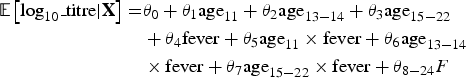Introduction
Fever and rash are common adverse events following measles vaccination [Reference Moss and Scott1]. Similar to natural disease, these reactions seem to be a consequence of the host response to the replication of the measles vaccine virus, with fever typically preceding antibody detection [Reference Moss and Griffin2, Reference Enders and Baron3]. Earlier studies on attenuated measles vaccines showed a correlation between vaccine reactions (fever, rash or malaise) and antibody response [Reference Lepow, Gray and Robbins4, 5].
The final antibody response to measles vaccine reflects a complex interaction between the immunogenicity of the vaccine (depending on strain and type of combined measles vaccine), the age-related host immune system (depending on the presence of maternal antibodies and the maturity of the immune system) and individual genetic factors [Reference Moss and Scott1, Reference Hambidge6]. Older age at first vaccination is associated with increased reactogenicity and antibody response [Reference Klinge7, Reference Vesikari8]. Similarly, Measles-Mumps-Rubella-Varicella vaccine (MMRV) is more reactogenic and immunogenic compared with Measles-Mumps-Rubella vaccine (MMR) [Reference Ma9]. Finally, Kuter et al. have suggested an association between antibody response after the first dose of measles-containing vaccine (MCV) and the incidence of fever and measles-like rash [Reference Kuter10].
Considering the hypotheses that post-vaccination fever is (1) a marker of innate and/or cell-mediated immunity preceding humoral response [Reference Ohga11, Reference Griffin12] and (2) associated with age at first dose (MCV1) and type of MCV, we aimed to explore fever as a mediator in the pathways between each of these two expositions (age at vaccination and type of vaccine) and antibody response. A mediation analysis allowed us to decompose the total effect of each exposition into its direct effect, not mediated by fever, plus its indirect effect, mediated by fever (Fig. 1). In case of exposure–mediator interaction, there are two measures of direct effect: the controlled direct effect (CDE) and the natural direct effect (NDE). The CDE expresses the direct effect of the exposure for a fixed level of fever and will potentially vary for each level of the mediator. The NDE corresponds to the direct effect observed if fever distribution is set to that of the reference exposure category (e.g. 12 month-olds) [Reference Valeri and Vanderweele13].
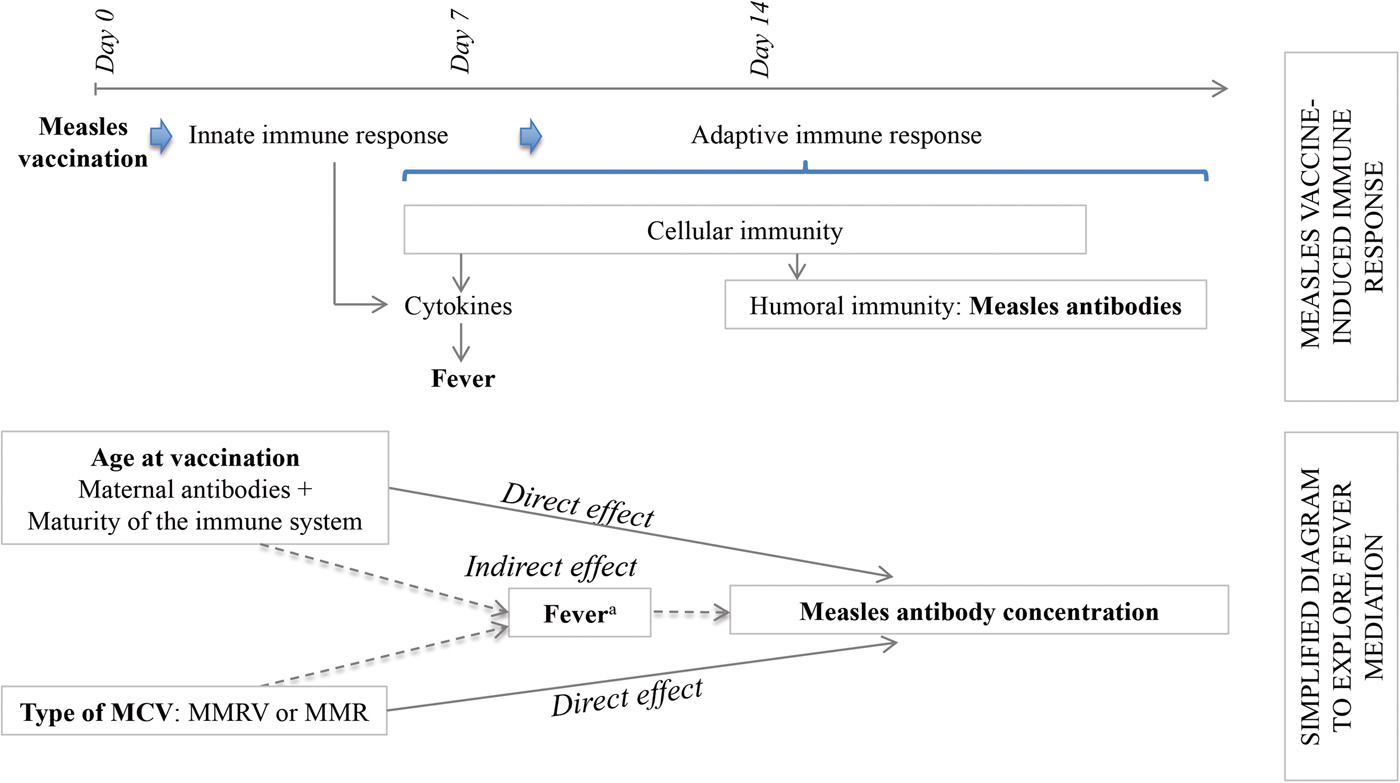
Fig. 1. Conceptual frame of the determinants of the antibody response to measles vaccination. MMR, Measles, Mumps and Rubella vaccine; MMRV, Measles, Mumps, Rubella and Varicella vaccine. aFever as a marker of the immune processes preceding the humoral response.
We performed a post-hoc pooled analysis of data of five randomised controlled trials (RCT) including more than 5000 children vaccinated with one or two doses of MMRV or MMR in order to:
(a) Describe the frequency of adverse events following immunisation with MCV and their association with the type of vaccine and age at vaccination.
(b) Evaluate the association between post-immunisation fever and rash and the antibody response.
(c) Explore if fever is a mediator in the pathway between age at first vaccination or type of MCV and the antibody response.
Methods
Study design, population, intervention and immunogenicity assessment
We pooled the data of five multicenter RCTs conducted in Europe between 2004 and 2008 and in the USA between 2010 and 2012, which compared the immunogenicity and the safety of MMRV to MMR. The detailed methodology of the studies has been described elsewhere [Reference Carazo Perez14]. In short, healthy children aged 11–22 months were vaccinated with one or two doses of MMRV or MMR separated by 6 weeks. Measles antibody concentrations were measured before and 6 weeks after each dose using the Enzygnost (Behring) ELISA assay. The cut-off value for seropositivity corresponds to a concentration ⩾150 mIU/ml of measles IgG antibody. Geometric mean concentrations (GMCs) were calculated as the antilog of the mean of the log10 transformations of the concentrations. By convention, negative results were assigned a value of 75 mIU/ml. For this analysis, only subjects with undetectable antibodies pre-vaccination and immunised with MMRV Priorix-tetra ® and MMR Priorix ® containing the Schwarz measles strain and manufactured by GlaxoSmithKline (GSK) were included. This study was approved by the ethics committee of Centre Hospitalier Universitaire de Quebec Research Center.
Safety data
The parents recorded daily children's temperature during 15 days following each vaccination. Daily incidence of fever was defined as the presence of an axillary temperature ⩾38 °C in a child previously afebrile. Cumulative incidence of fever was calculated as the number of children with temperature ⩾38 °C measured at least once during the at-risk period, predefined as days 4–11 post-immunisation with day 0 being the day of vaccination, divided by all immunised subjects. An investigator examined any child with rash appearing during the 43 days after each dose and determined the nature of the rash (measles/rubella-like, varicella-like or other) and its likelihood of being related to vaccination.
Statistical analysis
Daily incidence of fever and cumulative incidence of fever and measles/rubella-like rash after each dose of MCV were computed with two-sided 95% confidence intervals (CI) and stratified by type of vaccine (MMRV or MMR) and age at first vaccination (seven categories).
The association between fever following MCV1 and the antibody response post-MCV1 was assessed with a linear regression model comparing GMCs and with a log-binomial model for the risk of seronegativity. Models were adjusted for the type of vaccine, age at MCV1, study, country and season. A robust Poisson regression was used when the convergence of binomial likelihood maximisation failed due to a small number of events (seronegativity) compared with the number of covariates; adjustment for the country was not possible in this last model. Similar models were constructed to evaluate seronegativity risk ratio (RR) and GMC ratio according to the onset of measles/rubella-like rash.
The associations of the determinants of MCV's immunogenicity examined in the mediation analysis are presented in a conceptual frame (Fig. 1). For the mediation analysis, fever was coded as five ordered categories (<38, 38 < 38.5, 38.5 < 39, 39 < 39.5 and ⩾39.5 °C) according to the maximum temperature measured during the at-risk period and age was categorised in four groups (11, 12, 13–14 and 15–22 months) using indicator variables. After testing its linear relationship with the antibody concentrations, the temperature was introduced as an ordinal variable in the models, which increased power and simplified analyses. The models’ assumptions were respected; multicollinearity between study and country did not affect the outcome's variance. The interaction terms for age × fever and vaccine × fever were sequentially tested in the previously defined linear regression model. In case of interaction between fever and age, we considered the following regression models, adjusted for the covariates type of vaccine, country and study (F):
(a)
 $$\eqalign{ {\rm {\opf E}}\left[ {{\rm fever} \vert {\bf X}} \right] = & \beta _0 + \beta _1 {\rm age}_{11} + \beta _2 {\rm age}_{13 - 14} + \beta _3 {\rm age}_{15 - 22} \cr & + \beta _{4 - 20}F} $$
$$\eqalign{ {\rm {\opf E}}\left[ {{\rm fever} \vert {\bf X}} \right] = & \beta _0 + \beta _1 {\rm age}_{11} + \beta _2 {\rm age}_{13 - 14} + \beta _3 {\rm age}_{15 - 22} \cr & + \beta _{4 - 20}F} $$(b)
 $$\eqalign{{\rm {\opf E}}\left[ {{\rm log}_{10}\_{\rm titre} \vert {\bf X}} \right] = & \theta _0 + \theta _1 {\rm age}_{11} + \theta _2 {\rm age}_{13 - 14} + \theta _3 {\rm age}_{15 - 22} \cr & + \theta _4 {\rm fever} + \theta _5 {\rm age}_{11} \times {\rm fever} + \theta _6 {\rm age}_{13 - 14} \cr & \times {\rm fever} + \theta _7 {\rm age}_{15 - 22} \times {\rm fever} + \theta _{8 - 24}F} $$
$$\eqalign{{\rm {\opf E}}\left[ {{\rm log}_{10}\_{\rm titre} \vert {\bf X}} \right] = & \theta _0 + \theta _1 {\rm age}_{11} + \theta _2 {\rm age}_{13 - 14} + \theta _3 {\rm age}_{15 - 22} \cr & + \theta _4 {\rm fever} + \theta _5 {\rm age}_{11} \times {\rm fever} + \theta _6 {\rm age}_{13 - 14} \cr & \times {\rm fever} + \theta _7 {\rm age}_{15 - 22} \times {\rm fever} + \theta _{8 - 24}F} $$
Based on these results, the direct and indirect effects were assessed using the following equations to calculate the CDE (c), NDE or pure NDE (d) and the natural indirect effect (NIE) or total NIE (e) of age at 11 months, with 12 months and a temperature <38 °C as the reference levels and the covariates set to their average level. For each age category β 1, θ 1 and θ 5 were replaced by the corresponding coefficient. The total effect was the sum of NDE and NIE [Reference Valeri and Vanderweele13, Reference VanderWeele15].
(c) CDE = θ 1 + θ 5 fever
(d)
 ${\rm NDE} = \theta _1 + \theta _5 (\beta _0 + \beta _{4 - 20} F)$
${\rm NDE} = \theta _1 + \theta _5 (\beta _0 + \beta _{4 - 20} F)$(e)
 ${\rm NIE} = \theta _4 \beta _1 + \theta _5 \beta _1 $
${\rm NIE} = \theta _4 \beta _1 + \theta _5 \beta _1 $
In the absence of interaction (θ 5 = 0), the formulae were simplified and CDE = NDE. The proportion of the total effect mediated by fever was calculated as NIE/Total effect × 100. Equivalent models and equations were applied to decompose the effect of the type of vaccine.
If no mediation was demonstrated, the contributions of fever, age at first vaccination and type of vaccine to the variation of GMCs post-MCV1 were estimated calculating the difference in R 2 when each variable was removed from the fully adjusted model. The sum would be less than the R 2 of the model if the variables were correlated, being the remaining variance explained by more than one variable.
All analyses were performed using SAS/STAT® software version 9.4.
Results
Population
From the 6041 subjects recruited for the five serological studies, 6001 (99.3%) received a first dose of MCV and 3883 (64.3%) received a second dose; 5216 and 3631 were analysed in this study. Reasons for exclusion were (n): detectable antibodies pre-vaccination (72), no serological results pre or post-vaccination (373), age <11 or >22 months at first dose (14) and vaccinated with M-M-R®II containing the Moraten strain (260). Additionally, 66 (1.1%) and 55 (1.2%) children, who did not have any temperature recorded during days 4 to 11 after MCV1 and after the second dose (MCV2), were also excluded from the respective analyses.
Reactogenicity
Following MCV1, the daily incidence of fever was low in days 0–3 (2.5%), higher during the at-risk period between days 4 and 11 (6.0%), highest (>8%) on days 7–9 and returned to low levels days 12–14 (0.8%). After MCV2, there was no increase of daily incidence of fever during days 4–11 compared with other days, ranging between 1.0% and 3.1%. The daily incidence post-MCV1 was higher with MMRV than MMR vaccine (Fig. 2).
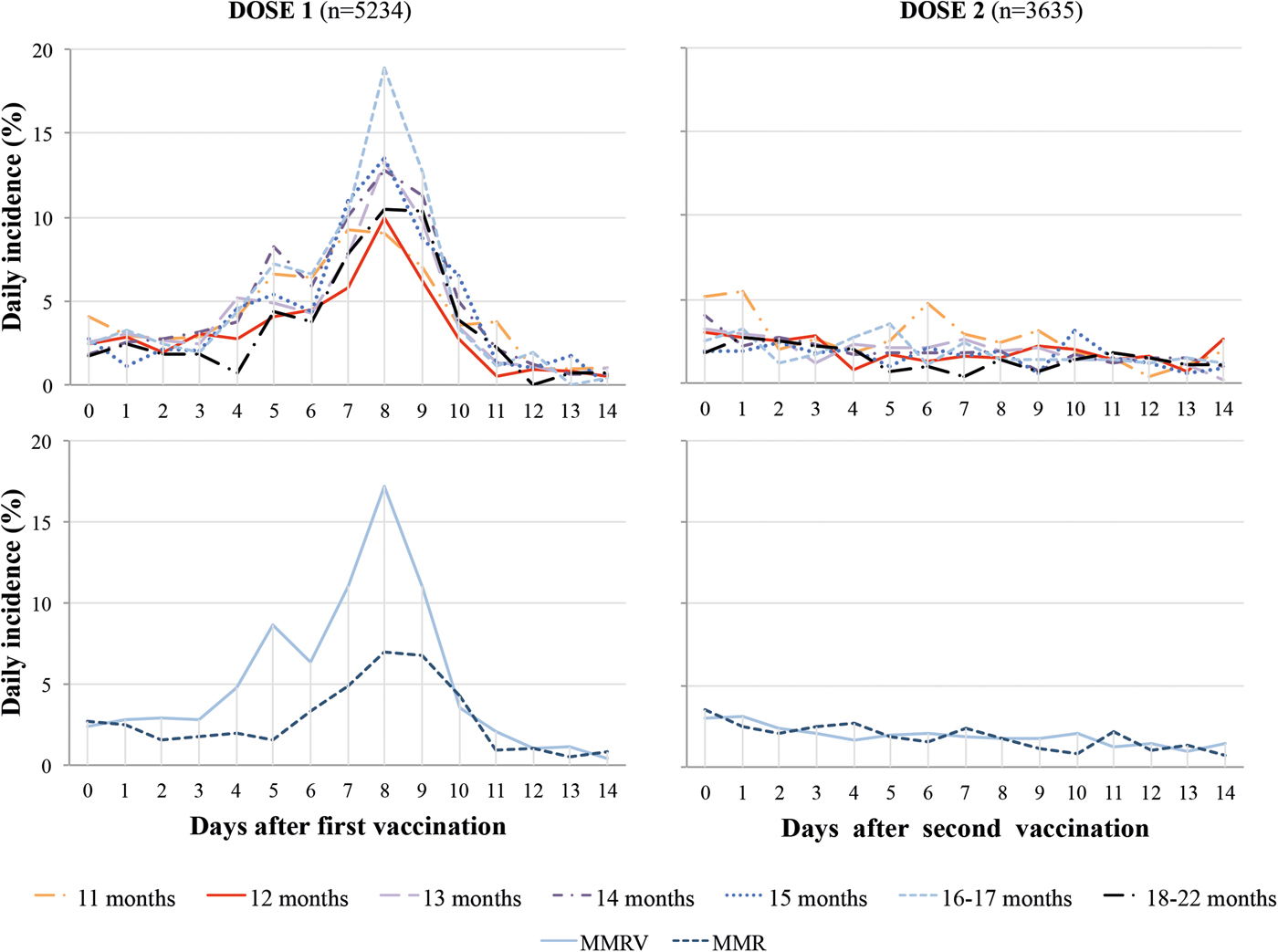
Fig. 2. Daily incidence of fever post dose one and two of measles-containing vaccine by age at first immunisation and type of vaccine. MMR, Measles, Mumps and Rubella vaccine; MMRV, Measles, Mumps, Rubella and Varicella vaccine.
During the at-risk period the crude overall cumulative incidence of fever post-MCV1 increased with older age at vaccination from 11 (34.7%) to 16–17 months (45.4%) (P < 0.001) but decreased at 18–22 months (36.3%). This pattern persisted when adjusting for type of vaccine and country, but the difference in fever incidence was more pronounced for 11- vs. 12-month-old children and attenuated for older ages. Post-immunisation fever was 1.8 times higher with MMRV rather than MMR (51.8%, 95% CI 50.1–53.7% vs. 29.4%, 95% CI 27.6–31.3%) (Fig. 3).

Fig. 3. Cumulative incidence of fever during days 4–11 post-dose one and two of measles-containing vaccine by age at first immunisation and type of vaccine. MMR, Measles, Mumps and Rubella vaccine; MMRV, Measles, Mumps, Rubella and Varicella vaccine. Note: Total cumulative incidences of fever are adjusted for the type of combined vaccine and the country.
Measles/rubella-like rash occurred in 8.1% (95% CI 7.4–8.9%) and 4.4% (95% CI 3.9–5.1%) of subjects following MCV1 and MCV2, respectively. However, rash considered by the investigators as vaccine-related was observed in only 4.3% (95% CI 3.8–4.9%) post-MCV1 (83% occurring between days 4 and 11) and 1.2% (95% CI 0.9–1.6%) post-MCV2. The cumulative incidence of vaccine-related rash post-MCV1 was lower for those vaccinated with MMR (3.2%, 95% CI 2.6–4.0%) than for those receiving MMRV (5.1%, 95% CI 4.4–6.0) but was not different post-MCV2 (0.8% vs. 1.4%, P = 0.06). Age at first vaccination was not associated with the occurrence of vaccine-related rash (P = 0.17 post-MCV1 and P = 0.51 post-MCV2) (data not shown).
Fever and antibody response
The crude and adjusted GMCs post-MCV1 were 60% higher for febrile vs. afebrile children (adjusted GMCs: 4416 mIU/ml and 2766 mIU/ml, respectively) and increased with higher recorded temperature from 2775 mIU/ml (<38 °C) to 3828 mIU/ml (38–<38.5 °C) and 5290 mIU/ml (⩾39.5 °C) (Table 1). This difference was present for each type of vaccine and each age category (Fig. 4). Conversely, the adjusted risk of seronegativity was 70% lower for febrile (0.8%) compared with afebrile subjects (2.7%) (P < 0.001) (Table 1). Antibody concentrations post-MCV2 were associated with age at MCV1 (P < 0.001) and type of vaccine (P < 0.001), but not with fever post-MCV2 (P = 0.75) (Fig. 4).

Fig. 4. GMC of antibodies 6 weeks after dose one and two of measles-containing vaccine by age at first immunisation, type of vaccine and onset of fever on days 4–11 after each dose. GMC, Geometric Mean Concentrations; MMR, Measles, Mumps and Rubella; MMRV, Measles, Mumps, Rubella and Varicella.
Table 1. Association between post-immunisation fever and measles/rubella-like rash after the first dose of a measles-containing vaccine and antibody response

CI, Confidence intervals; GMC, Geometric mean concentration; Ref, reference category.
a Robust Poisson model adjusted for age at vaccination (7 categories), type of vaccine, study and season, P-values correspond to type III tests.
b Linear regression model adjusted for age at vaccination (7 categories), type of vaccine, study, country and season, P-values correspond to test F.
c Measured in mIU/ml.
d Maximum temperature registered during the days 4–11 post-first vaccination (5 categories).
e Robust Poisson model restricted to febrile children is adjusted for age at vaccination (7 categories), type of vaccine and season.
Rash and antibody response
Subjects presenting vaccine-related measles/rubella-like rash had 30% higher GMCs post-MCV1 than vaccinees without rash, but no difference in seronegativity (Table 1). Among children presenting with rash, 66% also reported fever but given the low incidence of vaccine-related rash, the correlation between fever and rash was low (Pearson coefficient = 0.10). The association between rash and antibody response diminished from a GMC ratio of 1.30 (95% CI 1.15–1.48) to 1.18 (95% CI 1.05–1.34) when adjusting for fever. Stratified analysis showed an association between rash and GMCs among febrile but not afebrile children (Table 1). Adding rash to a model including fever, age, type of vaccine, study and country only improved the proportion of variability in antibody log-concentrations explained by the model by 0.1% (data not shown).
Fever as a mediator in the associations between age, type of vaccine and antibody response
There was an interaction in a fully adjusted log-additive model between age at MCV1 and temperature, but only for a change in age from 11 to 12 months (P < 0.001). Among afebrile children vaccinated at 11 vs. 12 months the GMC ratio was 0.73 (95% CI 0.64–0.83), but 0.86 (95% CI 0.76–0.95) among those with a temperature of 38–<38.5 °C and 1.38 (95% CI 1.04–1.84) among subjects with high fever (⩾39.5 °C). No interaction with fever was demonstrated for older age categories or type of vaccine (Supplementary Table S1).
The mediation analysis showed that the greater antibody concentrations with older age at MCV1 (from 12 months to 13–14 months or 15–22 months) were not mediated by the presence of fever (GMC ratios for NIE: 1.00 and 1.01 respectively). By contrast, for a change in age from 11 to 12 months, the proportion of the total effect mediated by fever was 26.5% without considering the age × fever interaction, or 44.5% when the interaction was specified. The latter analysis found that, for vaccination at 11 vs. 12 months the total effect was a 25% lower antibody concentration; natural direct and indirect effects corresponding to reductions of 15% and 12%, respectively; the total effect was equal to the sum of the NIE and the NDE in the log10 scale (Table 2). The CDE of this age change varied with the level of temperature, from a GMT ratio of 0.72 for <38 °C to a GMT ratio of 0.85 (95% CI 0.76–0.95) and 1.20 (95% CI 0.98–1.47) for temperatures of 38–<38.5 °C and 39–<39.5 °C, respectively.
Table 2. Mediation analysis of fever in the association between age at first vaccination or type of vaccine and antibody concentration

CDE, Controlled direct effect; CI, confidence intervals; GMC, geometric mean concentrations; Log10MC diff, difference of log base 10 mean concentrations; MMR, Measles-Mumps-Rubella vaccine; MMRV, Measles-Mumps-Rubella-Varicella vaccine; NDE, natural direct effect; NIE, natural indirect effect; TE, total effect; Ref, reference category.
a CDE for fever set at temperature <38 °C.
b NDE for covariates set at their average level.
c Percentage of the total effect mediated by fever = NIE/TE × 100.
d Models adjusted for type of vaccine, study and country.
e For models without interaction term CDE = NDE.
f Models adjusted for age at first vaccination, study and country.
Vaccination with MMRV induced 61% higher GMCs compared with vaccination with MMR (total effect); a rise in GMCs of 49% was due to the direct effect while an increase of 9% was mediated by fever (Table 2).
In an analysis restricted to children ⩾12-months-old at MCV1, the model including fever, age, type of vaccine, study and country explained 13% of the total variance; the estimated relative contributions on antibody log-concentrations were 5%, 2% and 3% for fever, age and type of vaccine, respectively (Table 3).
Table 3. Relative contribution of fever, age, type of vaccine, study and country on the antibody concentration 6 weeks after the administration of a MCV to children aged ⩾12 months

DF, Degrees of freedom; GMC, Geometric Mean Concentration; MCV, Measles-containing vaccine; MMR, Measles-Mumps-Rubella vaccine; MMRV, Measles-Mumps-Rubella-Varicella vaccine; Ref, reference category.
a GMC ratio for a change in each variable adjusted for the other variables of the model.
b Difference between the R 2 of the whole model and the R 2 of the model after removal of the selected variable.
Discussion
In this analysis, fever post-MCV1 was a strong predictor of higher antibody response whereas the independent effect of vaccine-related rash post-MCV1 was minimal. For children first vaccinated at ⩾12 months, the effect of age on antibody response was not modified by fever, which mediated only 2% to 3% of the total effect. The higher antibody response to MMRV vs. MMR was not modified by fever, which mediated 18% of the total effect of the type of vaccine. These results suggest that age at first dose, type of vaccine and fever have mostly independent but additive causal pathways on immunogenicity. While fever was the strongest predictor of antibody concentration, it explained only 5% of the 13% of the variation in a model including fever, age at first vaccination, type of vaccine, country and study. This suggests that individual genetic or other unmeasured factors determine most of the humoral response to MCV [Reference Haralambieva16].
Measles infection induces an initial innate response that stimulates adaptive immunity: T-cell activation and antibody production by B-cells, this latter being also influenced by CD4 T-helper cells [Reference Enders and Baron3, Reference Griffin12]. Both innate and cellular immune responses include the production of cytokines, some of them acting as endogenous pyrogens, directly or mediated by prostaglandins [Reference Ohga11, Reference Conti17, Reference Evans, Repasky and Fisher18]. However, the pathogenesis of the immunity activation after measles vaccination and the relative contribution of post-vaccination cellular and humoral immunity in protection against measles are less known [Reference Dhiman19]. Fever has been shown to boost innate and adaptive immunity during infections in general [Reference Evans, Repasky and Fisher18], but its role after measles infection or measles immunisation has not been well defined [Reference Griffin12, Reference Dianzani, Baron and Baron20]. Our mediation analysis was based on the hypothesis that fever was a marker of the innate or cellular immune response in the pathway to the antibody response to MCV. However, we found that the enhanced antibody response among children vaccinated older were not explained by an increased incidence of fever.
Higher immunogenicity and a 1.4-fold increased risk of fever and rash have been described after vaccination with MMRV compared with MMR, findings with an unknown underlying mechanism but that might be a consequence of an increased local measles vaccine virus replication [Reference Ma9, Reference Kuter10, Reference Rowhani-Rahbar21]. Similarly, we found 80% and 60% significantly higher risk of fever and measles/rubella-like rash following MMRV vs. MMR. Our results support the positive association between fever post-MCV1 and antibody titres reported by Kuter et al. [Reference Kuter10] and we, therefore, expected fever to be a mediator in the association between type of MCV and antibody response. We found instead that 82% was due to the direct effect of MMRV and only 18% to the indirect effect mediated by fever, suggesting another mechanism.
Infants receiving MCV1 at 11 months develop a lower antibody response than those vaccinated after their first birthday [Reference Klinge7, Reference Vesikari8, Reference Carazo Perez14, Reference Dequadros22]. Our results show that fever is not only a marker of enhanced immune response among infants but also modifies the association between age at MCV1 (11 vs. 12 months) and antibody response, reaching similar GMCs among vaccinees at 11 or 12 months with fever ⩾39.5 °C (GMC ratio = 1.38; 95% CI 1.04–1.84). This underscores the need to better understand the relationship between post-immunisation fever and measles vaccine immunogenicity among children ⩽11 months old.
Similar to other safety studies [Reference Stokes23–Reference Blatter25], there was an increase in fever during days 4–11 after the first but not the second dose. Fever post-MCV1 increased with age from 11 to 17 months but decreased in children aged 18–22 months. This is consistent with other studies, which report greater fever incidence when comparing vaccination at 9 and/or 11 months vs. 12 months [Reference Klinge7, Reference Vesikari8, Reference Ceyhan26], but no difference or a decrease in fever when vaccinated at 15–17 [Reference Klinge7] or 16–23 months [Reference Rowhani-Rahbar21].
To our knowledge, this is the first study to explore the pathways underlying the associations between age at first vaccination, type of combined measles vaccine, reactogenicity and antibody response. The large sample size of this study permitted the analysis of several age categories and effect modification. Our study suffers from some limitations. The original trials were randomised for the type of vaccine and not for age at vaccination, but residual confounding seems unlikely. Definition of post-immunisation fever varies according to the measurement method, the period at risk and the threshold considered. As described in the literature, we predefined the at-risk period as days 4–11 post-vaccination, but on day 11, fever incidence had returned to baseline level. While our cut-off of 38 °C for fever was lower than in some other studies [Reference Peltola and Heinonen27], the analysis of temperature as an ordinal variable showed increased immunogenicity also for children with mild fever. There was a low incidence of rash and its attribution as vaccine-related could have varied between investigators; our conclusions about its association with measles vaccine immunogenicity are not robust. Other determinants of MCV antibody response, like vaccine strain or participant race, have not been studied, as our sample was mostly restricted to Caucasian children vaccinated with the Schwarz strain. Our results may not apply in settings where age at vaccination is recommended before 11 months of age.
The ideal measles vaccine would be non-reactogenic and highly immunogenic in very young infants but is not currently an option [Reference Griffin, Pan and Moss28]. Fever appears to induce stronger measles antibody response independently of age at vaccination and type of vaccine, but the underlying mechanisms remain unclear. Understanding the positive relationship between fever and immunogenicity may help parents, clinicians and public health decision-makers to better tolerate this adverse event.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268818001474
Acknowledgements
This work was supported by GlaxoSmithKline (GSK), but the manufacturer had no involvement in study design, analysis or interpretation of the data, writing of the report or the decision to submit the manuscript for publication.
Conflict of interest
G.D.S has received investigator-initiated grants from GSK and Pfizer; has received travel reimbursement to attend and ad hoc advisory board meeting of GSK. All other authors report no potential conflicts.