Iodine deficiency remains one of the commonest micronutrient deficiencies and is the leading cause, worldwide, of potentially preventable brain damage in children(Reference Hetzel1, 2). Based on urinary iodine concentrations (UIC) from samples representing 92 % of the world’s population, it is estimated that nearly two billion people have inadequate iodine nutrition (UIC < 100 μg/l)(Reference de Benoist, Andersson, Takkouche and Egli3–Reference Zimmermann5).
The soils of the Himalayas are severely iodine-deficient(Reference Hetzel4), thus those consuming foods originating from these soils are at high risk of iodine deficiency. An extensive study on iodine deficiency was conducted in the late 1960s by Ibbertson and colleagues among the Sherpas living in the Khumbu region of Nepal(Reference Ibbertson6–Reference Ibbertson9). The overall prevalence of goitre was more than 90 % and the mean urinary iodide concentration was 16·7 (sd 13·2) μg/g creatinine; 40 % of subjects had levels below 10 μg/g creatinine, classifying the population as severely iodine-deficient according to the criteria of WHO/UNICEF/International Council for Control of Iodine Deficiency Disorders (ICCIDD)(2). Approximately 6 % were classified as ‘classical cretins’, 5 % as ‘deaf mutes’ and 3 % had isolated deafness(Reference Ibbertson6–Reference Ibbertson9). Following limited intervention (potassium iodate tablets given to a small group of young children), a programme providing widespread iodised oil injections was implemented between 1966 and 1970(Reference Ibbertson6, Reference Ibbertson10). Iodised oil as capsules was again offered to 1900 women and children in the Khumbu region in 1999(Reference Litch and Bishop11). There have been no subsequent iodised oil programmes in Khumbu, and the main source of iodine in the community is now iodine-containing food products, including iodised salt which has been promoted in Nepal since 1973(Reference Jimba, Aitken, Joshi, Ohashi, Poudyal and Wakai12). Non-iodised Tibetan rock salt is also widely used(Reference Murdoch, Harding and Dunn13). A subsequent survey of iodine status in Khumbu Sherpas was carried out in the early 1990s by Murdoch and colleagues(Reference Murdoch, Harding and Dunn13). Goitre prevalence was 21 % and the median UIC of 35 μg/l classified the population as moderately iodine-deficient. Only one cretinous child had been born since the 1966 supplementation programme began, to an untreated woman. This indicated that iodine deficiency was much less severe, but still a significant public health problem. No further surveys have been conducted.
The results are reported herein of a survey undertaken in 2005, 35 years after the last intensive population-wide intervention programme and 6 years following the more limited intervention involving the offer of iodised oil capsules to women and children. The aim of the survey was to assess the prevalence of iodine deficiency in a Sherpa population in the Khumbu region of Nepal and to investigate dietary habits that might influence iodine status.
Subjects and method
Study design
Random sampling of Khumbu Sherpas to select participants was considered both physically and socially impractical for this study. Instead, a single Khumbu village, Kunde (situated at 3840 m in the Mount Everest National Park), was selected as the location for the research. All Sherpas resident in Khumbu generally consume a similar traditional diet, growing their own potatoes (the staple food) or purchasing food from the same weekly market. Kunde village is located in the centre of the Khumbu region, where villages are spread over a confined area. The population of this village was considered to be representative of the overall population of Khumbu. Following a census of the entire village, an invitation to participate was extended to all Sherpa residents of Kunde. A participation rate of 98·2 % of those individuals present in Kunde during the study period was achieved.
Two experienced local medical assistants from Kunde Hospital, who were well known in the community, assisted with the research. They were able to use the Sherpa language to inform participants about the research, gain informed consent, and administer a questionnaire to gather demographic information, past and present medical history, and household dietary information concerning frequency of consumption of selected foods and salt intake.
Ethical approval was gained from the Chairman of the Himalayan Trust Advisory Committee, Nepal, the Medical Superintendent of Kunde Hospital, Nepal and the University of Otago Human Ethics Committee in New Zealand.
Measurement of goitre prevalence and iodine status
The thyroid glands of all participants were examined by palpation performed by one of the authors (E.E.H.). Classification of goitre was undertaken using the grading system(2): Grade 0 (no goitre), Grade 1 (goitre palpable but not visible), Grade 2 (visible goitre).
A casual morning urine sample was collected from each participant at the time of the researchers’ visit. In accordance with WHO/UNICEF/ICCIDD(2) recommendations, urine samples were stored in screw-top tubes in a cool, dark container to prevent evaporation until samples were transported to New Zealand for analysis. UIC was determined by rate of colour disappearance (the Sandell–Kolthoff reaction)(2, Reference Gnat, Dunn, Chaker, Delange, Vertongen and Dunn14). The accuracy of the method was monitored by the measurement of standards of known iodine concentration and control samples: Seronorm™ Trace Element Urine (LOT NO2525; Sero AS, Billingstad, Norway) with a certified value of 282 (95 % CI 264, 300) μg/l (UIC 273·4 (sd 9·4) μg/l; CV 3·4 %, n 112) and two samples of pooled urine (UIC 58·5 (sd 4·3) μg/l; CV 7·3 %, n 64) were analysed with each batch of analyses.
A finger-prick blood sample for measurement of thyroid-stimulating hormone (TSH) was collected and dried on specimen collection filter paper (Schleicher & Schuell BioScience 903®, Keene, NH, USA) from finger, heel or earlobe of all but three participants using a sterile, single-use lancet. To avoid deterioration of TSH, all blood spot cards were transported to New Zealand within 6 weeks of sample collection and stored at −20°C. TSH analysis was performed using a Coat-A-Count Neonatal TSH IRMA kit (Diagnostic Products Corporation, Los Angeles, CA, USA) with analytical precision of 0·5 μU/ml. Participants found to have abnormal TSH levels were referred to medical services.
Intake of iodine-containing foods
Nepalese packaged iodised salt and Tibetan rock salt were collected for analysis of iodine content. Samples of foods common to the Sherpa diet were also collected: two commonly used brands of milk powder, four types of cheese, buffalo meat, potatoes, instant noodles and the flavour sachets. All samples were obtained from households in the study village or from the local market and transported to New Zealand for analysis. Iodine content in foods was analysed by RJ Hill Laboratories Ltd (Hamilton, New Zealand) by a modification of the procedure of Fecher and colleagues(Reference Fecher, Goldmann and Nagengast15) using inductively coupled plasma–mass spectrometry.
Statistical analysis
The majority of the data were analysed using Microsoft Excel 2004 for Macintosh (Microsoft Corp., Redmond, WA, USA). InStat for MacIntosh version 2·01 (GraphPad Software, San Diego, CA, USA) was used to compare data using the χ 2 test and Fisher’s exact test. The STATA statistical software package release 9 (StataCorp, College Station, TX, USA) was used for regression analysis of the log-transformed urinary iodine data, adjusting for sex and age, and for sex, age, grade of goitre and time spent outside Khumbu, and to directly standardise the goitre prevalence against the Segi world population, to facilitate comparisons of sets of age-specific epidemiological and demographic rates across populations with different age composition(Reference Segi16).
Results
Of the 219 permanent residents of Kunde present in the village during the study period, 215 (98·2 %; 100 males, 115 females) agreed to participate. The age of the male participants ranged from 22 months to 85 years (mean 34 (sd 21) years) and the females’ age from 9 months to 94 years (mean 40 (sd 24) years). Twenty-three per cent of males and 30 % of females reported having received iodised oil injections at some stage in the past, but none in the last 5 years.
The prevalence of goitre in Kunde in relation to age and sex is summarised in Table 1. Standardised to the world population, the adjusted rate for the overall prevalence was 27·4 % (95 % CI 22·9, 31·9 %), and the adjusted prevalence of Grade 2 goitre was 2·8 % (95 % CI 1·0, 4·6 %). In both males and females, the prevalence of Grade 1 and Grade 2 goitres increased with age.
Table 1 Prevalence of goitreFootnote * in relation to age and sex in residents of Kunde village in Khumbu region, Nepal
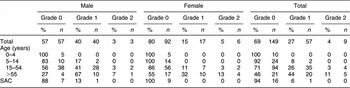
SAC, school-aged children (6–12 years).
* Grade 0 is no palpable or visible goitre (i.e. absence of goitre), Grade 1 is palpable but not visible, Grade 2 is clearly visible(2).
Median UIC values are presented in Table 2. Thirty per cent (22 % of males, 37 % of females) had UIC less than 50 μg/l and over half of the population (52 %) had concentrations below 100 μg/l. Excessive UIC (≥300 μg/l) was found in 16 % of males and 13 % females, but 31 % of children aged 14 years and under had levels in this range. Females had a lower median UIC than males (P = 0·020) and the median UIC for both sexes decreased with increasing age (P ≤ 0·001).
Table 2 Urinary iodine concentrations (μg/l) in relation to sex, age, goitre grade and time spent outside Khumbu: residents of Kunde village in Khumbu region, Nepal
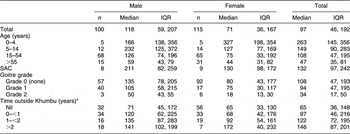
IQR, interquartile range; SAC, school-aged children (6–12 years).
*Estimated total time spent living outside Khumbu during the previous 5 years.
The results of the regression analysis of the log-transformed UIC values after adjustment for sex, age, grade of goitre and time spent outside Khumbu are presented in Table 3. The apparently higher UIC in males compared with females (P = 0·020) was no longer significant after adjusting for age, grade of goitre and time spent away from Khumbu (P = 0·166), whereas decreasing UIC with increasing age remained significant after adjustment (P = 0·01). Those with Grade 2 goitre had significantly lower UIC than those with no goitre after adjustment (P = 0·012). Time spent away from Khumbu was also associated with higher UIC, those spending 1–2 years away having significantly higher levels than those who had spent no time outside Khumbu (P = 0·024).
Table 3 Regression analysis of urinary iodine concentrationsFootnote *v. sex, age, grade of goitre and time outside Khumbu: residents of Kunde village in Khumbu region, Nepal
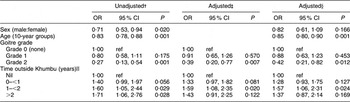
ref, referent category.
* Urinary iodine concentrations were log-transformed for the analysis.
† Ratio of geometric means.
‡ Adjusted for sex and age.
§ Adjusted for sex, age, grade of goitre and time outside Khumbu.
‖ Time outside Khumbu during the previous 5 years.
TSH concentrations in whole blood spots were available for 210 of the 215 participants. Two results were below the lower range of detection and three women did not provide blood samples. Median (interquartile range, IQR) TSH concentration was 2·1 (1·2, 3·5) μU/ml (males, 2·4 (1·3, 3·9) μU/ml; females, 1·9 (0·9, 3·2) μU/ml). Ten per cent of the participants had TSH concentrations above normal (greater than 5 μU/ml)(2, 17).
Iodine concentrations in the two types of salt available in Kunde households were 0·07 mg/kg in Tibetan rock salt compared with an average of 54·2 mg/kg in Nepalese iodised salt. Sixty-three of sixty-four households provided information on salt use; 75 % of the households used both types of salt, 16 % reported using only iodised salt, 8 % only rock salt and one household reported not using salt. The majority of households (95 %) always, or nearly always, added salt while cooking. Addition of salt to meals after preparation was variable, with 5 % always or nearly always adding salt, 51 % sometimes adding salt after tasting and 44 % rarely or never adding salt to meals. Of those households using iodised salt, the majority (93 %) stored their salt in sealed containers. Comparisons between those who did (n 199) and those who did not (n 16) use iodised salt are limited by the relatively small number who did not. Nevertheless, it is of interest that the prevalence of goitre (Grade 1 or 2) was greater among those not consuming iodised salt (50 %) than among those who did (29 %). The difference in median UIC (IQR) was striking between those who did (105 (44, 194) μg/l) and did not (33 (20, 88) μg/l) consume iodised salt. The percentage of participants with TSH concentrations greater than 5 μU/ml was only slightly greater in the group not consuming iodised salt (13 % v. 10 %).
The diet of Sherpas in Kunde comprised a limited number of foods. Rice, potatoes and green vegetables were consumed daily, red meat weekly and other meat or fish rarely. Milk powder was used three to four times per day (fresh milk being available for only a few months each year), cheese three to four times per week and yoghurt rarely. One of the most frequently consumed manufactured foods was instant noodles. Iodine analysis of the potatoes (<0·01 mg/kg) confirmed the expected low values in locally grown vegetables. Concentrations in buffalo meat (0·01 mg/kg) and dairy products (0·02 to 0·71 mg/kg) were also low. The two most popular brands of milk powder had iodine concentrations of 0·25 and 0·26 mg/kg. The two varieties of instant noodles tested contained 0·62 mg/kg and 1·4 mg/kg and their flavouring sachets, 32·9 mg/kg and 18·3 mg/kg, respectively. These noodles and their flavour sachets were consumed daily by children, uncooked straight from the packet. On the other hand, adults consumed boiled noodles and not generally on a daily basis.
Discussion
The prevalence of severe iodine deficiency among the Sherpas of Khumbu in Nepal has reduced dramatically since the 1960s(Reference Ibbertson, Tait, Pearl, Lim, McKinnon and Gill7, Reference Ibbertson, Tait, Pearl, Lim, McKinnon and Gill8), total goitre prevalence having decreased from over 90 % to 31 % and the prevalence of Grade 2 goitre from 63 % to 4·2 %. Total goitre prevalence was higher than in the study of Murdoch and colleagues in the 1990s(Reference Murdoch, Harding and Dunn13); however, participants in that study were not randomly selected nor did they constitute the entire population of a geographically defined area. The WHO/UNICEF/ICCIDD(2, 17) recommendations suggest that a total goitre prevalence of 5 % or more in school-aged children (6 to 12 years of age) indicates a public health problem. In the present survey only one of seventeen children in this age group had goitre, suggesting a relatively mild problem. However, because of the small number in this age group, this estimate may not be reliable.
Given that thyroid size is a good indicator of past but not necessarily present iodine status(Reference Delange, de Benoist, Pretell and Dunn18), UIC is generally considered to be the indicator of choice for assessment of current iodine status in a population group, but not in an individual. Optimum iodine nutrition has been defined as a median level for a population of 100 μg/l or greater(2) and a population frequency of low iodine concentration (<50 μg/l) less than 20 %(17). The median UIC of 96·5 μg/l in this population and the 30·2 % frequency of low concentrations both confirm the presence of mild iodine deficiency. However, WHO/UNICEF/ICCIDD(2) suggest that median UIC for school-aged children be used as a primary indicator for assessing iodine status. Using this measure alone would provide false reassurance about the adequacy of iodine status of the Sherpa people, in view of the median concentration in children of 131·5 μg/l. This was highlighted in one household with two grandparents and two parents, all with UIC <30 μg/l, a teenager with UIC of 71 μg/l, while the two youngest children had UIC of 544 and 300 μg/l. Particularly noteworthy is the decreasing UIC with increasing age such that women, including those of childbearing age who are particularly vulnerable to the effects of iodine deficiency, had a median UIC of 75 μg/l. Low UIC among those with visible goitre is also of concern since their thyroid glands are unlikely to reduce to normal size, and may develop nodules capable of secreting excess hormones and at risk of becoming malignant(Reference Dunn19). The prevalence of high TSH concentrations (10 %) also indicated the presence of mild iodine deficiency.
The continuing improvement in iodine status in this population must be due principally to the use of iodised salt, as iodised oil injections have a duration of efficacy of approximately 2 to 3 years(Reference Dunn19–Reference Wolff21) and no participants had received iodised oil injections within this time frame. Although participants were not asked about iodised oil capsules, which have a shorter duration of efficacy than injections, there have been no supplementation programmes involving iodised oil capsules since 1999 (KT Sherpa, personal communication). More than 90 % of households reported using iodised salt either alone or in combination with rock salt and storing their iodised salt in appropriate sealed containers to reduce potential iodine loss. This represents an appreciable improvement from previously reported consumption and the achievement of an international target that over 90 % of households should consume effectively iodised salt(17). WHO/UNICEF/ICCIDD recommend that, to allow for iodine losses, the iodine concentration of salt at the point of production should be within the range of 20–40 mg/kg to ensure adequate iodine intake by the consumer(2, 22). The iodine concentrations of salt measured at the consumer level (54 mg/kg) exceeded those required at production.
All locally produced, regularly consumed foods provided negligible amounts of iodine. Imported milk powder was the only important food source, consumed in modest quantities, which provided a regular source of iodine for Sherpas of all ages. The theory of recycling of iodine as a result of the practice of using human excreta as a fertiliser for the potato fields, proposed by Ibbertson(Reference Ibbertson10), appears not to operate to any extent. Regular daily consumption of uncooked instant noodles and their flavour sachets by children appears to provide a rich source of iodine for younger Sherpas and this is no doubt largely responsible for the relatively high UIC in the under 14 year age group, 30 % of whom have UIC greater than 300 μg/l and therefore are at increased risk of iodine-induced hyperthyroidism. Time spent outside Khumbu was another determinant of iodine status. However, it is not clear whether this was due to access to foods with higher iodine content in other regions or simply a marker for greater wealth, which enables some to purchase foods containing more iodine such as canned fish. Given the short-term nature of UIC, the latter is a more probable explanation.
A major strength of the present study was that it involved almost the entire population of a village, situated in the centre of the Khumbu region. However, we cannot be certain that this village is representative of the whole area, as a recent survey of urinary iodine excretion of children in five villages in Nepal showed significant differences among rural villages and schools in the same villages(Reference Jimba, Aitken, Joshi, Ohashi, Poudyal and Wakai12). It is conceivable that some misclassification occurred between Grade 0 and Grade 1 goitre(2). Nevertheless, there can be no doubt about the substantial overall improvement of iodine status, largely as a consequence of the use of iodised salt and, in children, consumption of iodine-rich instant noodles. Regular consumption of imported powdered milk with moderate iodine content may also have contributed.
While the overall findings are both encouraging and reassuring, they tend to obscure some potential causes for concern. First, it is clear that the overall status of women of childbearing age was at best marginal and many are likely to be iodine-deficient during pregnancy and lactation, times of greatest vulnerability for mother and infant. While the importance of using iodised salt should be reinforced at all times, it is inappropriate to recommend an increase in use of total salt, especially when there is evidence, admittedly largely anecdotal, of the emergence of chronic CVD even in rural Nepal. Several possible strategies could be considered depending on funding, iodised oil availability and local support. These include iodised oil injections every three years for all women of childbearing age, iodised oil injections for pregnant women presenting to antenatal clinics, or iodised oil capsules during pregnancy and lactation. Second, it is noteworthy that Sherpa children in the present study were consuming sufficient levels of iodine from their current diet, and that almost one-third of children were at increased risk of iodine-induced hyperthyroidism as a result of excessive iodine intake. Clearly it is inappropriate for them to increase iodine intake. On the other hand, future changes in snacking habits, such as eating cooked rather than uncooked noodles, or changes in the availability of instant noodles could have significant effects on iodine status of Sherpa children and increase the risk of iodine deficiency. Continuing monitoring of this population is an essential aspect of ensuring that iodine deficiency is eliminated and does not re-emerge, as has been the experience in some other traditionally iodine-deficient areas.
Acknowledgements
Funding from the Maurice and Phyllis Paykel Trust supported the project. There are no conflicts of interest. E.E.H. was responsible for study design, execution of the study including thyroid gland palpation and preparation of the paper. C.D.T. and J.M. contributed to study design and assisted in writing the paper. S.M.W. carried out the statistical analysis. S.A.S. and J.L.H. contributed to study design and commented on the paper. K.T.S. contributed to study design in Nepal. The authors thank Mingma Temba Sherpa, Gayse Nuru Sherpa and Edward Heydon for their assistance in Nepal, as well as the staff of Kunde Hospital and Ang Rita Sherpa for their support. We also thank Ashley Duncan, Kyle Doel and Christian Thoma for assistance with biochemical analyses. Most of all we thank the residents of Kunde village for their participation in the study.





