Elevated postprandial glucose and insulin levels are important determinants of metabolic risks that have been implicated in the aetiology of chronic metabolic diseases such as type 2 diabetes( Reference Blaak, Antoine and Benton 1 – Reference Alssema, Boers and Ceriello 3 ). In addition to pharmacological interventions, recent years have witnessed an emergence of dietary interventions and specific foods to reduce large glycaemic excursions. Starch digestibility in carbohydrate-containing foods is known to be affected by the nature of the starch as well as the food processing method. This effect is due to the degree of starch gelatinisation, particle size, food form and cellular structure( Reference Burton, Monro and Alvarez 4 ). These factors in turn influence glucose absorption and glycaemic response (GR)( Reference Jenkins, Wolever and Taylor 5 ).
Bread is one of the most commonly consumed carbohydrate food in the world, and strategies used to attenuate GR include restricting starch digestibility by increasing resistant starch content, increasing viscosity of food matrix to reduce gastric emptying rate and by manipulating its physical structure to make it more compact( Reference Burton, Monro and Alvarez 4 , Reference Fardet, Leenhardt and Lioger 6 , Reference Burton and Lightowler 7 ). One way to increase the resistant starch content is to enhance the formation of amylose–lipid complexes (ALC) during processing( Reference Taylor, Emmambux and Kruger 8 ). Complex formation between fats and amylose takes place readily during heat processing of starch, and has been reported to decrease the susceptibility of enzymatic digestion of starch( Reference Seneviratne and Biliaderis 9 – Reference Putseys, Derde and Lamberts 11 ). Fats play an important role in bread making and impart desirable attributes such as improved gas retention, moist mouthfeel and flavour to the final product( Reference Pareyt, Finnie and Putseys 12 ). It is generally accepted that fats lower GR( Reference Henry, Lightowler and Newens 13 – Reference Thomsen, Storm and Holst 15 ), but the effect of fat types on carbohydrate metabolism remains to be fully investigated. A number of studies have investigated the effect of co-ingestion of different fats with carbohydrate sources such as bread( Reference Henry, Lightowler and Newens 13 , Reference Gatti, Noe and Pazzucconi 16 ) and potato( Reference Macintosh, Holt and Brand-Miller 17 – Reference Rasmussen, Lauszus and Christiansen 20 ) on GR and/or insulinaemic responses (IR). Most studies did not observe significant differences in IR and/or GR between fats with different degree of saturation( Reference Henry, Lightowler and Newens 13 , Reference Gatti, Noe and Pazzucconi 16 , Reference Macintosh, Holt and Brand-Miller 17 , Reference Radulescu, Hassan and Gannon 19 ), although Rasmussen et al.( Reference Rasmussen, Lauszus and Christiansen 20 ) reported a significant reduction of GR and augmentation of IR with butter. However, these studies neither characterised the formation of ALC nor examined the link between ALC formation and GR. Most of the studies that investigated the effect of ALC formation on starch digestibility had been carried out on simple starch gels and were restricted to in vitro analysis( Reference Cui and Oates 10 , Reference Crowe, Seligman and Copeland 21 – Reference Guraya, Kadan and Champagne 23 ). Therefore, this may not reflect the true nature of the complex physiological processes that take place during human digestion.
Carbohydrates and fats of different degree of saturation and chain length form ALC to varying extents( Reference Kaur and Singh 24 ). Given that there is a knowledge gap in understanding how the extent of ALC formation influenced GR, it was of interest to use different types of fats/oils (with varying degree of saturation and chain length) for baking bread and measure the GR and IR after consumption. The aim of the present study was to investigate the influence of fat types on ALC formation during baking of bread and the corresponding GR and IR after consumption of bread.
Methods
Study design and experimental protocol
The present study was an acute, randomised, controlled, single-blinded trial that consisted of five types of bread, each tested on one occasion in a randomised order on separate days, with at least 3 washout days between test visits.
On the day before a test session, participants were requested to abstain from alcohol, restrict their intake of caffeine-containing drinks and avoid participation in intense physical activities. A standardised dinner was provided the evening before to reduce potential variations in GR that may arise because of the second meal effect( Reference Granfeldt, Wu and Bjorck 25 ). Participants were instructed to fast for 10–12 h before reporting to the centre the next morning between 08.00 and 09.00 hours. Upon arrival, participants rested for at least 15 min before starting the test session. An indwelling cannula was inserted into the antecubital vein of the forearm, and a fasting venous blood sample was collected at –5 min. In total, two fasting blood samples were obtained by the finger-prick method using a sterile and single-use lancing device (Accu-Chek Safe; Roche Diagnostics) at –5 and 0 min before consumption of a meal consisting of freshly baked test bread and 100 ml of potable water. The test meal was consumed at a comfortable pace within 15 min. Participants were provided with additional 300 ml of potable water for drinking throughout the remaining part of the test session. Following consumption of test bread, participants were asked to rate their liking of the bread on a 100-mm liking scale. Blood samples (both venous and capillary) were collected at 15, 30, 45, 60, 90, 120, 150 and 180 min after test bread consumption. The same protocol was repeated until the completion of all the five test sessions.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the National Healthcare Group Domain Specific Review Board, under registration number 2014/00849. All subjects provided written informed consent before their participation in the study.
Subjects
Participants were recruited through advertisements and personal communications. Inclusion criteria were as follows: (1) males aged between 21 and 50 years, (2) BMI values between 18·0 and 24·9 kg/m2, (3) blood pressure≤120/80 mg/dl and (4) fasting blood glucose≤6·0 mmol/l. People who had metabolic diseases, were on prescribed medication, were smokers, took part in sports at competitive levels or were concurrently participating in other clinical trials were excluded from the study. Females were excluded from the study to prevent differences in menstrual cycles from affecting carbohydrate metabolism( Reference Blaak 26 ). It is recommended that at least ten subjects are recruited to studies for in vivo assessment of GR( Reference Brouns, Bjorck and Frayn 27 , 28 ), and previous short-term acute studies have recruited between eight and fifteen participants for assessing the effect of fat on metabolic outcomes( Reference Henry, Lightowler and Newens 13 , Reference Macintosh, Holt and Brand-Miller 17 , Reference Radulescu, Hassan and Gannon 19 , Reference Clegg, Pratt and Markey 29 – Reference Joannic, Auboiron and Raison 31 ). A sample size of fifteen was therefore used in this study.
Test bread
The five types of bread used were as follows: control bread without any added fats (CTR) and breads baked with butter (BTR), coconut oil (COC), grapeseed oil (GRP) or olive oil (OLV). The ingredients used for test breads were as follows: 250 g bread flour (Prima), 125 g potable water, 10 g baker’s yeast (SAF), 40 g sugar (Fairprice) and 6 g salt (Fairprice). These ingredients were mixed at speed 1 for 8 min (Kitchenaid) to form base dough, of which 320 g was weighed and then fat/oil was added. The fats/oils added were 96 g butter that contained predominantly SFA (Anchor), 87 g coconut oil that was rich in medium-chain TAG (Titi Ecofarm), 80 g grapeseed oil containing predominantly PUFA (Borges) and 76 g olive oil containing predominantly MUFA (Naturel). The amount of fats/oils added was calculated based on the percentage fat as stated on the nutritional panel on the packaging, and was added at 20 %, w/w of dough. Oil was not added into the control bread. The dough mixture was kneaded for a further 12 min, and was then allowed to rest at room temperature for 10 min. Following this, the dough was moulded into serving portions and proofed in the oven (EOB98000; Electrolux) at 40±1°C for 30 min in a fan-assisted mode. Baking was carried out in the same oven at 200°C for 18 min, and bread was allowed to stand for 10 min before being served warm.
Moisture content of the bread was determined by drying bread in an oven at 105°C overnight for 16 h and by expressing the difference in weight as a percentage of initial weight. Protein content was determined by the Kjeldahl method (Tecator™ Digestor and Kjeltec™ 8200 Auto Distillation Unit; FOSS), using a standard nitrogen-to-protein conversion factor of 5·7. Freeze-dried samples were used for all analyses. Crude fat content was determined gravimetrically by the solvent extraction method (with petroleum ether) using the Soxtec System (Soxtec™ 2055 Manual System; FOSS). The total available carbohydrate content of each type of bread was determined using the Megazyme assay kit (Megazyme).
Measures
Amylose–lipid complex formation
The extent of ALC formation was measured using the complexing index (CI) to relate ALC formation with starch digestibility( Reference Singh, Kaur and Singh 32 ). I solution (2 %, w/w potassium iodide and 1·3 % w/w iodine in deionised water) was prepared for analysis. A 5-g sample of bread crumb, cut into approximately 1 cm×1 cm×1 cm cubes, was mixed with 25 ml of deionised water in a 50-ml falcon tube. The contents were vortexed for 5 min, and then centrifuged for 15 min at 1500 g . The supernatant was filtered through a Whatman No. 3 filter to remove the top layer of fat. The filtrate (0·5 ml) was added to distilled water (7·5 ml) and iodine solution (1 ml). The solution was inverted several times, and absorbance was measured at 690 nm (UV–2600; Shimadzu). All tests were carried out in triplicate (two replicates per set). Pure wheat starch dissolved in deionised water (6 %) was used as a control to ensure reproducibility for all sets. ALC formation was compared using the following equation: CI=(absorbancecontrol bread–absorbancetest bread)/(absorbancecontrol bread)×100. CTR had a reference CI value of 0, as all amylose molecules present were assumed to be freely available for binding with I.
Anthropometric measurements
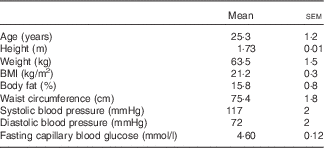
Anthropometric measurements were obtained in a fasting state using standardised methods on the morning of a screening session. Height was recorded to the nearest centimetre using a stadiometer (Seca 763; Medical Scales and Measuring Systems) with the subject standing erect and without shoes. Body weight was recorded to the nearest 0·1 kg using the same stadiometer, with the subject wearing light clothing and no shoes. Height and weight were measured twice and an average reading was used. BMI was calculated using the standard formula: weight (kg)/height (m2), using average height and weight values. Blood pressure readings were obtained using a digital blood pressure monitor (Omron HEM-907). Waist circumference was measured using a standard measuring tape at the midpoint between the coastal margins of the ribs and the upper margin of the iliac crest to the nearest centimetre. Hip circumference was measured at the widest level of the greater trochanters on both sides to the nearest centimetre. Percentage of body fat was measured using a bioimpedance instrument (Tanita BC 418) to the nearest 0·1 %. Physical activity level was quantified using the questionnaire of Baecke et al.( Reference Baecke, Burema and Frijters 33 ).
Glycaemic response
The protocol used to measure blood glucose response was adapted from the method described by Brouns et al.( Reference Brouns, Bjorck and Frayn 27 ) and was in line with the procedures recommended by the Food and Agricultural Organization & the World Health Organization( 28 ). Blood samples were obtained by the finger-prick method using the Unistik® 3 single-use lancing device (Owen Mumford). Participants were encouraged to warm their hand to increase blood flow before a finger-prick test. To minimise plasma dilution, fingertips were gently massaged starting from the base of the hand moving towards the tips. The first two drops of expressed blood were discarded, and the third drop was used for testing. Capillary blood was used for glucose measurements for increased sensitivity to fluctuations in blood glucose concentrations( Reference Granfeldt, Wu and Bjorck 34 ). Blood glucose levels were measured using a HemoCue Glucose 201+ analyzer (HemoCue® Ltd), which was calibrated daily using standard control solutions provided by the manufacturer.
As baseline blood glucose concentrations were not different between test visits, blood glucose concentrations after bread consumption were expressed as ‘change in blood glucose concentrations’ from baseline. These values were obtained by calculating the difference between blood glucose concentrations at each time point and mean baseline values (calculated from the average of two fasting blood glucose values measured at –5 min and 0 min), and represented the relative increment in blood glucose concentrations at each time point. Peak change in blood glucose (Δglucosemax) concentrations was defined as the greatest increment above baseline. The incremental AUC (IAUC) of change in blood glucose concentrations over time (IAUCglucose) was calculated using the trapezoidal rule, and any area under the baseline was ignored( Reference Brouns, Bjorck and Frayn 27 , Reference Allison, Paultre and Maggio 35 ).
Insulinaemic response
Venous blood samples for insulin analysis were transferred to collection tubes containing di-potassium EDTA and centrifuged at 1500 g at 4°C for 10 min (Rotina 420R; Hettich). Plasma samples were stored in Eppendorf tubes at –80°C until analysis. For the analysis, samples were defrosted, and plasma insulin concentrations were determined by electrochemiluminescence immunoassay using an automated analyser (Cobas E411; Roche Diagnostics).
Similar to GR, plasma insulin concentrations after bread consumption were converted into ‘change in plasma insulin’ from baseline. Peak change in plasma insulin concentrations (Δinsulinmax) and IAUC of change in plasma insulin concentrations over time (IAUCinsulin) were calculated in a similar manner as for blood glucose concentrations. Postprandial β-cell function was estimated by two methods as described by Tura et al.( Reference Tura, Kautzky-Willer and Pacini 36 ) and Bermudez et al.( Reference Bermudez, Ortega-Gomez and Varela 37 ): (1) the insulinogenic index (IGI), which is a surrogate measure of first-phase insulin secretion, and was computed as IGI30=(I30–I0)/(G30–G0), where G x and I x are blood glucose and plasma insulin concentrations at x min, respectively; (2) insulin to glucose net incremental area under the time-concentration curve, and was computed as (IAUCinsulin/IAUCglucose×100).
Statistical analyses
Statistical analysis was performed using SPSS version 23 (SPSS Inc.). Data are presented as mean values with their standard errors. The CI values between types of test bread were compared using one-way ANOVA along with Tukey’s test for pair-wise comparisons. Postprandial glucose and insulin data were analysed using the general linear model for repeated-measures ANOVA with post hoc Bonferroni’s corrections. A repeated-measures ANOVA was also performed to determine whether there were significant differences between types of test bread on GR and IR parameters (Δglucosemax, Δinsulinmax, IAUCglucose, IAUCinsulin, IGI30 and insulin:glucose ratio (IGR)). Pearson’s correlation analysis was performed to determine associations between CI and IAUCinsulin and IAUCglucose, and a linear regression model was used to predict IAUCglucose with IAUCinsulin and CI. Statistical significance was set at P≤0·05.
Results
In total, fifteen participants completed the study and the baseline characteristics of the subjects are shown in Table 1.
Table 1 Anthropometric characteristics of healthy, male subjects (Mean values with their standard errors; n 15)
The CI and macronutrient compositions of the types of test bread are shown in Fig. 1 and Table 2, respectively. COC and OLV had significantly lower amylose–lipid complex forming ability as compared with BTR and GRP (P<0·05).

Fig. 1 Complexing index results for different types of bread. Values are means (n 6), with standard errors represented by vertical bars. a,b Mean values with unlike letters were significantly different (P<0·05; one-way ANOVA with post hoc Tukey’s test). BTR, bread baked with butter; COC, bread baked with coconut oil; GRP, bread baked with grapeseed oil; OLV, bread baked with olive oil.
Table 2 Serving size, energy content and macronutrient composition of each type of test bread (per serving basis)
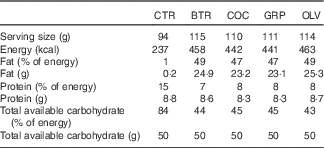
CTR, control bread without added fats; BTR, bread baked with butter; COC, bread baked with coconut oil; GRP, bread baked with grapeseed oil; OLV, bread baked with olive oil.
There were significant time (P<0·001), type of bread (P<0·001) and bread×time interaction (P=0·002) effects on GR (Fig. 2(a)). The significant interaction effects in GR were primarily due to COC, which had a lower GR as compared with CTR (P=0·016). A comparison of GR parameters is shown in Table 3. Significant reductions in Δglucosemax were seen in bread with added fats/oils as compared with CTR (P<0·05), but the effect of different types of fat/oil was not observed. There was an overall significant effect of different breads on IAUCglucose (P=0·027), but post hoc comparisons did not show significant differences between fat types, where the difference between COC and CTR approached statistical significance (P=0·059).

Fig. 2 (a) Postprandial response curves for change in blood glucose and (b) plasma insulin levels after consumption of 50 g available carbohydrate portion of test bread. Values are means (n 15), with standard errors represented by vertical bars. For glucose response, there were significant time (P<0·001), bread (P<0·001) and bread×time interaction effects (P=0·002) when analysed by two-way, repeated-measures ANOVA. For insulin response, two-way, repeated-measures ANOVA showed a significant time effect (P<0·001) and bread×time interaction effect at near significant levels (P=0·074), but no effect of bread was seen (P=0·195). ![]() , Control bread without oil;
, Control bread without oil; ![]() , bread with butter;
, bread with butter; ![]() , bread with coconut oil;
, bread with coconut oil; ![]() , bread with grapeseed oil;
, bread with grapeseed oil; ![]() , bread with olive oil.
, bread with olive oil.
Table 3 Fasting and postprandial parameters for glycaemic and insulinaemic responses after consumption of test bread (Mean values with their standard errors for fifteen healthy young men)
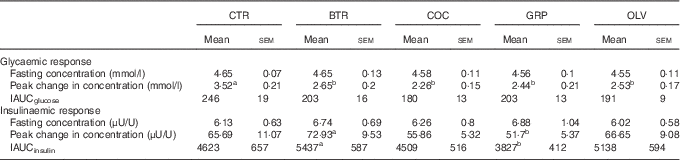
CTR, control bread without added fats; BTR, bread baked with butter; COC, bread baked with coconut oil; GRP, bread baked with grapeseed oil; OLV, bread baked with olive oil; IAUC, net incremental area under curve from 0 to 180 min calculated using trapezoid rule.
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05; one-way, repeated-measures ANOVA with post hoc Bonferroni’s corrections).
IR changed significantly over time (P<0·001) but no bread×time interaction or type of bread effects were found (P=0·074 and P=0·195, respectively) (Fig. 2(b)). Comparisons of Δinsulinmax and IAUCinsulin for the different types of bread are shown in Table 3. There was a significant effect of type of bread on Δinsulinmax (P=0·043) and IAUCinsulin (P=0·016), and post hoc analysis showed differences between BTR and GRP (P<0·05). There were significant effects of adding fats to bread dough on IGI30 (P=0·030), where IGI30 was higher in BTR and OLV than in CTR (P=0·045 and P=0·004, respectively) (Fig. 3). IGR was significantly different between types of bread (P=0·031), with OLV having higher IGR than CTR (P=0·030) (Fig. 3). BTR had higher IGR than CTR at near significant levels (P=0·061). No significant effect of fat types was found for IGI30 and IGR.

Fig. 3 Surrogate measures of postprandial β-cell function after consumption of 50 g available carbohydrate portion of test bread. Values are means (n 15), with standard errors represented by vertical bars. Repeated-measures ANOVA with Bonferroni’s correction was used to analyse significance. Mean values were significantly different from each other: * P<0·05. CTR, control bread without added fats; BTR, bread baked with butter; COC, bread baked with coconut oil; GRP, bread baked with grapeseed oil; OLV, bread baked with olive oil. ![]() , Insulinogenic index at 30 min (IGI30), calculated as IGI30=(I30−I0)/(G30−G0);
, Insulinogenic index at 30 min (IGI30), calculated as IGI30=(I30−I0)/(G30−G0); ![]() , insulin to glucose net incremental area under the curve ratios, insulin:glucose ratio (IGR), calculated as IGR=IAUCinsulin/IAUCglucose.
, insulin to glucose net incremental area under the curve ratios, insulin:glucose ratio (IGR), calculated as IGR=IAUCinsulin/IAUCglucose.
CI was significantly negatively correlated with IAUCglucose (r –0·365, P=0·001), but no significant correlation was found between IAUCglucose and IAUCinsulin (r –0·421, P=0·480). Linear regression analysis showed that CI explained 13·3 % of the variance (P=0·001) and was a significant predictor of IAUCglucose (β=–1·265, P=0·001), but IAUCinsulin did not predict IAUCglucose.
Discussion
Postprandial blood glucose level is a function of both the rate of absorption of glucose into the blood circulation and the rate of removal of glucose due to uptake from circulation by tissue( Reference Schenk, Davidson and Zderic 38 ). Fat is known to reduce GR via reduced absorption into circulation by delaying gastric emptying( Reference Clegg, Pratt and Markey 29 , Reference Gentilcore, Chaikomin and Jones 39 – Reference Owen and Wolever 42 ) or reducing carbohydrate digestibility through the formation of ALC with starch that are resistant to enzymatic digestion( Reference Cui and Oates 10 , Reference Guraya, Kadan and Champagne 23 , Reference Kawai, Takato and Sasaki 43 , Reference Ai, Hasjim and Jane 44 ). This study measured the extent of ALC formation in baked bread using different fat types, and directly correlated ALC formation with GR in healthy subjects. ALC formation was assessed using CI, an index derived from the reduction in the iodine-binding capacity of amylose( Reference Guraya, Kadan and Champagne 23 , Reference Kaur and Singh 24 , Reference Kawai, Takato and Sasaki 43 , Reference Tang and Copeland 45 ). A higher CI value indicated greater formation of ALC. Formation of ALC at temperatures above 90°C allows for the creation of well-defined crystalline structures that are more resistant towards digestion( Reference Putseys, Lamberts and Delcour 46 ). Other than the gelatinisation condition, ALC formation is also influenced by lipid chain length, solubility of lipid in water and degree of saturation( Reference Putseys, Lamberts and Delcour 46 ). The ranking of CI of fats used in this study (OLV>COC>GRP>BTR) differed from earlier studies, which reported lauric acid as having the highest complex forming ability( Reference Kawai, Takato and Sasaki 43 , Reference Tang and Copeland 45 ). This could be due to differences in the nature of starch used, type and concentration of lipids and the conditions for formation of ALC affecting heat stability of complexes. Kawai et al.( Reference Kawai, Takato and Sasaki 43 ) found that oleic acid and lauric acid formed complexes with starch that significantly reduced in vitro starch digestibility, and this corroborated with our results in baked bread, in which OLV (containing oleic acid) and COC (containing MCT such as lauric and myristic acid) had significantly higher CI as compared with BTR and GRP. In addition, CI was negatively and significantly correlated with IAUCglucose, a measure of GR. Linear regression analysis further confirmed that CI was a significant predictor of GR, although it only accounted for 13·3 % of the observed variability. Fats have been shown to lower GR of various carbohydrate sources( Reference Henry, Lightowler and Newens 13 , Reference Macintosh, Holt and Brand-Miller 17 , Reference Gannon, Nuttall and Westphal 18 , Reference Rasmussen, Lauszus and Christiansen 20 , Reference Clegg, Pratt and Markey 29 , Reference Owen and Wolever 42 ), and all test breads with added fat reduced the peaks of GR significantly when compared with CTR (Table 3). When examined as IAUC, COC showed the greatest attenuation of GR in baked bread. A similar study by Clegg et al.( Reference Clegg, Pratt and Markey 29 ) showed that high-fat pancakes containing MCT had the slowest gastric emptying rate as compared with other fats/oils over a 4-h period. The low GR of COC in this study could be due to a combination of factors. These include delay in gastric emptying rates due to MCT having a higher osmolarity( Reference Clegg, Pratt and Markey 29 ) and formation of ALC resulting in resistant starch( Reference Kaur and Singh 24 ).
Elevated glucose concentrations in the intestinal lumen promote insulin secretion, which in turn mediates the uptake of glucose in peripheral tissues( Reference Schenk, Davidson and Zderic 38 , Reference Eelderink, Schepers and Preston 47 – Reference Saltiel and Kahn 49 ). Addition of fats/oils did not alter IR, but a greater IR was observed for BTR than GRP despite having similar GR, indicating that SFA of animal origin were more insulinotropic than PUFA. This was similarly reported in earlier studies by Rasmussen et al.( Reference Rasmussen, Lauszus and Christiansen 20 ), although conflicting results that do not show effects of fat type on IR have also been reported( Reference Thomsen, Storm and Holst 15 , Reference Burdge, Powell and Calder 50 , Reference Brynes, Frost and Edwards 51 ). Surrogate measures of postprandial β-cell function (IGI30 and IGR) were also used to compare IR in relation to GR. IGI30 compared first-phase insulin concentrations in relation to rise in glucose concentrations( Reference Tura, Kautzky-Willer and Pacini 36 ), whereas IGR compared glucose sensitivity with early-phase insulin secretion in terms of IAUC areas( Reference Bermudez, Ortega-Gomez and Varela 37 ). Addition of fat showed a trend for having higher IGI30 and IGR, which was due to similar insulin increments and IAUCinsulin between CTR and bread with added fats, despite the latter showing reduced GR. Although BTR had a greater IR than GRP, insulin sensitivity, as measured with IGI30 and IGR, was not significantly different. This corroborated with the linear regression analysis that insulin secretion did not predict GR in this study, suggesting that the GR effects observed were not due to insulin release or glucose uptake mechanisms, but partially due to rate of appearance of glucose as a result of carbohydrate digestibility.
The fat content of the test bread was higher than commercial bread. However, the amount of fat used attempted to represent a typical serving size of 25 g of butter applied onto two slices of bread. A potential limitation of this study is that gastric emptying was not measured. There are also a number of strengths to this study. A variety of common dietary fats/oils differing in the degree of saturation and carbon chain length was included in this study, which contributed to the understanding of how different fat types alter carbohydrate digestibility, as well as postprandial GR and IR. It was one of the first studies designed to incorporate dietary fats during baking of bread (as actual food system) to understand how formation of ALC could affect GR and IR in humans, rather than using model starch gel systems. The provision of a standardised dinner before test sessions also minimised the potential influences of dietary variations on GR on test days.
Conclusions
Carbohydrate-rich foods are rarely consumed on their own. It is therefore important to understand how the inclusion of the other two macronutrients (proteins and fats) influences glycaemia. In this study, we have focused our attention on incorporating four different types of fats/oils (butter, coconut oil, grapeseed oil and olive oil) varying in chain length and degree of saturation into baked bread. The incorporation of fats during bread baking reduces GR, with the greatest attenuation seen in COC. Lower GR observed in this study was predicted by carbohydrate digestibility via ALC formation, but not by IR. The observation that coconut oil has the greatest impact on GR points to the view that lauric acid and myristic acid in coconut oil may have a contributory influence on reducing GR. These results open up a new avenue of research in identifying other MCT that could be used to lower GR of baked products and other food systems. The use of simple dietary interventions (addition of functional lipids during cooking of carbohydrate-rich staple foods) may be an effective and practical strategy for improving glycaemic control, and may help in the prevention and management of chronic diseases such as type 2 diabetes and CVD.
Acknowledgements
The authors’ thank all the volunteers for their participation in the study, Susanna Lim for her phlebotomy assistance and Dr Tan Sze Yen for his helpful comments on the manuscript.
This research was funded by the Agency for Science, Technology and Research Health and Lifestyle Grant (project number 1121770033).
The authors’ contributions are as follows: C. J. H. and E. L. designed the study; E. L. conducted the study; E. L. analysed the data; C. J. H., W. Z. and E. L. evaluated the results and wrote the paper; and C. J. H., W. Z. and E. L. had primary responsibility for the final content. All the authors have read and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114516001458









