T. alexandrinum L. cv. ‘Alex’), site (warm, hot semi-arid), sowing date (early, late) and harvest date (hd). A total of four harvesting dates (hd1–hd4) were recorded for the early sowing while three harvesting dates (hd1–hd3) were realized for the late sowing (Table 1). This was done to simulate different harvesting/defoliation opportunities for farmers to extend feed provision hd1: early harvesting, hd2: medium harvesting, hd3: late harvesting and hd4: very late harvesting.
Table 1. A summary of harvesting dates (hd) according to early and late sowing with the corresponding number of days after sowing (DAS, in days), and temperature sum (TS, in °Cd) between the warm semi-arid and hot semi-arid sites

Consequently, one season per year refers to the period from establishment in late autumn until early spring. The experimental setup represented a randomized block design with four replications. In 2019, early sowing was on the 3rd of May at the hot site and on the 10th of May at the warm site. The late sowing was approximately 4 weeks after the early sowing, on the 31st of May and on the 7th of June at the hot and warm sites, respectively. In 2021, early sowing was done on the 4th of May, while late sowing was done on the 6th of June at both sites. As the winter season starts in June the later sowing dates represent drier conditions than the early ones. Average monthly day lengths during the experimental periods were similar at both sites which were about 11.3 h in May and decreased in the winter period to about 10.7 h before increasing in August and September to 11.0 and 11.7 h, respectively. Accumulated temperature over each sowing date until each harvest date for each site was computed as ∑[(Tmax − Tmin)/2] − Tbase (here Tbase = 0°C) according to Bartholomew and Williams (Reference Bartholomew and Williams2006) (Table 1).
Prior to sowing, the soil was tilled to a soil depth of 30 cm after which a smooth seedbed was prepared. The area was demarcated after ploughing and rows for seeding of 15 cm apart were prepared manually. For each season and site, bulk seeding rates were 35 kg/ha for clover and 30 kg/ha for vetch. Both sowings were carried out manually in the prepared seed-beds and phosphorus fertilizers were applied at sowing in the form of superphosphate (10.5% P), at a rate of 30 kg P/ha by broadcasting. Irrigation was carried out using a sprinkler irrigation system consisting of four sprinklers that were assigned independently between blocks in a way to minimize edge effects. Targeted monthly water was fixed to about 100 mm taking into account water scarcity in the study region. We based our estimation on the rainfall patterns and previous studies in the study region (Rapholo et al., Reference Rapholo, Odhiambo, Nelson, Rötter, Ayisi, Koch and Hoffmann2019), and on the production of winter forages under similar conditions (Xu et al., Reference Xu, Gichuki, Shan and Li2006; Muzangwa et al., Reference Muzangwa, Chiduza and Muchaonyerwa2013). The application was 2–3 h, once to twice a week and the amount was determined using measurable water cans installed randomly at the sites. For each month, irrigation was supplied and regulated to avoid sensitivity in the first weeks, and deficit irrigation was applied in the last week to make up the target. Weeds were controlled manually using basic uprooting and chopping techniques in the plots. Aboveground biomass was harvested once per month between June and September for the early sowing, and August and September for the late sowing (Table 1). Harvesting was done from each plot across all four replications. Cordless grass shears were used as a tool to cut the total aboveground biomass at the soil surface inside a 50 cm × 50 cm quadrat frame. The fresh matter was dried at 60°C until constant weight and weighed afterward to determine the dry matter (DM). Former harvesting points were not used again at the subsequent sampling dates to balance the treatment effects across the two sites and seasons. Total water (irrigation + precipitation) was collected during the experimental periods to determine the DM response to water referred to as water use (WU, defined here as dry matter production divided by total water) at each harvest date.
Statistical analyses
Firstly, we calculated the average growth rate (GR, in kg DM/ha/day) of the selected species to provide the growth trends between sowing (early, late) and harvest dates (hd1, hd2, hd3 and hd4) across sites using the following equation:
where GR is the average growth rate (kg DM/ha/day), DM (hd1) is the aboveground dry biomass at a given harvest 1 (in kg/ha), DM (hd0) is the aboveground dry biomass at the previous harvest (in kg/ha), t 1 is the number of days until a given harvest (days) and t 0 is the number of days at the previous harvest date (days).
Secondly, we analysed the DM accumulation using linear mixed-effects models and analysis of variance (ANOVA) in the R Studio 4.2.2 statistical software (R Core Team, 2022). However, to capture the effects of the sowing date more clearly across the distinct environments and species, we subset the aboveground DM based on days after sowing (DAS) and harvesting date (hd) as given in Table 1. Though there were minor variations between the sampling dates across sites we grouped the aboveground biomass per the first three harvest dates only (i.e. hd1, hd2 and hd3) for the statistical analyses. For this, we included only hd1 (~49 DAS), hd2 (~72 DAS), hd3 (~89 DAS) for the early sowing and hd1 (~48 DAS), hd2 (~71 DAS), hd3 (~97 DAS) for the late sowing.
The analyses of variances were conducted using linear mixed-effects models (Pinheiro et al., Reference Pinheiro, Bates, DebRoy and Sarkar2021). For each harvest date (hd1, hd2, hd3), we analysed the fixed and interaction effects of site, sowing date and species on the aboveground biomass accumulation across the seasons. We used block as a factor of replication and year as a random effect to account for the repeated harvesting over time. We also included sites as nested random effects to account for variation when estimating the treatment effects. No singular fit was caused in the model. We proceeded to fit the Maximum Likelihood and Likelihood ratio tests through each model using the ‘MuMIn’ package (Barton, Reference Barton2020) for the ANOVA. We checked the normality of the model residuals graphically in qqplots. Data were transformed when needed to ensure normality and homogeneity of variance. Post-hoc comparisons of means were followed by Tukey's HSD test using the ‘emmeans’ package (Lenth et al., Reference Lenth, Buerkner, Herve, Love, Riebl and Singmann2021) for significant influencing factors. Considering drought as a major challenge for winter forage production in the region, we lastly determined the DM response to total water per harvest date (hd1, hd2, hd3). Similar to the aboveground biomass, the data were analysed in a linear mixed-effects model considering the fixed and interaction effects of site, sowing date, species and site nested in block as random effects for each harvest date. Post-hoc comparisons of means were followed by Tukey's HSD test for significant influencing factors.
Results
Weather conditions and the average growth rate of aboveground biomass
There was a little variation in the timing of rainfall between the two seasons across the sites. During the experimental period, it was consistently dry at the warm site with a total rainfall of less than 5 mm in both 2019 and 2021. Though the hot site was also dry in 2019, in 2021 it received about 50 mm more rainfall. Across sites, the monthly amount of water irrigated was relatively well distributed (Fig. 1). However, the hot environment received a slightly larger total amount of water due to potentially higher evapotranspiration (Table 1). Generally, the coolest site was the warm site with an average monthly minimum temperature dropping to 1.6°C in July 2021.
The growth rate was slow at the beginning for both clover and vetch (usually below 10 kg DM/ha/day) (Fig. 2) with a smaller value of 4.5 kg DM/ha/day when sown later. However, the growth rate increased between hd1 and hd2 to values ranging between 30 and 36 kg DM/ha/day. Late sowing achieved slightly higher values than early sowing for clover while the opposite was true for hairy vetch. At hd3, a clear increase in growth rate was specifically observed for hairy vetch though higher values were recorded after late sowing (Fig. 2). Hairy vetch achieved approximately 60 and 73 kg DM/ha/day on average at hd3 for the early and late sowing, respectively (Fig. 2). On the contrary, the growth rate decreased drastically for clover at hd3 for both sowing dates with a relative slight increase at hd4. However, for vetch, we observed a decrease in the growth rate at hd4 indicating hd3 (~90–97 DAS) as the peak growth rate (Fig. 2).
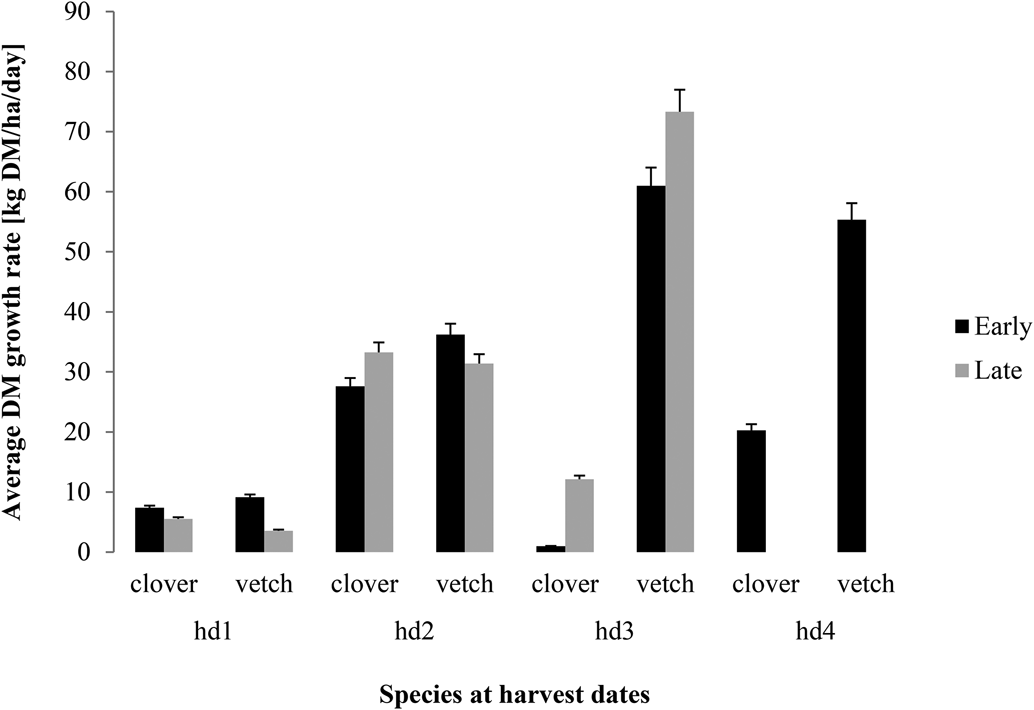
Figure 2. Arithmetic means of the average growth rate of clover and vetch (kg DM/ha/day) ± 5% standard error as affected by sowing date (black: early sowing, grey: late sowing) between harvesting dates (hd1: ~50, hd2: ~70, hd3: ~90 and hd4: ~120 days after sowing for early, hd1: ~50, hd2: ~70, hd3: ~97 days after sowing for late) pooled over sites and years.
Aboveground biomass production and DM response to water application
The statistical models for the aboveground biomass accumulation indicated a significant influence of the interaction of site × sowing date across the harvest dates (hd1: P = 0.01, hd2: P = 0.02 and hd3: P = 0.01; Table 2). Particularly for hd1 and hd2, the effect of site × sowing date is shown by a larger aboveground biomass at the warm site after early sowing compared to late sowing (Table 3). Meanwhile, at the hot site for these harvest dates (hd1, hd2), the accumulated aboveground biomass was not statistically different between early and late sowing (Table 3). The aboveground biomass increased from 461 to 1400 kg DM/ha and from 184 to 775 kg/ha after early and late sowing, at the warm site respectively. At the hot site, on the other hand, aboveground biomass was greater for late- than early-sowing, increasing from 114 to approximately 641 kg DM/ha (early) and 142 to 864 kg DM/ha (late) (Table 3) reflecting the growth rates.
Table 2. Output of the linear mixed-effects models of the aboveground dry matter (DM) accumulation at each harvest date (hd) separately: DM hd1, DM hd2 and DM hd3
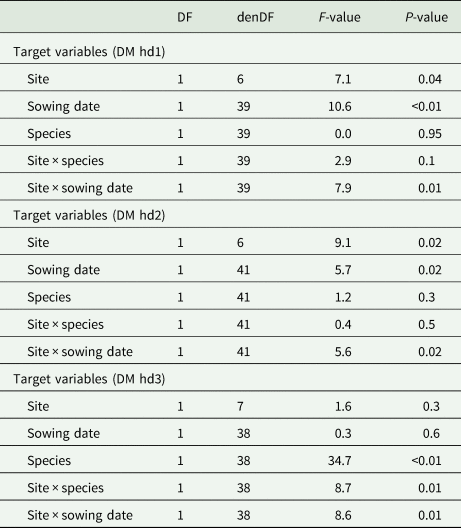
DF, degrees of freedom; denDF, denominator degrees of freedom; F-value, variance ratio.
Table 3. Estimated means of accumulated aboveground dry matter (DM) biomass (kg DM/ha) at the different harvest dates (hd) across sites (warm and hot semi-arid) between sowing date (early, late) (DM hd1: 50 days after sowing, DM hd2: 70 days after sowing and DM hd3a: 90–97 days after sowing) or species (clover, vetch) for DM hd3b (90-97 days after sowing)
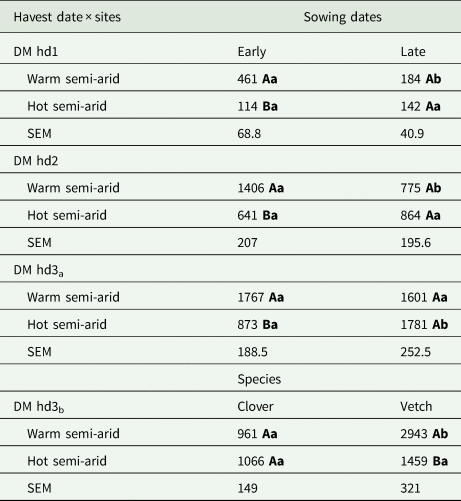
SEM, standard error of the means. Means followed by different uppercase letters within a column are significantly different between site within sowing date, and the different lowercase letters within a row differ significantly between sowing dates within site by Tukey's test (P < 0.05).
The significant interaction effect of site × sowing at hd3 is first demonstrated by similar aboveground biomass at the warm site between early (1767 kg DM/ha) and late sowing (1601 kg DM/ha), whereas at the hot site, significant differences were found between early 873 kg/ha and late sowing 1781 kg DM/ha (Table 3). Additionally, the ANOVA of the finally accumulated aboveground biomass (hd3) was significantly influenced by the interaction effect of site × species (P = 0.01, Table 2). This effect is indicated by no significant differences between means of clover (1066 kg DM/ha) and vetch (1459 kg DM/ha) at the hot site, whereas the inverse was true at the warm site with the aboveground biomass of vetch (2943 kg DM/ha) significantly higher compared to clover (961 kg DM/ha) (Table 3).
Similarly to the aboveground biomass, the analyses of variance for the response of DM to water were affected by the interaction effect of site × sowing date for WU1 i.e. water use at hd1 (P = 0.02), and WU2 i.e. water use at hd2 (P = 0.01, Table 4). Water use at hd3 was not only affected by the effect of site × sowing date (WU3a, P = 0.03) but was also strongly affected by the interaction effect of site × species (WU3b, P < 0.01) (Table 4). At the first two harvest dates hd1 and hd2, the interaction effect was indicated by WU at the warm site that increased from 3.5 to 6.9 and later to 8.3 kg DM/ha/mm at hd3 for earlier sown species (Table 5). In addition, the interaction effect of site × species at hd3 showed that water use varied significantly between species across sites (P < 0.01); as indicated by higher water use for vetch at the warm site (13.5 kg DM/ha/mm), compared to the hot site (6.5 kg DM/ha/mm). On the contrary, for clover, no significant difference in WU was found (4.3 and 4.7 kg DM/ha/mm at the warm and hot sites, respectively Table 5).
Table 4. Output of the linear mixed-effects models of water use (WU) at each harvest date (hd) separately: WU1: water use at hd1, WU2: water use at hd2 and WU3: water use at hd3
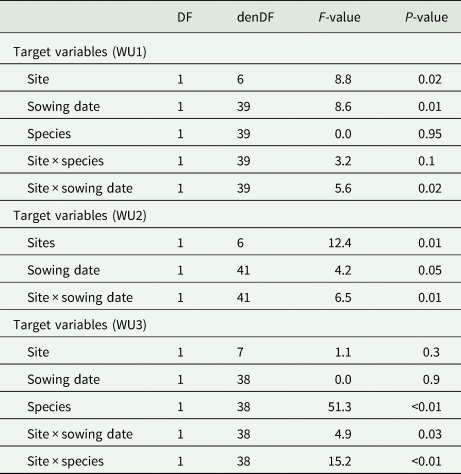
DF, degrees of freedom; denDF, denominator degrees of freedom; F-value, variance ratio.
Table 5. Estimated means of DM response to water (WU, kg DM/ha/mm) at the different harvest dates: WU1: water use at hd1, WU2: water use at hd2, WU3: water use at hd3a across sites (warm and hot semi-arid) between sowing date (early, late) or species (clover, vetch) for WU3b

SEM, standard error of the mean. Means followed by different uppercase letters within a column are significantly different between site within sowing date, and the different lowercase letters within a row differ significantly between sowing dates within site by Tukey's test (P < 0.05).
Discussion
In this study, we conducted and analysed the effects of different sowing dates, sites and harvest dates on the aboveground biomass production of two potentially important forage legumes for the Limpopo province in South Africa namely Egyptian clover and hairy vetch. This study is intended to increase the understanding of the potential of integrating these annual forage crops in dryland mixed crop–livestock farming systems, with a wider application to the southern African region. Furthermore, the results are expected to assist farmer's decision-making for positive impacts concerning improved livestock feed-base systems.
Environmental effects on the aboveground biomass accumulation and water use
One of the criteria for improved livestock productivity is the provision of high-quality forage in the winter–spring period, which is the most critical period to mitigate feed gaps in the dry areas of southern Africa (Lamega et al., Reference Lamega, Klinck, Komainda, Odhiambo, Ayisi, Isselstein, von Maltitz, Midgley, Veitch, Brümmer, Rötter, Viehberg and Veste2024b). Overall, our results show considerable potential for both hairy vetch and Egyptian clover when grown under the given semi-arid climatic conditions and sufficiently supplied with irrigation. We observed accumulated aboveground biomass of about 2943 kg DM/ha for vetch, and 1066 kg DM/ha for clover, 90–100 days after sowing. These values are in the range of those reported under similar climatic conditions in earlier studies for hairy vetch (e.g. China, Zhang et al., Reference Zhang, Duan, Nie, Yang, Ren, van der Werf, Evers, Zhang, Su and Zhang2019) and Egyptian clover (e.g. Egypt, Ibrahim and Nagy, Reference Ibrahim and Nagy2024). For longer growing periods (e.g. hd4), the growth rate indicated higher aboveground DM accumulation, particularly for vetch (Fig. 2). However, under semi-arid conditions, a longer growing period for a very late harvest will increase the exposure to high temperatures (accumulation of radiation) which may reduce forage quality (Sita et al., Reference Sita, Sehgal, HanumanthaRao, Nair, Vara Prasad, Kumar, Gaur, Farooq, Siddique, Varshney and Nayyar2017; Sanderson et al., Reference Sanderson, Johnson and Hendrickson2018). In general, the biomass accumulation of clover was substantially lower than that of the vetch. Contrary to our results, Egyptian clover pure stands grown under semi-arid conditions in Egypt (Rady et al., Reference Rady, Attia, Kholif, Sallam and Vargas-Bello-Pérez2022) or in Iran (Balazadeh et al., Reference Balazadeh, Zamanian, Golzardi and Torkashvand2021), accumulated significantly more biomass (about 6000 kg DM/ha, and up to 14 000 kg DM/ha, respectively). This discrepancy between those values and the results from the present study is likely related to high phosphorus and nitrogen application which may be required as a starter, to boost leguminous forage production at marginal sites (e.g. in Pakistan Ul-Allah et al., Reference Ul-Allah, Khan, Fricke, Buerkert and Wachendorf2015).
One major result of our study is that the effect of the sowing date was strongest under the warm semi-arid environment: the early sowing date produced more biomass than late sowing irrespective of harvest date. Under the hot semi-arid climate the only significant difference between sowing dates was observed at hd3 (97 DAS) with considerably greater biomass for late-sown forage crops. The reason for this pattern at hd3 at the hot semi-arid site was probably related to the accumulated daily temperatures for late sowing. Site-specific sensitivity to temperature and photoperiod are key factors that influence phenological development (Iannucci et al., Reference Iannucci, Terribile and Martiniello2008). Gao et al. (Reference Gao, Su, Chen, Tian and Shen2021), who conducted a comprehensive meta-analysis on the effect of temperature sums on forage legume biomass, concluded that biomass was generally greater at semi-arid sites where accumulated temperatures were lower than 2000°C. The temperature sums in our study were in that range (<2000°C) at the warm semi-arid environment and could explain why biomass accumulation was generally better at the warm semi-arid site after early sowing. Moreover, higher temperatures affect germination and establishment of cool-season forage legumes under semi-arid conditions (Butler et al., Reference Butler, Celen, Webb, Krstic and Interrante2014). This could explain why the growth rates and biomass accumulation, especially under the hot semi-arid environment, were poorer at the earlier harvests. Nevertheless, increased accumulated biomass observed for late sowing at hd3 for the hot site could be explained by photoperiod requirements. As explained by Sennhenn et al. (Reference Sennhenn, Odhiambo, Maass and Whitbread2017), as long as photoperiod requirements are met under warmer conditions, crop development is dominated by temperature only. Sowing date remains, therefore, an important factor for forage growth dynamics and harvest management. Previous research under similar semi-arid environments of e.g. Australia, China or South Africa has shown that delayed sowing decreased biomass accumulation of cool-season annual forages (Muzangwa et al., Reference Muzangwa, Chiduza and Muchaonyerwa2013; Zhang et al., Reference Zhang, Whish, Bell and Nan2017, Reference Zhang, Duan, Nie, Yang, Ren, van der Werf, Evers, Zhang, Su and Zhang2019; Cann et al., Reference Cann, Schillinger, Hunt, Porker and Harris2020; Lamega et al., Reference Lamega, Komainda, Hoffmann, Ayisi, Odhiambo and Isselstein2021b). However, the present study goes beyond the scope of earlier studies as it considers different environmental conditions enabling a more sophisticated evaluation of the importance of either the sowing date or the harvest date for optimized production. Essentially, across harvest dates, species, and sites, late sowing decreased the overall biomass accumulation by 40%. In addition, under hot semi-arid conditions, early sowing increased the biomass accumulation of clover and vetch by 40 and 55%, respectively. Meanwhile, under warm semi-arid conditions, early sowing increased the biomass of vetch and clover by 70 and 64%, respectively. This shows that an early sowing is more decisive for an improved production.
On the other hand, the high variation in the accumulated biomass between the warm and the hot semi-arid environment could be linked to site-specific soil conditions (Phelan et al., Reference Phelan, Moloney, McGeough, Humphreys, Bertilsson, O'Riordan and O'Kiely2015). Here, we found that the highest amount of accumulated biomass was recorded at the sandy-loam warm semi-arid site for vetch. Under warmer climates, varied biomass accumulation of forage legumes was attributed to soil texture and nutrient availability. In detail, Hayes et al. (Reference Hayes, Ara, Badgery, Culvenor, Haling, Harris, Li, Norton, Orgill, Penrose and Smith2019) suggested that in semi-arid regions, soil physicochemical constraints, especially with regard to soil acidity, may have a direct effect on the establishment of forage legumes such as clover spp. Soil pH at the hot site was slightly acidic (5.5) and may explain site-specific species responses. Also, though the clay soil at the hot site may have good water holding capacity, Rapholo et al. (Reference Rapholo, Odhiambo, Nelson, Rötter, Ayisi, Koch and Hoffmann2019) reported for this same study environment that the higher temperatures may lead to a considerable reduction in the water supply due to higher evapotranspiration. This may add to the reasons for a poorer crop establishment at the hot site. Besides, semi-arid soils require for specific forage legumes the adequate N fixing rhizobia bacteria species to improve crop establishment and growth (Phelan et al., Reference Phelan, Moloney, McGeough, Humphreys, Bertilsson, O'Riordan and O'Kiely2015), which may differ from one site to the other (Tumbure et al., Reference Tumbure, Wuta and Mapanda2013). The interaction of climatic conditions with the soil will therefore contribute to variation in the range of biomass production on the farm level. Additionally, as explained by Sennhenn et al. (Reference Sennhenn, Odhiambo, Maass and Whitbread2017), the phenological plasticity of legumes adds to the complexity of interpreting the interaction effects between species and environments. Therefore, further exploration is needed in this context to assess the clear effect of soil types on the selected crop species.
In water-limited environments, water use is an important indicator for evaluating crop-specific adaptability (DeLaune and Mubvumba, Reference DeLaune and Mubvumba2020; Lai et al., Reference Lai, Shen, Wang, Ma, Yang and Ma2022). On average, we found that the response of clover to water applied was lower, while vetch had a significantly greater response, thus higher aboveground biomass. Furthermore, our study suggests this response is influenced by the type of forage crop in interaction with the site. Especially at hd3, higher DM was produced per mm water used of vetch under sandy-loam warm semi-arid, while lower DM per mm was recorded for both clover and vetch under hot conditions. This pattern is consistent with other studies on the generally higher water use of vetch under semi-arid conditions (Xu et al., Reference Xu, Gichuki, Shan and Li2006; DeLaune and Mubvumba, Reference DeLaune and Mubvumba2020). Besides, clover spp. are very sensitive to water availability in dry areas (Hayes et al., Reference Hayes, Ara, Badgery, Culvenor, Haling, Harris, Li, Norton, Orgill, Penrose and Smith2019) which limits its herbage production, unlike vetch. This adds to why the high aboveground biomass production of Egyptian clover under similar environments such as in Pakistan, Iran or Egypt is mainly due to the additive effect of higher irrigation water and fertilizer inputs (Ul-Allah et al., Reference Ul-Allah, Khan, Fricke, Buerkert and Wachendorf2015; Balazadeh et al., Reference Balazadeh, Zamanian, Golzardi and Torkashvand2021; Rady et al., Reference Rady, Attia, Kholif, Sallam and Vargas-Bello-Pérez2022). In our study, irrigation was the main water source in the winter period; therefore, in terms of forage production for livestock grazing, hairy vetch is well adapted and could offer herbage advantages over Egyptian clover.
Implications for mixed crop–livestock farmers
In the semi-arid southern African region, the issue of the forage gap is particularly linked to the lack of forage quality in summer, while in winter both quantity and quality are scarce (Lamega et al., Reference Lamega, Komainda, Ayisi and Isselstein2024a). To maximize growth duration and rates, adjusting the planting date to the onset of the winter period (April/May) is optimum for the selected species. As observed in other studies conducted under similar semi-arid conditions, the high-yielding potential of species such as hairy vetch or Egyptian clover is obtained with early sowing (Muzangwa et al., Reference Muzangwa, Chiduza and Muchaonyerwa2013; Mirsky et al., Reference Mirsky, Ackroyd, Cordeau, Curran, Hashemi, Reberg‐Horton, Ryan and Spargo2017). In semi-arid regions, where the soil is often drier due to soil surface evaporation, particular attention should be paid to forage species that produce considerable aboveground biomass with minimal water input. From a practical point of view, the different growth patterns between species and sites are to be considered to maximize site-specific production. In this regard, clover showed the highest risk in water-limited, low-input environments since higher herbage production will require higher water use and perhaps high nutrient inputs (e.g. phosphorus). Vetch production is therefore well adapted under these marginal conditions (e.g. high temperature, soil constraints) and thus an important cool-season forage legume for the southern African region. Moreover, we are aware that crop yields in semi-arid and arid areas of South Africa are already critically affected by the variation in rainfall patterns and drought (Rapholo et al., Reference Rapholo, Odhiambo, Nelson, Rötter, Ayisi, Koch and Hoffmann2019; Lam et al., Reference Lam, Rötter, Rapholo, Ayisi, Nelson, Odhiambo and Foord2023; Moyo and Ravhuhali, Reference Moyo and Ravhuhali2023), yet, efficient winter forage production can only be successful through irrigation.
Conclusion
In this study, we showed that the development and growth rates of hairy vetch and Egyptian clover in warm and hot semi-arid environments were influenced by pedo-climatic conditions and growth duration. As such, an early sowing date is more important in maximizing aboveground DM production. Moreover, the results suggest vetch as the better adapted cool season forage legume providing adequate DM at about 50, 70 or 90 days after sowing under warm sandy-loam and hot clayey environments; whereas clover showed higher sensitivity to water deficit.
Nevertheless, further studies in this context need to consider the effects of other important factors such as soil water dynamics, evaporation rates, root development and genetic variability of cultivars on biomass production of both clover and vetch. In addition, modelling approaches to evaluate and improve site-specific production and integration of cool-legume species in mixed farm systems may be necessary before adoption.
Data
The data that support the findings of this study are available on request.
Acknowledgements
The authors are thankful for the support of Kabisheng Mabitsela of the Risk and Vulnerability Science Centre, University of Limpopo, and Segolo Pasha of the University of Venda, department of plant and soil sciences during field trials in South Africa.
Author contributions
S. A. L., M. K., K. K. A., J. J. O. O. and J. I. conceived and designed the study. S. A. L. conducted trials, data gathering, performed statistical analyses and wrote the article. J. I. fund acquisition and supervision. M. K., K. K. A., J. J. O. O. and J. I. edited and revised the article. All authors approved the final version of this article.
Funding statement
This work was conducted as part of the South African Limpopo Landscapes Network (SALLnet) financed by the German Federal Ministry of Education and Research (grant number 01LL1802A).
Competing interests
None.
Ethical standards
Not applicable.










