Obesity is an escalating health concern imposed on millions of people worldwide, which is the accumulation of excessive body fat(Reference Tremblay, Clinchamps and Pereira1). Obesity-associated co-morbidities are complicatedly linked with an expansion in reactive oxygen species and consequent oxidative stress, which grounds a state of redox imbalance(Reference Savini, Catani and Evangelista2,Reference Asbaghi, Ghanavati and Ashtary-Larky3) . To preserve reactive oxygen species at sufficient levels, tissues containing antioxidant components work synergically to reduce free radical cytotoxicity(Reference Halliwell4,Reference Pérez-Torres, Guarner-Lans and Rubio-Ruiz5) . The pro-oxidants are significantly generated, and the antioxidant defence mechanisms are weakened, simplifying chronic inflammation, which first dysregulates the vital communication system that adipocytes have within the body(Reference Wensveen, Valentić and Šestan6). The interweaving relation between redox imbalance and the production of inflammatory markers generates an inflammatory milieu influencing metabolic pathways, which can advance impaired physiological functions in obesity and associated consequences(Reference Sankhla, Sharma and Mathur7). In individuals with obesity, the balance of the signalling adipokines is considerably interrupted(Reference Sirico, Bianco and D’Alicandro8). Pro-inflammatory adipokines (IL-6, TNF-α and monocyte chemoattractant protein-1) and leptin, associated with the preservation of obesity, are raised during obesity(Reference Eskandari, Hooshmand Moghadam and Bagheri9). In contrast, adiponectin, which plays an essential role in insulin sensitivity(Reference Bagheri, Rashidlamir and Ashtary-Larky10), is reduced, linking its role to insulin resistance and type 2 diabetes mellitus(Reference Sirico, Bianco and D’Alicandro8).
Evidence suggests that the disorders above may be lessened through a multimodal approach, including diet(Reference Tremblay, Clinchamps and Pereira1,Reference Zouhal, Bagheri and Ashtary-Larky11) , physical activity(Reference Bagheri, Rashidlamir and Ashtary-Larky12,Reference Hooshmand Moghadam, Bagheri and Ghanavati13) and medical treatments(Reference Achkasov, Razina and Runenko14). For instance, natural dietary polyphenolic compounds in green tea are potent antioxidants and anti-inflammatory ingredients that lessen oxidative stress and inflammation and protect the body in opposition to various oxidative stress and inflammation-associated diseases(Reference Lasaite, Spadiene and Savickiene15–Reference Medina-Remón, Casas and Tressserra-Rimbau17). As a matter of fact, the major polyphenols with antioxidant and anti-inflammatory properties in green tea are epicatechin, epigallocatechin-3-gallate (EGCG), epicatechin-3-gallate, epigallocatechin and gallocatechin gallate, which are also called catechins(Reference Sirichaiwetchakoon, Lowe and Eumkeb18). Though several investigations have shown that EGCG and other catechins in green tea augment the activity of antioxidant enzymes and reduce oxidative stress markers and inflammatory markers(Reference Ye, Ye and Xu19–Reference Bogdanski, Suliburska and Szulinska22), there are equivocal effects on markers of oxidative stress, and inflammation in diabetes, metabolic syndrome, β-thalassemia major, and obesity(Reference Lasaite, Spadiene and Savickiene15,Reference Azizbeigi, Stannard and Atashak23–Reference Basu, Du and Sanchez28) . Regarding meta-analysis studies on the roles of green tea extract (GTE) supplementation on inflammation and oxidative stress markers, we should note that a study indicated that GTE supplementation failed to alter inflammatory markers (C-reactive protein, IL-6 and TNF-α) in adults(Reference Haghighatdoost and Hariri29). In contrast, our study revealed decreased C-reactive protein and malondialdehyde (MDA) in type 2 diabetes mellitus patients following GTE supplementation(Reference Asbaghi, Fouladvand and Gonzalez30). Our most recent study indicated that GTE supplementation significantly increased total antioxidant capacity (TAC); moreover, meta-regression analysis revealed a linear inverse association between the dosage and significant change in MDA(Reference Rasaei, Asbaghi and Samadi31). Moreover, while some studies reported that GTE supplementation improves body fatness or mass in individuals with obesity(Reference Wang, Wen and Du32–Reference Yang, Yang and Chao34), some studies did not find any favourable effects(Reference Stendell-Hollis, Thomson and Thompson35,Reference Janssens, Hursel and Westerterp-Plantenga36) . Due to discrepancies in reported results among studies, we aimed to conduct an up-to-date systematic review and meta-analysis study to evaluate the effects of GTE supplementation on inflammatory markers (adiponectin, ghrelin and leptin), oxidative stress (MDA and TAC) and body composition (body mass (BM), BMI, waist circumference (WC), fat mass (FM) and body fat percentage (BFP)) in adults. We hypothesised that our systematic review and meta-analysis would show favourable effects of GTE supplementation on body composition, obesity-related hormones and oxidative stress markers.
Materials and methods
Search strategy and study selection
This meta-analysis was performed based on the Preferred Reporting Item for Systematic Review and Meta-analysis (PRISMA) guideline(Reference Moher, Liberati and Tetzlaff37). A systematic search of studies was performed from inception to July 2022 in databases, including PubMed, ISI Web of Science, and Scopus, without any limitations to language and date. Therefore, these databases were searched using the following search MeSH and non-MeSH terms in titles and abstracts: (‘green tea’(Title/Abstract) OR ‘green tea extract’(Title/Abstract) OR ‘catechin’(Title/Abstract) OR ‘catechins’(Title/Abstract) OR ‘Camellia sinensis’(Title/Abstract) OR ‘Thea sinensis’(Title/Abstract)) AND (Intervention(Title/Abstract) OR ‘controlled trial’(Title/Abstract) OR randomized(Title/Abstract) OR randomised(Title/Abstract) OR random(Title/Abstract) OR randomly(Title/Abstract) OR placebo(Title/Abstract) OR ‘clinical trial’(Title/Abstract) OR Trial(Title/Abstract) OR ‘randomized controlled trial’(Title/Abstract) OR ‘randomized clinical trial’(Title/Abstract) OR RCT(Title/Abstract) OR blinded(Title/Abstract) OR ‘double blind’(Title/Abstract) OR ‘double blinded’(Title/Abstract) OR trial(Title/Abstract) OR trials(Title/Abstract) OR ‘Cross-Over’(Title/Abstract) OR parallel(Title/Abstract) OR). Finally, all searched studies were included in the Endnote software for screening.
Eligibility criteria
All studies that met the following criteria were included in the study: (1) randomised controlled trials (RCT) that evaluated the effects of GTE supplementation on body composition and anthropometric measurements (BM, BMI, WC, BFP and FM), adiponectin, MDA, TAC, leptin, and ghrelin with a control group, (2) parallel or cross-over design, (3) trial duration more than 2 weeks, (4) studies that reported outcomes at the baseline and the end of the intervention, and (5) studies that conducted on adult population (> 18 years old).
Excluded studies
Exclusion criteria included in the study: (1) RCT without a control group, (2) lack of sufficient data on the baseline or follow-up, (3) animal, review and observational studies, (4) Studies were performed on children (< 18 years old) and (5) intervening green tea products in combination with other ingredients.
Data extraction
Initially, records were screened based on title and abstract to determine eligibility for the meta-analysis. Then, to verify eligibility for inclusion, the full text of potential articles was reviewed. Lastly, the following data were extracted: the name of the first author, publication year, location of the study, study design, sample size in each group, individuals’ characteristics such as mean age, sex, and BMI, the GTE doses used for intervention, duration of interventions, mean changes and standard deviation of markers throughout the trial for both intervention and control groups. If a study provided multiple data at different time points, only the latest were considered.
Quality assessment
Two independent reviewers assessed the quality of qualified studies using Version 2 of the Cochrane risk-of-bias tool for randomised trials (RoB 2)(Reference Eldridge, Campbell and Campbell38). It consists of six criteria to evaluate the risk of bias, which are as follows: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and other biases. Consequently, terms such as ‘Low’, ‘High’ or ‘Unclear’ were used to judge each domain. Furthermore, any dissimilarity was resolved by the corresponding authors.
Data synthesis and statistical analysis
In the present study, weighted mean differences (WMD) and the sd of measures from both intervention and control groups were extracted and used to determine the overall effect sizes using the random effects model following DerSimonian And Laird method(Reference DerSimonian and Laird39). Additionally, when mean changes were not reported, we calculated them by using this formula: mean change = final values−baseline values, and s d changes were calculated by the following formula(Reference Borenstein, Hedges and Higgins40):
 $${\eqalign{& {\!\rm{SD\;change}} \cr & \ \ \ = \sqrt {[(\left( {{\rm{SD\;baseline}}} \right)\,\,\unicode{x0302}{2}{\rm{\;}} + {\rm{\;}}\left( {{\rm{SD\;final}}} \right)\,\,\unicode{x0302}{2}{\rm{\;}} - {\rm{\;}}\left( {2{\rm{R\;}} \times {\rm{\;SD\;baseline\;}} \times {\rm{\;SD\;final}}} \right)}} }$$
$${\eqalign{& {\!\rm{SD\;change}} \cr & \ \ \ = \sqrt {[(\left( {{\rm{SD\;baseline}}} \right)\,\,\unicode{x0302}{2}{\rm{\;}} + {\rm{\;}}\left( {{\rm{SD\;final}}} \right)\,\,\unicode{x0302}{2}{\rm{\;}} - {\rm{\;}}\left( {2{\rm{R\;}} \times {\rm{\;SD\;baseline\;}} \times {\rm{\;SD\;final}}} \right)}} }$$
The correlation coefficient or R was considered 0·8 in this formula(Reference Higgins, Thomas and Chandler41). Moreover, we converted se, 95 % CI and interquartile ranges to sd using the method of Hozo et al. which includes the following formulas(Reference Hozo, Djulbegovic and Hozo42):
We applied a random effects model, which considers between-study variations to find the overall effect size. The overall effect size of each variable is shown in forest plots (Fig. 2). Furthermore, we tested between-study heterogeneity by Cochran’s Q test and measured by I-square (I 2) statistic(Reference Higgins, Thompson and Deeks43). I 2 > 40 % or P-value < 0·05 was considered as high between-study heterogeneity. To detect potential sources of heterogeneity(Reference Higgins and Thompson44), subgroup analyses were performed according to pre-planned criteria, including study duration (≤ 12 and > 12 weeks), baseline BMI (overweight (25–29·9 kg.m–2) and obese (> 30 kg.m–2)), intervention doses (mg/d), sex (female, male and both) and general risk of bias (low/unclear/high). We accomplished sensitivity analysis to find the effect of each particular study on the overall estimation(Reference Tobias45). The potential non-linear effects of GTE (mg/d) supplementation and treatment duration (weeks) were investigated using fractional polynomial modelling. Also, we enforced the meta-regression to differentiate the confounders and linear relations among the effect size and duration of intervention, and intervention dosage(Reference Mitchell46). The overall certainty of evidence across the studies was graded according to the guidelines of the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) Working Group. The quality of evidence was classified into four categories, according to the corresponding evaluation criteria: high, moderate, low and very low(Reference Gordon, Oxman and Vist47).
The possibility of publication bias was checked through Egger’s regression test and the visually inspected funnel plot test(Reference Egger, Smith and Schneider48). Statistical analysis was carried out using STATA, version 11.2 (StataCorp). In all analyses, the P-values < 0·05 were considered statistically significant.
Results
Study selection
The databases’ primary search detected 11 286 records. Three thousand five hundred twenty-nine studies were excluded after duplication removal. At this stage, 7689 articles were excluded following evaluating the title and abstract, and the full text of the remaining sixty-eight records was reviewed to confirm eligibility. Nine articles were excluded due to a lack of desired data, including not having desired data. Finally, fifty-nine studies(Reference Azizbeigi, Stannard and Atashak23,Reference Basu, Du and Sanchez28,Reference Freese, Basu and Hietanen49–Reference Bazyar, Hosseini and Saradar105) were included in this systematic review and meta-analysis. The flow chart of the study selection for inclusion trials is shown in Fig. 1.

Fig. 1. Flow chart of study selection for inclusion trials in the systematic review.

Fig. 2. Forest plot detailing weighted mean difference and 95 % CI for the effect of green tea consumption on: (a) body weight (kg); (b) BMI (kg/m2); (c) WC (cm); (d) BFP (%); (e) FM (kg); (f) adiponectin (μg/ml); (g) MDA (µmol/l); (h) TAC (mmol/l); (i) Leptin (ng/ml); and (j) Ghrelin (pg/ml).
Characteristics of the included studies
The detailed characteristics of the included studies are summarised in Table 1. Studies were published between 1999 and 2021 and were carried out in the USA(Reference Basu, Du and Sanchez28,Reference Nantz, Rowe and Bukowski64,Reference Wu, Spicer and Stanczyk72,Reference Dostal, Arikawa and Espejo83,Reference Dostal, Samavat and Espejo84,Reference Kumar, Patel and Pow-Sang91) , UK(Reference Brown, Lane and Coverly58,Reference Frank, George and Lodge61,Reference Brown, Lane and Holyoak66) , Finland(Reference Freese, Basu and Hietanen49), the Netherlands(Reference Kovacs, Lejeune and Nijs50,Reference Westerterp-Plantenga, Lejeune and Kovacs52,Reference Diepvens, Kovacs and Vogels54,Reference Hursel and Westerterp-Plantenga62,Reference Janssens, Hursel and Westerterp-Plantenga78) , Japan(Reference Fukino, Shimbo and Aoki51,Reference Nagao, Hase and Tokimitsu56,Reference Fukino, Ikeda and Maruyama59,Reference Nagao, Meguro and Hase63,Reference Sone, Kuriyama and Nakaya69,Reference Miyazaki, Kotani and Ayabe73) , China(Reference Chan, Koo and Ng53), Australia(Reference Hill, Coates and Buckley55), Taiwan(Reference Hsu, Tsai and Kao60,Reference Hsu, Liao and Lin67,Reference Liu, Huang and Huang75,Reference Kuo, Lin and Bernard79,Reference Chen, Liu and Chiu82,Reference Lu and Hsu86,Reference Huang, Liu and Wang99) , Thailand(Reference Auvichayapat, Prapochanung and Tunkamnerdthai57), Iran(Reference Azizbeigi, Stannard and Atashak23,Reference Mohammadi, Hasseinzadeh Attar and Karimi65,Reference Mirzaei, Hossein-Nezhad and Karimi80,Reference Hovanloo, Fallah Huseini and Hedayati85,Reference Pezeshki, Safi and Feizi87–Reference Hadi, Pourmasoumi and Kafeshani89,Reference Mombaini, Jafarirad and Husain92,Reference Rostamian Mashhadi and Bijeh94–Reference Amozadeh, Shabani and Nazari97,Reference Zandi Dareh Gharibi, Faramarzi and Banitalebi100,Reference Bagheri, Rashidlamir and Ashtary-Larky102–Reference Bazyar, Hosseini and Saradar105) , Poland(Reference Jówko, Sacharuk and Balasińska68,Reference Bogdanski, Suliburska and Szulinska70,Reference Suliburska, Bogdanski and Szulinska71) , Lithuania(Reference Lasaite, Spadiene and Savickiene74,Reference Spadiene, Savickiene and Ivanauskas77) , Spain(Reference Mielgo-Ayuso, Barrenechea and Alcorta76), Brazil(Reference Borges, Papadimitriou and Duarte81,Reference Nogueira, Nogueira Neto and Klein93,Reference de Amorim, Vaz and Cesário98) , Pakistan(Reference Hussain, Habib Ur and Akhtar90) and Mexico(Reference Quezada-Fernández, Trujillo-Quiros and Pascoe-González101). Out of these sixty RCT, five studies(Reference Fukino, Ikeda and Maruyama59,Reference Brown, Lane and Holyoak66,Reference Mombaini, Jafarirad and Husain92,Reference Nogueira, Nogueira Neto and Klein93,Reference Huang, Liu and Wang99) performed as crossover and fifty-four studies(Reference Azizbeigi, Stannard and Atashak23,Reference Basu, Du and Sanchez28,Reference Freese, Basu and Hietanen49–Reference Brown, Lane and Coverly58,Reference Hsu, Tsai and Kao60–Reference Mohammadi, Hasseinzadeh Attar and Karimi65,Reference Hsu, Liao and Lin67–Reference Kumar, Patel and Pow-Sang91,Reference Rostamian Mashhadi and Bijeh94–Reference de Amorim, Vaz and Cesário98,Reference Zandi Dareh Gharibi, Faramarzi and Banitalebi100–Reference Bazyar, Hosseini and Saradar105) as parallel. The follow-up period ranged from 2 to 48 weeks. The intervention dose of GTE supplementation varied between 60 and 3000 mg/d. One thousand nine hundred thirty-one individuals were allocated to the intervention, and 1871 participants were in the control group. Twenty-nine(Reference Basu, Du and Sanchez28,Reference Kovacs, Lejeune and Nijs50–Reference Westerterp-Plantenga, Lejeune and Kovacs52,Reference Nagao, Hase and Tokimitsu56,Reference Auvichayapat, Prapochanung and Tunkamnerdthai57,Reference Fukino, Ikeda and Maruyama59,Reference Hursel and Westerterp-Plantenga62–Reference Mohammadi, Hasseinzadeh Attar and Karimi65,Reference Hsu, Liao and Lin67,Reference Sone, Kuriyama and Nakaya69–Reference Suliburska, Bogdanski and Szulinska71,Reference Miyazaki, Kotani and Ayabe73–Reference Liu, Huang and Huang75,Reference Spadiene, Savickiene and Ivanauskas77,Reference Janssens, Hursel and Westerterp-Plantenga78,Reference Mirzaei, Hossein-Nezhad and Karimi80,Reference Borges, Papadimitriou and Duarte81,Reference Pezeshki, Safi and Feizi87,Reference Hussain, Habib Ur and Akhtar90,Reference Soeizi, Rafraf and Asghari-Jafarabadi95,Reference Tabatabaee, Alavian and Ghalichi96,Reference de Amorim, Vaz and Cesário98,Reference Quezada-Fernández, Trujillo-Quiros and Pascoe-González101,Reference Bazyar, Hosseini and Saradar105) were performed on both sexes, twenty studies were performed only on females(Reference Freese, Basu and Hietanen49,Reference Chan, Koo and Ng53–Reference Hill, Coates and Buckley55,Reference Hsu, Tsai and Kao60,Reference Wu, Spicer and Stanczyk72,Reference Mielgo-Ayuso, Barrenechea and Alcorta76,Reference Chen, Liu and Chiu82–Reference Lu and Hsu86,Reference Afzalpour, Ghasemi and Zarban88,Reference Mombaini, Jafarirad and Husain92–Reference Rostamian Mashhadi and Bijeh94,Reference Amozadeh, Shabani and Nazari97,Reference Huang, Liu and Wang99,Reference Zandi Dareh Gharibi, Faramarzi and Banitalebi100,Reference Bagheri, Rashidlamir and Ashtary-Larky102) and ten study were conducted on males only(Reference Azizbeigi, Stannard and Atashak23,Reference Brown, Lane and Coverly58,Reference Frank, George and Lodge61,Reference Brown, Lane and Holyoak66,Reference Jówko, Sacharuk and Balasińska68,Reference Kuo, Lin and Bernard79,Reference Hadi, Pourmasoumi and Kafeshani89,Reference Kumar, Patel and Pow-Sang91,Reference Bagheri, Rashidlamir and Ashtary-Larky103,Reference Sobhani, Mehrtash and Shirvani104) . The quality assessment of the included studies is presented in Table 2.
Table 1. Characteristics of the included studies

IG, intervention group; CG, control group; GTE, green tea extract; EGCG, epigallocatechin-3-gallate; DB, double-blind; R, randomised; PC, placebo-controlled; F, female; RCT, randomised controlled trial; M, male; GT, green tea; T2DM, type 2 diabetes mellitus; PCOS, polycystic ovary syndrome.
Table 2. Risk of bias assessment

General low risk < 2 unclear risk of bias and no high risk of bias; ⊕.
General moderate risk = 2 unclear risk of bias and no high risk of bias; ?.
General high risk > 2 unclear risk of bias or more than one high risk of bias; ⊖.
Meta-Analysis
Effects of green tea extract supplementation on body mass
Thirty-eight studies reported BM as an outcome measure. Overall results from the random effects model indicated that GTE supplementation resulted in a significant reduction in BM (WMD: −0·64 kg; 95 % CI −0·97, −0·30; P < 0·001) without any significant heterogeneity among studies (I2 = 22·0 %, P = 0·120; Fig. 2(a)). Moreover, subgroup analysis showed that high doses (≥ 1000 mg/d) and interventions on female and male participants with obesity and normal BM individuals did not significantly affect BM (Table 3).
Table 3. Subgroup analyses of GT supplementation on anthropometric measurements, adiponectin, leptin and oxidative stress in adults

GT, green tea; WMD, weighted mean differences; GTE, green tea extract; BM, body mass; WC, waist circumference; BFP, body fat percentage; FM, fat mass; MDA, malondialdehyde; TAC, total antioxidant capacity. Bold values represent statistically significance at the p<0.05
Effects of green tea extract supplementation on BMI
Pooled data from forty-six studies indicated that GTE supplementation reduced BMI (WMD: −0·16 kg.m–2; 95 % CI −0·25, −0·07; P < 0·001) with high heterogeneity among the studies (I2 = 79·6 %, P < 0·001; Fig. 2(b)). Moreover, subgroup analysis showed that high doses (≥ 1000 mg/d), interventions on female participants with obesity and younger than 50 years, and normal BM individuals did not have a significant effect on BMI (Table 3).
Effects of green tea extract supplementation on waist circumference
Results from twenty-six studies demonstrated that GTE supplementation failed to alter WC (WMD: −0·44 cm; 95 % CI −1·19, 0·30; P = 0·244). However, there was significant heterogeneity among the studies (I2 = 90·9 %, P < 0·001; Fig. 2(c)). Moreover, subgroup analyses indicated that GTE supplementation significantly reduced WC in the short term (≤ 12 weeks) and trials conducted on males (Table 3).
Effect of green tea extract supplementation on body fat percentage
Effects of GTE supplementation on BFP were reported in nineteen studies. Combined results from the random effects model indicated that BFP significantly decreased following GTE supplementation (WMD: −0·62 %; 95% CI −1·02, −0·23; P = 0·002); Fig. 2(d)) with high heterogeneity among the studies (I2 = 90·5%, P < 0·001). Furthermore, subgroup analysis revealed that short-term interventions (≤ 12 weeks), lower doses of GTE (< 1000 mg/d) and intervention in people younger than 50 years significantly decreased BFP (Table 3).
Effect of green tea extract supplementation on fat mass
The effects of GTE supplementation on BFP were evaluated in eleven studies. Combined results from the random effects model indicated that GTE supplementation failed to alter FM (WMD: −0·39 kg; 95 % CI −1·19, 0·39; P = 0·324) with high heterogeneity among the studies (I2 = 78·8 %, P < 0·001; Fig. 2(e)). Subgroup analysis indicated a significant reduction in FM in studies conducted on overweight and people younger than 50 years (Table 3).
Effect of green tea extract supplementation on adiponectin
A total of nineteen studies investigated the effects of GTE supplementation on adiponectin. Pooled results from the random effects model indicated that adiponectin significantly increased following GTE supplementation (WMD: 0·62 μg/ml; 95 % CI 0·09, 1·14; P = 0·020) with significant heterogeneity among the studies (I2 = 74·3 %, P < 0·001; Fig. 2(f)). Subgroup analysis showed that GTE supplementation significantly increased adiponectin when GTE was supplemented with higher dosages (≥ 1000 mg/d), in participants with overweight and men older than 50 years.
Effect of green tea extract supplementation on malondialdehyde
Analysis of data from ten studies showed a significant decrease in MDA after GTE supplementation (WMD: −0·32 µmol/l; 95 % CI −0·46, −0·19; P < 0·001) with significant heterogeneity between the studies (I2 = 90·3 %, P < 0·001; Fig. 2(g)). Moreover, subgroup analysis revealed that MDA was significantly reduced following GTE when supplemented by dosages of < 1000 and ≥ 1000 (mg/d) in studies that enrolled females and in short-term studies (≤ 12 weeks) and intervention in people younger than 50 years old (Table 3).
Effect of green tea extract supplementation on total antioxidant capacity
Overall results from eleven studies showed a significant increase in TAC (WMD: 0·10 mmol/l; 95 % CI 0·06, 0·15; P < 0·001) with significant heterogeneity between the studies (I2 = 87·6 %, P < 0·001; Fig. 2(h)). In addition, subgroup analysis showed that TAC significantly increased in studies enrolled in men and short-term studies (≤ 12 weeks) and intervention in people younger than 50 years (Table 3).
Effect of green tea extract supplementation on leptin
Overall results from sixteen studies did not reveal significant alterations in leptin (WMD: −1·01 ng/ml; 95 % CI −3·13, 1·09; P = 0·347), with high between-study heterogeneity (I2 = 92·2 %, P < 0·001; Fig. 2(i)). Subgroup analysis did not reveal any significant effect (Table 3).
Effect of green tea extract supplementation on ghrelin
Results from seven studies did not demonstrate a significant alteration in ghrelin (WMD: −40·09 pg/ml; 95 % CI −117·72, 37·54; P = 0·311), with high between-study heterogeneity (I2 = 84·2 %, P < 0·001; Fig. 2(j)). Subgroup analysis did not reveal any significant effect except for studies conducted on females (Table 3).
Publication bias
Begg’s test did not indicate publication bias for BM (P = 0·087), BMI (P = 0·373), FM (P = 0·889), BFP (P = 0·876), adiponectin (P = 0·184), MDA (P = 0·348), TAC (P = 0·755), leptin (P = 0·685) and ghrelin (P = 0·072), except for WC (P = 0·047). In addition, we conducted Egger’s regression test; however, we did not observe significant publication bias for BM (P = 0·170), WC (P = 0·837), FM (P = 0·155), BFP (P = 0·973), adiponectin (P = 0·051), MDA (P = 0·057), TAC (P = 0·428) and ghrelin (P = 0·111). Nevertheless, there was significant publication bias for BMI (P = 0·015) and leptin (P = 0·048). Funnel plots indicated no evidence of asymmetry in the effects of GTE supplementation on analysed markers except for BMI and leptin (Fig. 3(a)–(j)).

Fig. 3. Funnel plots for the effect of green tea consumption on (a) body weight (kg); (b) BMI (kg/m2); (c) WC (cm); (d) BFP (%); (e) FM (kg); (f) Adiponectin (μg/ml); (g) MDA (µmol/l); (h) TAC (mmol/l); (i) leptin (ng/ml) and (j) ghrelin (pg/ml).
Non-Linear dose–response and meta-regression between dose and duration of green tea extract supplementation
Based on dose and duration, the dose–response analysis did not show significant associations between GTE supplementation and changes in BM, BMI, BFP, adiponectin, TAC, leptin, and ghrelin (Fig. 4(a)–(j)). However, the dose–response analysis showed that GTE supplementation significantly altered WC, FM and MDA based on duration (r = 0·065, P non-linearity < 0·001, r = 0·155, P non-linearity = 0·017 and r = 0·1·768, P non-linearity = 0·03, respectively) in a non-linear fashion (Fig. 5(a)–(j)). Meta-regression showed an inverse association between the intervention doses and the mean difference in MDA (Fig. 6(a)–(j)). Moreover, there is an inverse association between the duration of intervention and the mean difference in TAC (Fig. 7(a)–(j)).

Fig. 4. Non-linear dose–response relations between green tea consumption and absolute mean differences. Dose–response relations between dose (mg/d) and absolute mean differences in: (a) body weight (kg); (b) BMI (kg/m2); (c) WC (cm); (d) BFP (%); (e) FM (kg); (f) adiponectin (μg/ml); (g) MDA (µmol/l); (h) TAC (mmol/l); (i) leptin (ng/ml) and (j) ghrelin (pg/ml).

Fig. 5. Non-linear dose–response relations between green tea consumption and absolute mean differences. Dose–response relations between duration of intervention (week) and absolute mean differences in: (a) body weight (kg); (b) BMI (kg/m2); (c) WC (cm); (d) BFP (%); (e) FM (kg); (f) adiponectin (μg/ml); (g) MDA (µmol/l); (h) TAC (mmol/l); (i) leptin (ng/ml) and (j) ghrelin (pg/ml).

Fig. 6. Linear dose–response relations between green tea consumption and absolute mean differences. Dose–response relations between dose (mg/d) and absolute mean differences in: (a) body weight (kg); (b) BMI (kg/m2); (c) WC (cm); (d) BFP (%); (e) FM (kg); (f) adiponectin (μg/ml); (g) MDA (µmol/l); (h) TAC (mmol/l); (i) leptin (ng/ml) and (j) ghrelin (pg/ml).

Fig. 7. Linear dose–response relations between green tea consumption and absolute mean differences. Dose–response relations between duration of intervention (week) and absolute mean differences in: (a) body weight (kg); (b) BMI (kg/m2); (c) WC (cm); (d) BFP (%); (e) FM (kg); (f) adiponectin (μg/ml); (g) MDA (µmol/l); (h) TAC (mmol/l); (i) leptin (ng/ml) and (j) ghrelin (pg/ml).
Sensitivity analysis
To explore each study’s impact on the overall effect size, we omitted each trial from the analysis step by step. After the removal of the study by Hussain et al. 2017 and Bagheri et al. 2019 et al., respectively, the overall result of adiponectin (WMD: 0·50 μg/ml; 95 % CI −0·02, 1·03) and (WMD: 0·51 μg/ml; 95 % CI −0·02, 1·03) were significantly changed.
Grading of evidence
To assess the quality of evidence for outcomes, the GRADE framework was performed and determined the effect of BM to be of moderate quality. The evidence about BMI, FM, adiponectin, MDA and TAC was downgraded to low. According to the GRADE protocol, evidence regarding WC, BFP, leptin, and ghrelin was of very low quality (Table 4).
Table 4. GRADE profile of GTE supplementation on body composition, adiponectin, leptin, oxidative stress in adults

GRADE, Grading of Recommendations Assessment, Development, and Evaluation; GTE, green tea extract; WMD, weighted mean differences; GT, green tea; WC, waist circumference; BFP, body fat percentage.
* The most of the included studies have high risk of bias.
† There is significant heterogeneity (I2 > 40).
‡ There is no evidence of significant effects of GT intake on WC, BFP, leptin and ghrelin.
Discussion
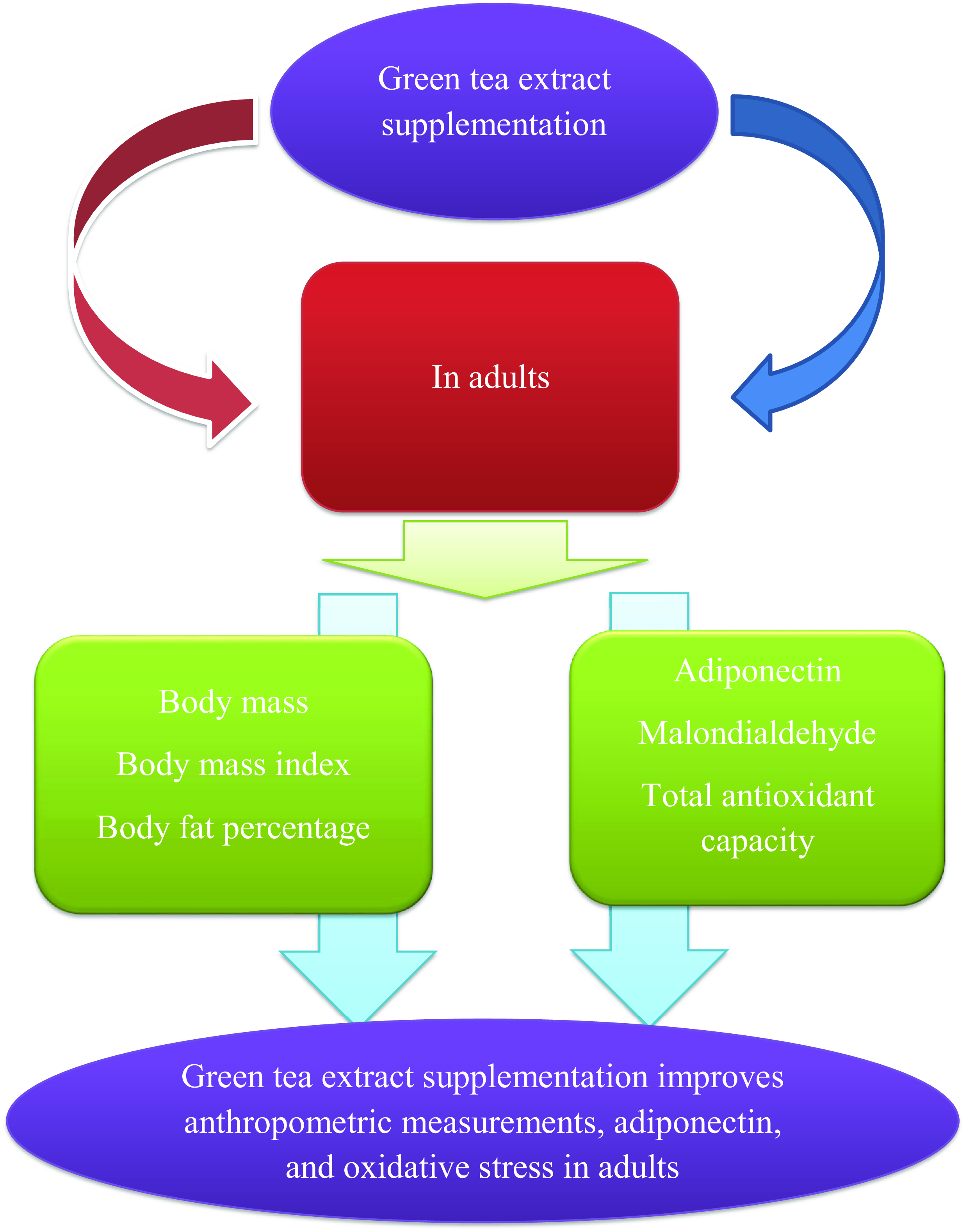
This meta-analysis, including fifty-nine eligible studies with sixty-three arms, was conducted to determine the effects of GTE supplementation, catechin-enriched green tea, EGCG, and other forms of GTE supplementation on body composition, adipose tissue-derived hormones, and oxidative stress markers. The pooled analysis revealed significant lowering effects of GTE supplementation on BM, BMI, BFP and MDA. In addition, a significant increase in adiponectin and TAC was observed.
Prior meta-analytic works by Rasaei et al. (Reference Rasaei, Asbaghi and Samadi31) and Lin et al. (Reference Lin, Shi and Su106) reported the effects of GTE supplementation on antioxidant status and obesity, respectively. The present findings exhibit discrepancies with the results of these two recently published meta-analyses, which provided new knowledge on several relevant topics. First, the number of included studies in our meta-analysis is much higher than in the previous meta-analysis. For example, Rasaei et al. included only 22, 22 and 13 effect sizes for assessing the effects of GTE supplementation on BM, BMI and WC, while we included 37, 45 and 26 trials, respectively. Second, selected variables in Rasaei et al. meta-analysis were limited to BM, BMI, WC and obesity-related markers. They did not evaluate the influence of GTE supplementation on BFP and FM. However, we added more accurate obesity-related markers, including BFP and FM(Reference Ashtary-Larky, Ghanavati and Lamuchi-Deli107). It is well known that obesity is defined as an excess accumulation of body fat not only excess BM. Therefore, FM measurement is the best way to determine obesity and its classification(Reference Ashtary-Larky, Daneghian and Alipour108). Therefore, our analysis presents a better picture of the effects of GTE supplementation on body composition and obesity-related anthropometric indices. Moreover, we analysed hormones that regulated appetite (leptin and ghrelin) to discuss the anti-obesity effects of green tea.
Regarding the antioxidant status, Lin et al. only analysed TAC and MDA as the antioxidant biomarkers, while we determined more variables that indirectly related to antioxidant defiance, including C-reactive protein and adiponectin. Third, the present meta-analysis is the first and only existing meta-analysis that graded the overall certainty of evidence across the studies according to the GRADE guidelines. Due to the mentioned differences in the methodology of our meta-analysis compared with previous studies, we revealed different findings, as mentioned below in the discussion section of the manuscript.
Effects of green tea extract supplementation on body composition
Results showed that GTE supplementation significantly lowered BM, BMI and BFP, while non-significantly reduced WC and FM. However, subgroup analyses by putative influencing factors found considerable variance across subgroups. In this regard, a reduction of BM and BMI was observed in studies with obese and overweight, while studies with normal BM participants were unable to show any significant BM changes. These findings imply that the BM reduction effect of GTE supplementation may depend on participants’ oxidative and inflammatory status, as obesity has been characterised by a low-grade inflammatory state and oxidative stress status(Reference Pereira and Alvarez-Leite109). Numerous epidemiological studies have demonstrated that drinking three to four cups of tea (600–900 mg of tea catechins) can reduce BM, indicators of the metabolic syndrome, and the risk for diabetes and CVD(Reference Sae-tan, Grove and Lambert110–Reference Huang, Wang and Xie112). The results from our study are in line with previous studies, which observed the positive effects of GTE supplementation on BM. In this regard, in eighty overweight, non-diabetic, and dyslipidemic patients with non-alcoholic fatty liver disease, Hussain et al. (Reference Hussain, Habib Ur and Akhtar90) showed that supplementation with 500 mg of GTE twice a day for 3 months caused a significant decrease in BM and BMI. In another study, Tabatabaee et al. (Reference Tabatabaee, Alavian and Ghalichi113) concluded that 3 months of supplementation with 550 mg/d of GTE significantly reduced BMI in obese patients with non-alcoholic fatty liver disease. Another research has also observed similar findings with longer intervention duration(Reference Liu, Huang and Huang75). Even though there have been several studies, the underlying molecular mechanisms of the action of green tea polyphenols in BM loss and reducing the metabolic syndrome are still unclear(Reference Yang, Zhang and Zhang114). It has been proposed that catechins, as the dominant polyphenols in green tea, may reduce digestion and absorption of lipids and proteins in the gastrointestinal tract suggesting their potential role in BM management(Reference Koo and Noh115). Inhibitory activities of catechins against digestive enzymes such as α-amylase, glucosidases and glucose transporters have been observed in previous in vitro studies(Reference Huang, Wang and Xie112,Reference Forester, Gu and Lambert116,Reference Park, Jin and Baek117) . The increase of the probiotic population in the intestine following GTE supplementation has been shown in animal and human studies(Reference Axling, Olsson and Xu118,Reference Jin, Touyama and Hisada119) , demonstrating its possible effects on changing intestinal microbiota correlated with body fatness(Reference Remely, Tesar and Hippe120). In addition, EGCG, the major catechin in green tea and accounts for 50 % to 80 %(Reference Khan, Afaq and Saleem121), can act as an AMP-activated protein kinase activator via altering the AMP:ADP:ATP ratio(Reference Pournourmohammadi, Grimaldi and Stridh122,Reference Li, Gao and Yan123) . The activation of AMP-activated protein kinase can result in BM loss and increase energy metabolism by decreasing gluconeogenesis and fatty acid production and boosting catabolism(Reference Long and Zierath124). However, there are still challenges facing the advantages and adverse effects of GTE, while various health-promoting benefits of tea outweigh its few observed unfavourable effects(Reference Hayat, Iqbal and Malik125).
Subgroup analysis by study duration indicated a significant reduction of WC and BFP in studies with a duration less than or equal to 12 weeks, while the changes in studies lasting more than 12 weeks were not statistically significant. The lack of beneficial effects of GTE supplementation in studies lasting more than 12 weeks is possibly due to the relatively low dose of EGCG, especially in participants previously treated with the aim of BM reduction. In addition, our subgroup analysis by supplementation dosage of less than or equal to 1000 mg/d showed a significant reduction in BM, BMI and BFP. Due to the pharmaceutical properties and observed hepatotoxicity potential of green tea or its specific polyphenols in animal studies(Reference Hu, Webster and Cao126), the effective doses of GTE in our study (< 1000 mg/d) seem to be sufficient for the attributed health effects of green tea. Also, green tea contains tannin, which affects Fe absorption; therefore, nutrient–nutrient interactions following GTE supplementation must be considered(Reference Hambidge127).
Effects of green tea extract supplementation on antioxidants
Our results showed that TAC and MDA significantly changed after GTE supplementation, specifically for periods less than or equal to 12 weeks. In the subgroup analysis, a greater increase in TAC was revealed in studies conducted on men, but only a study among women showed a significant reduction in MDA. Our recent meta-analysis on the antioxidant effects of supplementing with all types of green tea demonstrated beneficial effects on TAC in adults; however, in contrast to our results, no reduction in MDA was observed except for low-dose supplementation and individuals with obesity(Reference Rasaei, Asbaghi and Samadi31). Although it is becoming increasingly evident that GTE supplementation has a potential role in enhancing TAC(Reference Bogdanski, Suliburska and Szulinska70,Reference Suliburska, Bogdanski and Szulinska71,Reference Yang, Lambert and Sang128) , its effects on MDA as a lipid peroxidation indicator have yet to be established. In conflict with our findings, various studies indicated low or no effects of GTE supplementation on MDA(Reference Azizbeigi, Stannard and Atashak23,Reference Kuo, Lin and Bernard79,Reference Tabatabaee, Alavian and Ghalichi113) . More research investigating the impact of GTE supplementation on MDA is needed to expand on our findings. It is well known that the antioxidant-promoting effects of GTE are mainly attributed to its catechins content(Reference Isemura129). Catechins may assist in preventing and protecting against oxidative stress-induced diseases. Catechins exert direct antioxidant effects by scavenging reactive oxygen species and chelating metal ions in which catechins’ phenolic hydroxyl groups can undergo a termination reaction with reactive oxygen species or reactive nitrogen species (RNS), breaking the cycle of radical formation(Reference Bernatoniene and Kopustinskiene130). In this regard, as the dominant catechin in green tea, EGCG has been reported as the most efficient scavenger for a variety of radical species, including superoxide anions, 2,2-diphenyl-1-picrylhydrazyl and hydroxyl radicals(Reference Azman, Peiró and Fajarí131,Reference Fujisawa and Kadoma132) . As indirect impacts, catechins affect protein synthesis and signalling cascades involved in inducing antioxidant enzymes, repressing pro-oxidant enzymes, and generating phase II detoxification enzymes and antioxidant enzymes(Reference Bernatoniene and Kopustinskiene130).
Effects of green tea extract supplementation on adipose tissue-derived hormones and ghrelin
Among adipose tissue-derived hormones assessed in the present study, adiponectin was significantly increased following GTE supplementation. The subgroup analyses further revealed that GTE supplementation enhanced adiponectin in studies with intervention dosages more than or equal to 1000 mg/d in overweight individuals and men compared with other studies. Our results revealed novel findings regarding the effects of GTE supplementation on ghrelin, indicating the lowering effects of GTE on ghrelin in studies conducted on women. The higher ghrelin might partially explain the reason for green tea’s potential effects on ghrelin at the beginning of the study among women. Research suggests that GTE supplementation imparts numerous beneficial effects on adipose tissue-derived hormones. Chen et al. showed that 12 weeks of supplementation with 856·8 mg/d of GTE in women with central obesity significantly increased adiponectin while lowering ghrelin(Reference Chen, Liu and Chiu82). The observed increase in adiponectin in this study may be a consequence of BM loss following GTE supplementation. In support of this claim, the results of a meta-analysis noted that a low-energy diet and its consequence BM loss could substantially enhance adiponectin(Reference Salehi-Abargouei, Izadi and Azadbakht133). Another important factor to be considered is the increasing adiponectin gene expression in animal pre-adipocyte cells after the consumption of green tea catechins(Reference Wu, Hung and Chen134,Reference Cho, Park and Shin135) . These effects of GTE supplementation may be the reason behind the BM reduction-independent effects on adiponectin secretion observed in previous studies(Reference Nagao, Meguro and Hase63,Reference Wu, Spicer and Stanczyk72,Reference Bagheri, Rashidlamir and Ashtary-Larky103) .
Sex and age differences in the effects of green tea extract supplementation
The subgroup analysis revealed sex and age group differences in the effects of GTE supplementation on analysed variables. For example, body composition improvement was significant only in men and when both sexes were included, and there were no statistically significant effects of GTE supplementation in women. It should be noted that sex and age differences in our analysis were minor and may not reach clinical importance. Regarding the low number of included studies to perform subgroup analysis for obesity-related hormones and oxidative stress markers, it is impossible to reveal a clear conclusion on sex differences effects of GTE supplementation. Regarding age differences in the effects of GTE supplementation on body composition, for the first time, we demonstrated that body composition variables, including BM, BMI, FM and BFP, significantly decreased in the younger population (< 50) compared with older individuals (> 50). This phenomenon may be related to a lower BMR, which decreases almost linearly with age(Reference Shimokata and Kuzuya136).
Practical applications
Our findings underlined that GTE supplementation has potential anti-obesity properties in both anthropometrical and hormonal aspects. Although these favourable effects of GTE supplementation were clinically small, coaches and nutritionists could recommend moderate consumption of GTE in athletes and patients with obesity as a part of their lifestyle modification interventions.
Strengths and limitations
This meta-analysis contains some strengths and limitations. The main strength of this study is the relatively acceptable number of studies and high sample size. Another advantage of this meta-analysis is the lack of publication bias and heterogeneity for most variables in the analysis. Moreover, we performed non-linear dose–response and meta-regression between dose and duration of GTE supplementation, significant associations between GTE supplementation, and changes in obesity-related markers. Finally, we performed grading of evidence to assess the quality of evidence for outcomes. However, several limitations to this study need to be acknowledged. First, most studies did not report any data related to body shape or distribution of body fat which are more important risk factors for developing chronic disease. Second, most studies did not report any data on nutrient intake, which does not allow us to rule out their confounding effect. Third, most analyses had high levels of heterogeneity cause of differences in included studies like different types of participants, doses and intervention durations. However, subgroup analysis was performed to examine the possible dose duration and sex and participant differences. Lastly, unfortunately, we did not register the protocol for this review. Also, in the search strategy, we did not use a validated search strategy.
Conclusion
In conclusion, this systematic review and meta-analysis highlighted that GTE supplementation significantly decreased BM, BMI, BFP, and MDA, while increasing TAC and adiponectin. However, it had no significant effect on FM, WC, leptin and ghrelin. An optimal dose of GTE can alleviate cardiometabolic risk factors in the present study.
Acknowledgements
None.
O. A. contributed to the conception and design of the study; M. Z. and M. R. K. contributed to data extraction; N. A. and K. G. screened articles for inclusion criteria; O. A. contributed to data analysis; D. A. L., F. K., M. G., R. B. and O. A. contributed to manuscript drafting; O. A. and M. G. supervised the study; M. Z., N. A., R. B. and F. K. revised the manuscript. All authors approved the final version of the manuscript.
The authors declared that there is no conflict of interest.