INTRODUCTION
Drinking water contaminated with Cryptosporidium oocysts is an established risk factor for human illness [Reference McAnulty1–Reference Goh3]. Contamination of drinking water can arise from a variety of sources [Reference Chalmers4] including raw water and post-treatment contamination with oocysts from infected humans, livestock and feral animals. Oocysts can remain infectious in the environment and water for prolonged periods and are resistant to most disinfectants used to treat drinking water. Inadequate treatment of drinking water can permit transmission of infectious oocysts to susceptible consumers [Reference MacKenzie5–Reference Mason7]. Although infection in immunocompetent people generally results in a self-limiting diarrhoeal illness, cryptosporidiosis in the immunodeficient can result in extra-intestinal disease and life-threatening wasting and malabsorption [Reference Hunter and Nichols8].
The response by the water industry to Cryptosporidium contamination of drinking water has been to establish effective multiple barrier water treatment systems, using a variety of methods, in an effort to reduce this pathogen from drinking-water supplies. These include coagulation, rapid gravity filtration (RGF), dissolved air flotation (DAF) and membrane filtration [Reference Goh3]. We previously performed a retrospective analysis of Cryptosporidium incidence in a population supplied with drinking water, where introduction of both coagulation and RGF had a demonstrable association with a reduction in local incidence rates of cryptosporidiosis [Reference Pollock6].
Low levels of oocysts have been detected in 65–97% of surface-water supplies, suggesting that many populations may be at risk of waterborne infection [Reference Mason7, Reference Juranek9]. In Scotland, the majority of drinking-water supplies to urban areas have had effective forms of water treatment, capable of significantly reducing the Cryptosporidium load in final drinking water. Until September 2007, the Loch Katrine system, which supplies much of the Glasgow and Clyde population had only micro-straining and chlorination but not filtration (Fig. 1). In 2000, an outbreak of cryptosporidiosis in Glasgow residents was associated with drinking water from Loch Katrine [10]. The catchment area around Loch Katrine had a large sheep population, as well as cattle. Livestock were removed from the catchment area by the water authorities, following the outbreak although there was no microbiological evidence that these animals were the source of the outbreak [Reference Elwin and Chalmers11]. Furthermore, in order to eliminate Cryptosporidium from Loch Katrine-sourced water, coagulation and RGF was introduced as a treatment to the Loch Katrine supply in September 2007.

Fig. 1 [colour online]. Layout of Loch Katrine supply to Glasgow.
In order to determine whether Loch Katrine-sourced water was associated with the local incidence of cryptosporidiosis, we performed a retrospective cohort study of all laboratory-confirmed cases in the Glasgow & Clyde area between 2004 and 2010, 3 years either side of the introduction of water filtration. Our hypothesis was that if a proportion of clinical illness was attributable to drinking unfiltered Loch Katrine water, then the incidence should have decreased after the introduction of enhanced water treatment. However, rates of reported cryptosporidiosis in the Glasgow & Clyde NHS Board area were known to be much lower than the mean rate for Scotland. Detecting small changes in incidence might therefore be difficult.
METHODS
The methodology has been previously described elsewhere [Reference Pollock6]. Briefly, a retrospective cohort study of microbiologically confirmed cases of cryptosporidiosis was conducted involving the population of the Glasgow & Clyde NHS Board area. The period of study (2004–2010) was chosen to enable the detection of any epidemiological trends in cases and in risk factor exposures associated with cryptosporidiosis, straddling the introduction of enhanced water treatment. In order to improve the potential power of the analysis and to improve the ability to detect a true difference, if one existed, we pooled all cases.
Both demographic and potential risk factor data from original NHS Health Board case investigation records were reviewed, and were entered into an Excel database. Using home location postal codes, we linked cases to their drinking-water supply zone and water source, details of which were supplied by Scottish Water. For analysis, cases were divided into two groups according to the source of drinking water at the home address (Loch Katrine or other source, i.e. not Loch Katrine). Water treatment for the ‘not Loch Katrine’ drinking water supply did not change from 2004 to 2010. We used the date of laboratory confirmation to code the variables before/after filtration, based on 1 October 2007 as the cut-off.
The potential waterborne oocyst exposure during the study period was addressed using weekly data collected on the levels of oocyst contamination detected in the Loch Katrine drinking-water supply. Large sample volumes (about 1000 litres) were used for oocyst detection to reduce scope for sampling bias, taken at points before and after filtration, i.e. raw and final, treated water. ‘Genera’ filters were used for final water sampling; ‘Cuno’ filters (3M, UK & Ireland) were used for raw water samples due to the higher turbidity of these samples.
Descriptive statistics were used to describe the characteristics of cases and the distribution of cases by water source, before and after the introduction of enhanced physical treatment at Loch Katrine. Cases associated with different water sources were compared using Mann–Whitney tests for quantitative variables and χ 2 tests for qualitative variables. A χ 2 test was then performed to investigate any difference in the distribution of cases in people who received water from Loch Katrine or from other sources, before and after the introduction of enhanced treatment of Loch Katrine water. The factors with χ 2 P values < 0·10 were included in a multiple logistic regression model and we used backward selection by removing the least significant factor at each stage. Unless otherwise stated, Minitab statistical software, version 14 (www.minitab.com) was employed at a significance level of 5% for all analyses.
RESULTS
Of the 452 microbiologically confirmed cases, 15 had no date of laboratory confirmation and were excluded from further analysis. Although there were no reported outbreaks in Glasgow and Clyde between 2004 and 2010, there was a large outbreak in 2005, associated with a wildlife centre in Perthshire, 50 km distant from Glasgow [Reference McGuigan, Steven and Pollock12]. Nineteen cases from Glasgow and Clyde were known to have visited this wildlife park during the exposure period; both microbiological and epidemiologicalal investigations defined these as outbreak-associated cases and so these were also excluded. A small number of cases (n = 23) had no defined water source or were supplied via a private water source, therefore the actual number in the final analysis was 395 (Table 1).
Table 1. Distribution of cases of cryptosporidiosis in Greater Glasgow & Clyde NHS Board area by drinking-water source, before and after the introduction of filtration on Loch Katrine water, Scotland
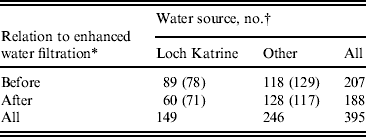
* Coagulation and rapid gravity filtration, introduced in September 2007.
† Numbers in parentheses are cases expected under the null hypothesis (i.e. no association between water supply and filtration).
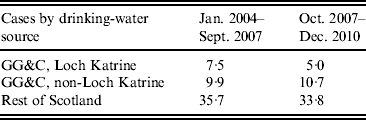
There was no association between water source, gender or age, i.e. those living in the area supplied by Loch Katrine had a similar demographic profile to non-Loch Katrine distributed water. This did not change after filtration was introduced in September 2007. The period incidence of cases supplied with water from Loch Katrine was 7·5 cases/100 000 population before filtration (September 2007) and 5 cases/100 000 after filtration (Table 2). The period incidence of cases who received water from other water sources in the Glasgow & Clyde region was 9·9/100 000 before October 2007 and 10·7/100 000 after. By comparison, the period incidence of cases in the rest of Scotland was 35·7/100 000 before October 2007 and 33·8/100 000 after.
Table 2. Period incidence of cryptosporidiosis cases/100 000 population in Greater Glasgow & Clyde (GG&C) NHS Board area and Scotland, 2004–2010
The period incidence of cases of Cryptosporidium spp. for the Loch Katrine supply area in the Glasgow & Clyde region was analysed for cases before and after filtration (Table 3). Comparison of the observed and expected data illustrates that fewer cases of C. parvum occurred in the area supplied by Loch Katrine after enhanced treatment than would have been expected (P = 0·025). Consequently, there were more cases of C. hominis in the Loch Katrine supply area, after filtration.
Table 3. Period incidence of Cryptosporidium spp., per 100 000 population in Loch Katrine supply area of Greater Glasgow & Clyde NHS Board, 2005–2010

* Health Protection Scotland only received speciated data from April 2005 via the Cryptosporidium Reference Unit, Swansea.
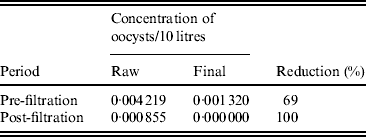
Before September 2007 (the introduction of filtration to Loch Katrine) the oocyst detection rate in Glasgow (final water) averaged 13·2 × 10−4/10 litres (Table 4). Following the introduction of filtration at Loch Katrine, the oocyst count decreased to zero in final water, representing complete removal of waterborne oocysts.
Table 4. Rate of oocysts in raw and final water before and after the installation of a filtration system in Glasgow
Comparison of the observed and expected values (Table 1) illustrates that fewer cases occurred in the area supplied by Loch Katrine after enhanced treatment than would have been expected if there had been no association between the incidence of cases and introduction of drinking-water filtration. The analysis shows a significant association between being a case and living in the area supplied by Loch Katrine water before filtration was introduced (P = 0·023) (Table 5).
Table 5. Reported exposure variables for cryptosporidiosis, Greater Glasgow & Clyde NHS Board area – univariate analysis and logistic regression analysis
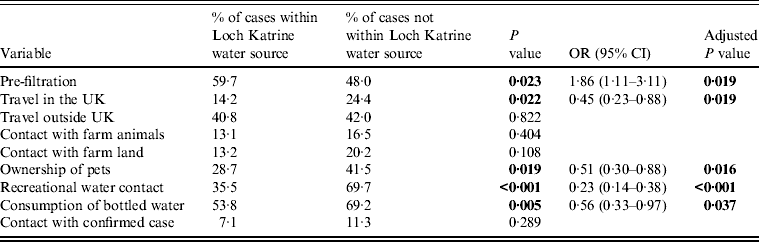
OR, Odds ratio; CI, confidence interval.
There were a number of other putative risk factors associated with being a confirmed case of cryptosporidiosis (Table 5). Fewer cases than expected from the Loch Katrine-supplied area were associated with travel within the UK (P = 0·022), while fewer cases than expected supplied by Loch Katrine, owned pets (P = 0·019). Fewer cases than expected from the Loch Katrine area had recreational water contact compared to cases from non-Loch Katrine areas (P < 0·001). Finally, cases from the Loch Katrine supply area were significantly less likely to consume bottled water compared to their non-Loch Katrine counterparts (P < 0·005).
In the logistic regression analysis, the most significant factor associated with being a Loch Katrine-supplied case was consumption of unfiltered drinking water (P = 0·019) (Table 5), people who consumed Loch Katrine water prior to the introduction of filtration (vs. other studies) were significantly more likely to be a confirmed case of cryptosporidiosis [odds ratio (OR) 1·86, 95% confidence interval (CI) 1·11–3·11]; significantly less likely to have consumed bottled water (OR 0·56, 95% CI 0·33–0·97), owned pets (OR 0·51, 95% CI 0·30–0·88) or had recreational water contact (OR 0·23, 95% CI 0·14–0·38).
DISCUSSION
In Scotland, there has been an intense public interest in the microbial quality of drinking water, largely due to a number of high-profile outbreaks of cryptosporidiosis and incidents resulting in boil-water notices [10, Reference Smith13, Reference Mukherjee14]. The legacy of the Central Scotland E. coli O157 outbreak in 1996 [Reference Cowden15] still prompts significant media and political interest in cases of infectious intestinal disease. Multiple barrier methods have been developed for source and treatment especially in urban areas [Reference Cassady16]. The Loch Katrine distribution system supplying much of the Glasgow and Clyde population was an exception until September 2007.
In order to reduce the risk of waterborne cryptosporidiosis from Loch Katrine, coagulation and RGF was introduced to the Loch Katrine distribution system in September 2007. A sero-epidemiological study was conducted to determine whether there was evidence of an association between the seroprevalence to Cryptosporidium and the type of treatment (Ramsay et al., unpublished data). As a follow-up to this work, we collated data on all laboratory-confirmed cases of cryptosporidiosis in the Greater Glasgow & Clyde NHS Board area from 2004 to 2010 and performed a retrospective cohort study to ascertain whether there was an ecological association between exposure to unfiltered Loch Katrine-sourced drinking water and the incidence of cryptosporidiosis.
The study provided evidence that consumers were being exposed to infectious oocysts in minimally treated tap water. Upgrading the water treatment, using coagulation and RGF was associated with significantly reduced numbers of laboratory-confirmed cases. This is consistent with findings in similar natural experiments involving other drinking water supplies in the UK [Reference Goh3, Reference Pollock6, 17]. The concurrent findings that bottled water consumption, recreational water contact and pet ownership were inversely associated with being a Loch Katrine supply area case are of interest. While consumption of bottled water might suggest reduced consumption of tap water and therefore less potential for waterborne oocyst exposure in the Loch Katrine case cohort, the ‘protective’ element of recreational water contact and pet ownership is unclear although there is some evidence to suggest that pet ownership may be protective [Reference Robertson18].
Chappell and others [Reference Chappell19, Reference Frost20] have postulated that protective immunity may be acquired by repeated low-level exposure to oocysts. However, the same group suggests that clinically apparent infection may develop with ingestion of as little as 10 oocysts [Reference Chappell21]. Since recreational water exposure is considered one of the strongest aetiological risk factors for infection with Cryptosporidium [Reference Yoder, Harral and Beach22, Reference Valderrama23], our finding of previous recreational water exposure being apparently ‘protective’ against developing cryptosporidiosis in the Loch Katrine case cohort, is paradoxical but substantiated elsewhere [Reference Schijven and de Roda Husman24]. Host immunity is multi-factorial and depends on both infectious dose and viability of the consumed oocysts, as well as the immunological status of the host at the time of ingestion and during the incubation period. It is probable that susceptibility/resistance to infection is due to key interactions in the immunological milieu (both cellular and physical) being significantly influenced by subtle extrinsic and intrinsic factors, e.g. steroidal medications for underlying disease or endogenous stress cortisol levels [Reference Tatar, Haziroglu and Hascelik25, Reference Gopal, Birdsell and Monroy26]. This argument could be applied to pet ownership but the situation is likely to be even more complex [Reference Overgaauw27].
We previously performed a study assessing filtration in the Loch Lomond supply to Glasgow and other parts of Central Scotland and observed that the period incidence decreased from 12·6/100 000 to 6·5/100 000 [Reference Pollock6]. It is interesting to note that the period incidence per 100 000 for the Loch Katrine case cohort (before and after filtration) was lower than that for the non-Loch Katrine case cohort. Given that the non-Loch Katrine case cohort received filtered water from Loch Lomond and other sources in the Glasgow area and has done for at least 7 years, this would suggest that rates of cryptosporidiosis are actually increasing in these areas (from 6·5/100 000 for 2000–2003, to 10·7 cases/100 000 for 2007–2010).
While filtration appears to be associated with initially reduced rates of cryptosporidiosis, it may paradoxically make those consumers more susceptible to other transmission routes as demonstrated by an increasing period incidence of C. hominis in the area supplied by Loch Katrine. This finding corroborates previous studies [Reference Frost20] and suggests that a reduction in exposure to one species (C. parvum) through improved physical treatment of drinking water might lead to increased susceptibility to other species (e.g. C. hominis) through other transmission routes such as foreign travel and swimming [Reference Mayne28, Reference Chalmers29].
Given the ecological study design, our conclusions are valid only at the population level; the study was based on the water supply solely to the home location of cases. Data on the water consumption patterns of individuals were insufficient for us to be able to comment on other sources of drinking-water exposure. The ecological study method is, however, widely accepted in environmental epidemiology, as opportunities to collect precise environmental exposure data on individuals are often limited. The inferences gleaned from this study highlight the complexities associated with exposure, infection and immunity to Cryptosporidium.
While all known outbreak cases were excluded from this study, it is plausible that we may have included cases that were associated with undetected mini-outbreaks. Since 2005, most positive isolates for Cryptosporidium spp. have been forwarded to the Cryptosporidium Reference Unit for speciation and/or genotyping. In summary, our data suggest that drinking unfiltered tap water supplied from Loch Katrine was associated with infection with Cryptosporidium spp. at the population level. Upgrading the treatment using a RGF system was associated with a marked reduction in the numbers of cryptosporidiosis cases. These findings support implementation of similar treatment for other unfiltered drinking-water supplies, as a means of reducing clinical cryptosporidiosis.
ACKNOWLEDGEMENTS
The authors acknowledge the Scottish Government (Drinking Water Quality Directorate) for their generous funding of this project.
DECLARATION OF INTEREST
None.








