Carotenoids form a group of more than 600 fat-soluble compounds synthesised by higher plants and some yeasts, fungi, algae and bacteria (Goodwin, Reference Goodwin1980). Based on their chemical structure, the members of the carotenoid family are usually divided into two subclasses by the presence or absence of O in the molecule: if at least one O atom is present, the substances are called xanthophylls; otherwise they are termed carotenes. In recent years, xanthophylls such as lutein and zeaxanthin have received increasing interest because of positive correlations between their consumption and the prevention of eye diseases such as age-related macular degeneration and cataract formation (Landrum & Bone, Reference Landrum and Bone2001; Olmedilla et al. Reference Olmedilla, Granado, Blanco, Vaquero and Cajigal2001; Broekmans et al. Reference Broekmans, Berendschot, Klöpping-Ketelaars, de Vries, Goldbohm, Tijburg, Kardinaal and van Poppel2002; Alves-Rodrigues & Shao, Reference Alves-Rodrigues and Shao2004). Unlike α-/β-carotene and α-/β-cryptoxanthin, lutein and zeaxanthin are not considered to be provitamin A active as they are not converted in the human body into retinol.
Several factors influence the bioavailability of xanthophylls, among them their chemical nature (E/Z-isomers or esters) and dietary factors such as fat amount and fibre content, as well as the level of food processing (Roodenburg et al. Reference Roodenburg, Leenen, van het Hof, Weststrate and Tijburg2000; Zaripheh & Erdman, Reference Zaripheh and Erdman2002). As xanthophylls in human blood are typically found in their free form, it was suggested that the saponification of ative xanthophyll esters could improve their bioavailability in dietary supplements. The discussion of this topic is highly controversial and is still ongoing, especially with respect to human dietary supplements containing free or esterified lutein (Bowen et al. Reference Bowen, Herbst-Espinosa, Hussain and Stacewicz-Sapuntzakis2002). To date, long-term human intervention studies of zeaxanthin bioavailability are rare (Hartmann et al. Reference Hartmann, Thürmann, Spitzer, Schalch, Manner and Cohn2004; Cheng et al. Reference Cheng, Chung, Szeto and Benzie2005), whereas corresponding studies with lutein are much more frequent (Granado et al. Reference Granado, Olmedilla, Gil-Martínez and Blanco1998; Roodenburg et al. Reference Roodenburg, Leenen, van het Hof, Weststrate and Tijburg2000; Olmedilla et al. Reference Olmedilla, Granado, Blanco, Vaquero and Cajigal2001; Lienau et al. Reference Lienau, Glaser, Tang, Dolnikowski, Grusak and Albert2003; Novotny et al. Reference Novotny, Kurilich, Britz and Clevidence2005).
With respect to β-cryptoxanthin, only a few studies have been conducted. Breithaupt et al. (Reference Breithaupt, Weller, Wolters and Hahn2003) applied free or esterified β-cryptoxanthin to human volunteers. Other studies have proved different physiological roles for β-cryptoxanthin: its ingestion – together with Zn – may reduce the risk of rheumatoid arthritis (Cerhan et al. Reference Cerhan, Saag, Merlino, Mikuls and Criswell2003), may inhibit chemically induced rat colon and mouse lung tumorigenesis (Tanaka & Kohno, Reference Tanaka and Kohno2002), and shows an anabolic effect on bone components in aged female rats in vivo and in vitro (Uchiyama et al. Reference Uchiyama, Sumida and Yamaguchi2004). Furthermore, β-cryptoxanthin may act as a chemopreventive agent for lung cancer in humans (Yuan et al. Reference Yuan, Stram, Arakawa, Lee and Yu2003) and may influence circulating bone metabolic markers (Yamaguchi et al. Reference Yamaguchi, Igarashi, Morita, Sumida and Sugawara2005). Current interest in this xanthophyll is clearly documented by two recent patents dealing with β-cryptoxanthin (Eichinger et al. Reference Eichinger, Goralczyk, Wertz and Wyss2005; Showalter et al. Reference Showalter, Khachik and Liu2005).
Little is known about a potential physiological role of α-cryptoxanthin: Craft et al. (Reference Craft, Haitema, Garnett, Fitch and Dorey2004) found that α- and β-cryptoxanthin belong to the major xanthophylls of the human brain. In particular, the frontal cortex, which is generally susceptible to Alzheimer's disease, had higher concentrations of antioxidants than other parts.
As far as we know, there has been no study directly comparing the absorption efficiency of the monohydroxylated counterparts of lutein and zeaxanthin, α- and β-cryptoxanthin (Fig. 1). To ensure a completely identical formulation, both xanthophylls were orally administered together in a minimally processed corn-oil-based supplement to female Wistar rats for 8 d. The main goal of the present study was to obtain basic data about the absorption efficiency of monohydroxylated xanthophylls.

Fig. 1 Chemical structures of xanthophylls: (1) lutein (β, ɛ-carotene-3,3′-diol); (2) zeaxanthin (β,β-carotene-3,3′-diol); (3) α-cryptoxanthin (β, ε-carotene-3′-ol); (4) β-cryptoxanthin (β,β-carotene-3-ol).
Methods
Materials
Light petroleum (boiling fraction 35–60°C, p.a.), methanol (99·99 %), tert-butyl methyl ether (99·0 %), ethyl acetate (100 %), ethanol (absolute, 99·9 %), n-hexane (85 %), KOH (p.a.) and NaCl (p.a.) were obtained from J. T. Baker (Philippsburg, NY, USA). Diethyl ether (anhydrous, 99 %), β-carotene (97 %), PBS (pH 7·4), protease from Streptomyces griseus type XIV (pronase E, 4·9 U/mg), and all-trans-retinol (vitamin A alcohol, 99 %) were obtained from Sigma (St Louis, MO, USA). BHT (2,6-di-tert-butyl-4-methyl phenol; 99 %) and silica gel 60 (0·063–0·200 mm) were purchased from Merck (Darmstadt, Germany). Distilled water for HPLC was membrane filtered (Barnstead International, Dubuque, IO, USA; 0·22 μm). Pure β-cryptoxanthin was generously provided by DSM (Kaiseraugst, Switzerland). Fresh green carrot leaves and corn oil were obtained from a supermarket in Stuttgart (Germany), fresh chicken liver was obtained from a local market in Querétaro (Mexico). Papaya purée was kindly provided by SGF International (Schutzgemeinschaft der Fruchtsaftindustrie e.V, Nieder-Olm, Germany).
Study design
The study was designed as a multiple-dose intervention study using ten female Wistar rats. The life phase of the study was conducted at the Faculty of Medicine, Autonomous University of Querétaro. The faculty is accredited by the Council of Mexico for Accreditation of Medical Education (El Consejo Mexicano para la Acreditación de la Educatión Médica; COMAEM), including housing, feeding and killing experimental animals, and is controlled by a committee that observes, revises and authorises all the methods used. All work with rats was in accordance with the Mexican Official Norm NOM-062-ZOO-1999.
All animals were housed singly in hanging numbered cages and were maintained on a 12 h light/dark cycle at a constant temperature of 20–24°C and were provided access to a NIH-31 basic diet (Zeigler, Gardners, PA, USA) and water ad libitum. The basic diet (20 g) and water (100 ml) were changed daily. Spilled food was collected and weighed. Food consumption was corrected by adding the weight of wasted food to the weight difference before and after feeding. Additionally, the rats were weighed every 3 d (Table 1).
Table 1 Consumption of the basic diet within the study period (days 1–23) of rats belonging to the control or treatment group
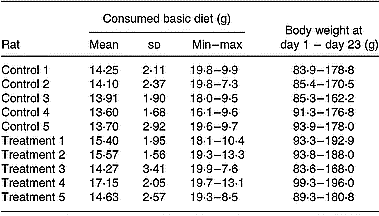
The average and standard deviation (8 measurement days) and the minimal (min) and maximal (max) amounts of the consumed diet are shown for each rat, as is the change in body weight during the study.
All animals (24 d of age, obtained from the breeding colony of the university) received the basic diet for the whole study period (23 d). After a clearance phase of 15 d, animals were randomly allocated to a control and a treatment group of five animals each. The control group was additionally fed the blank supplement, whereas the treatment group additionally received the xanthophyll supplement (0·5 ml/d) for 8 d each. A sterile syringe (DL Medica, S.A. de C.V., Mexico; 22 × 32 mm, 3 ml, calibrated in 0·1 ml units) equipped with a single-use sterile animal feeding needle (Popper and Sons, Inc., New Hyde Park, NY, USA; 18 × 2 mm) was used to apply the supplements. This procedure allowed for gentle feeding without wounding the oesophagus and without the application of anaesthetics. Both supplements were allowed to warm up to 20°C before application.
Preparation of the supplements
β-Cryptoxanthin was isolated from papaya purée (Carica papaya), as previously described, after saponification and purification of β-cryptoxanthin by open-column chromatography (Breithaupt et al. Reference Breithaupt, Weller, Wolters and Hahn2003). The β-cryptoxanthin-containing fraction was obtained by elution with light petroleum–acetone 95:5 (v/v). α-Cryptoxanthin was isolated from fresh green carrot leaves (Daucus carota) as recently described (Schlatterer & Breithaupt, Reference Schlatterer and Breithaupt2005). To remove xanthophylls, the final extract was saponified, and α-cryptoxanthin was purified by open-column chromatography. The concentrations of the final solutions obtained were determined by HPLC/DAD using the same calibration graph.
To prepare the xanthophyll supplement, aliquots of both solutions were combined to give a concentration ratio of 2:1 of β-:α-cryptoxanthin. The solvent was evaporated (35°C, 50 hPA), and the dry residue was suspended in ethanol (1 ml) and dissolved in corn oil (22 ml). The xanthophyll concentration of the supplement was determined after dilution in a volumetric flask (300 μl TBME–2 ml methanol, 1:1) and was as follows: β-cryptoxanthin 34·5 μmol/l, α-cryptoxanthin 18·3 μmol/l (corresponding to 17·3 nmol/0·5 ml and 9·2 nmol/0·5 ml, respectively). A blank supplement was obtained by mixing ethanol (1 ml) with corn oil (22 ml). Both supplements were stored in the dark at 4°C.
Blood and liver collection
Animals were killed by cervical dislocation according to NOM-062-ZOO-1999 and were necropsied. Terminal heart blood was obtained using a venous blood collection tube (BD Vacutainer Systems, Franklin Lakes, NJ, USA; 6 ml, sterile interior) containing an anticoagulant solution (trisodium citrate (13·2 g/l), citric acid (4·8 g/l), glucose (14·7 g/l); 1 ml). Immediately after collection, plasma was separated by centrifugation (5000 rpm, 10 min, 10°C) and stored in plastic caps at − 20°C until analysis. Livers were removed, washed with NaCl solution (0·9 %, w/v), dried with a tissue, weighed and stored in plastic bags at − 20°C until analysis. Plasma samples were analysed on the day of harvesting, and liver samples were stored for at least 1 week.
Extraction of xanthophylls and retinol from liver and plasma samples
Blood samples
An aliquot of an internal standard solution (0·25 ml) and 0·75 ml ethanol were added to aliquots of each plasma (0·80–1·40 ml). Xanthophyll extraction was performed with n-hexane (2 ml) in a screw-capped test tube (15 ml) using a vortex (10 s). After centrifugation (5000 rpm, 5 min, 10°C; Hermle Z323K; Hermle Labortechnik, Wehingen, Germany), the organic phase was removed and the extraction process repeated twice. To remove traces of water, ethanol (0·5 ml) was added to the combined organic phases and the solvent was evaporated (40°C, 50 hPA). The residue was dissolved in TBME–methanol (1:1, v/v; 0·5 ml), membrane filtered (0·45 μm; Millipore Corp., Bedford, MA, USA), and directly subjected to HPLC analysis (30 μl).
Liver samples
An aliquot of liver (500 mg, from different parts of the liver), 2 mg solid BHT and an aliquot of an internal standard solution (0·5 ml) were suspended in 2 ml PBS buffer solution and homogenised using a high-speed chopper T25 basic (IKA Works Inc., Wilmington, NC, USA; 8000 rpm, 10 s) in a screw-capped test tube (50 ml). For enzymatic protein digestion, an aliquot of a protease suspension (Streptomyces griseus type XIV; 40 mg/ml, 100 μl) was added. After vortexing the suspension (10 s), the homogenate was heated (37°C, 1 h) in a closed water bath. After cooling the samples under flowing water, the homogenate was again centrifuged (10 s), and ethanol (1 ml) was added to denature the proteins. Xanthophylls were extracted by the addition of light petroleum–ethyl acetate (1:1, v/v; 4 ml). After centrifugation (5000 rpm, 5 min, 10°C), the organic phase was removed and the extraction process repeated. To remove traces of water, ethanol (0·5 ml) was added to the combined organic phases and the solvent was evaporated (40°C, 50 hPA). The dry residue was dissolved in TBME–methanol (1:1, v/v; 0·5 ml). An aliquot was membrane-filtered and directly subjected to HPLC analysis (30 μl).
To determine the retinol, another liver aliquot was worked up as described above. For saponification of the retinol esters, the dry residue was dissolved in diethyl ether (2 ml), an aliquot of a methanolic KOH solution (20 %, w/v; 200 μl) was added, and the solution was stored overnight. The solution was washed with distilled water (3 ml). To assist phase separation, an aliquot of a saturated NaCl solution (100 μl) was added. After centrifugation (5000 rpm, 2 min, 10°C), the water layer was removed and the washing step repeated. The solvent was evaporated (40°C, 50 hPA), and the dry residue was dissolved in TBME–methanol (1:1, v/v; 2·0 ml). An aliquot was membrane-filtered and directly subjected to HPLC analysis (30 μl).
Validation of the extraction process
The reproducibility of the method was investigated by spiking fresh chicken liver samples (500 mg) with aliquots of a β-cryptoxanthin stock solution (c = 4·41 μmol/l in TBME–methanol (1:1, v/v); 0·5 ml each), resulting in a concentration of 4·41 nmol/g ( = 100 %). Blank and spiked samples were extracted and analysed by HPLC/DAD as described. Recoveries were calculated on the basis of an Association of Official Analytical Chemists method (Reference Cunniff1995) as follows:
![\%\,recovery = [(measured\,concentration\,in\,fortified\,sample -- measured\,concentration\,in\,unfortified\,sample)\times 100]/known\,concentration.](https://static.cambridge.org/binary/version/id/urn:cambridge.org:id:binary:20160321074955043-0444:S0007114507336751_eqnu1.gif?pub-status=live)
Quantification of xanthophylls and retinol
Preparation of an internal standard solution
A small amount of β-carotene was dissolved in dichlormethane (1 ml) in a volumetric flask (100 ml). The flask was made up to the volume with ethanol, and the concentration of the solution was determined photometrically on the basis of an ε-value of 118 800 (l/(mol × cm)) at 426 nm (c = 3·27 μmol/l; Köst, Reference Köst1988). The resulting peak area of multiple analyses was used to evaluate the extraction process.
Calibration of xanthophylls
A small amount of β-cryptoxanthin was dissolved in light petroleum (100 ml). The concentration of the resulting solution was determined photometrically on the basis of an ε-value of 131 000 (l/(mol × cm)) at 452 nm (Köst, Reference Köst1988). An aliquot (25 ml) was evaporated to dryness (40°C, 50 hPA), and the residue was dissolved in TBME–methanol (1:1, v/v), transferred to a volumetric flask (25 ml) and made up to the volume with the same solvent (stock solution). Further dilutions were made with TBME–methanol (1:1, v/v). Calibration was performed in the range of 0·01–0·22 μmol/l. A calibration graph was recorded by plotting the respective peak areas (450 nm (mAUs) v. concentrations (μmol/l)). Limits of quantitation (LOQ) and determination (LOD) were calculated from the calibration graph according to the recommendations of the Deutsche Forschungsgemeinschaft (Reference Lieferung1991).
Calibration of retinol
Retinol (68 mg) was dissolved in ethanol in a volumetric flask (100 ml) and diluted with ethanol 1:25 (stock solution). This solution was further diluted with ethanol (1:10), and the concentration was determined photometrically on the basis of an ε-value of 50 990 (l/(mol × cm)) at 325 nm (Taibi & Nicotra, Reference Taibi and Nicotra2002), revealing a concentration of 93·74 μmol/l. An aliquot of the stock solution (20 ml) was evaporated to dryness (40°C, 50 hPA), the residue dissolved in TBME–methanol (1:1, v/v; 20 ml) and the solution further diluted with the same solvent to allow for calibration in the range of 0·47–93·74 μmol/l.
Analysis and chromatography
A modular HP1100 (Hewlett-Packard GmbH, Waldbronn, Germany) system with a DAD was used for the analysis of xanthophylls and retinol. For separation, a C30 analytical column (YMC Europe GmbH, Dinslaken, Germany; 250 × 4·6 mm i.d., 5 μm) including a pre-column (10 × 4·0 mm i.d.) was used and kept at 35°C. The DAD was set to 450 nm for the quantification of xanthophylls and to 325 nm for the quantification of retinol. The mobile phases consisted of mixtures of tert-butyl methyl ether, methanol and water (15:81:4 v/v/v (A), 90:6:4 v/v/v (B)) using gradient elution (%A/min, 99/0, 44/39, 0/43, 99/45, 99/50; flow 1 ml/min). Data were processed with HPChem station software (Waldbronn, Germany) (rev. A 06·03.). HPLC-(APCI)MS was performed on an identical HPLC system coupled to a Micromass (Manchester, UK) VG platform II quadrupole mass spectrometer using an APCI interface. Mass spectra were acquired within a scan range of m/z 300–700, UV–visible spectra were recorded between 300 and 600 nm, and data were processed with MassLynx 3·2 software (Manchester, UK). The eluents were identical to those used for the HPLC/DAD analyses. Further MS parameters have previously been detailed (Schlatterer & Breithaupt, Reference Schlatterer and Breithaupt2006).
Results
A dietary supplement was obtained by combining xanthophylls isolated from saponified papaya purée (β-cryptoxanthin) and fresh carrot leaves (α-cryptoxanthin). After removal of the solvent, the xanthophylls were suspended in a small amount of ethanol and were dissolved in corn oil, resulting in a clear, dark red solution. A blank supplement was obtained by mixing analogue volumes of ethanol and oil. For unequivocal identification of the xanthophylls, LC-MS analyses were performed using an APCI interface operated in the positive mode, generating protonated xanthophyll ions. For identification, two characteristic mass traces were used (Fig. 2): the α-cryptoxanthin (3) was identified due to an intensive fragment ion at m/z 535·5, generated by a loss of water from the quasimolecular ion ([M+H − H2O]+), whereas the β-cryptoxanthin (4) was identified on the basis of its quasimolecular ion at m/z 553·5 ([M+H]+). This fragmentation of α-cryptoxanthin is typical for a xanthophyll comprising an ε-ionone ring (Schlatterer & Breithaupt, Reference Schlatterer and Breithaupt2005). The UV–visible maxima, determined in the solvents at time of elution, assisted peak assignment (α-cryptoxanthin: 424(sh)/446/474 nm; β-cryptoxanthin: 426(sh)/452/480 nm). Additionally, LC-(APCI)MS analyses suggested that some small peaks eluting in front of α-cryptoxanthin (3; Fig. 2) were due to (Z)-isomers of β-cryptoxanthin, possessing the same quasimolecular ion (m/z 553·5). These isomers are of natural origin as they were already in the papaya and could not be separated from all-E-β-cryptoxanthin by open-column chromatography.

Fig. 2 HPLC-(APCI)MS analysis (extended sections) of the xanthophyll supplement. The lower trace corresponds to UV–visible detection (450 nm, diode array detector), the other traces show selected molecular masses, suitable for detection of α-cryptoxanthin (3; m/z 535·5, [M+H − H2O]+) and β-cryptoxanthin (4) (m/z 553·5, [M+H]+).
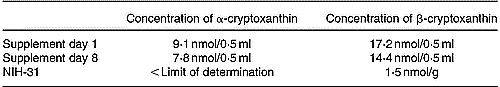
The xanthophyll concentrations of the supplement were determined before and after finishing the feeding period (8 d; Table 2). (Z)-Isomers of β-cryptoxanthin were not quantified as the identification is currently only tentative. A comparison of the results revealed a loss of 14 % α-cryptoxanthin and 16 % β-cryptoxanthin. These losses may be due to multiple opening and closing steps of the vials (access of O) as well as changes in the ambient temperature (storage at 4°C, application at 20°C), leading to unspecific degradation reactions. These losses were, however, regarded as acceptable since no additional antioxidants were applied and the feeding period did not exceed 8 d.
Table 2 Concentrations of α- and β-cryptoxanthin determined in the supplement before (day 1) and after finishing (day 8) the feeding period, and the β-cryptoxanthin concentration found in the basic NIH-31 diet
Pure β-cryptoxanthin was used for construction of a calibration graph. As it is supposed that the molar extinction coefficients of α- and β-cryptoxanthin are similar, the same graph was applied for quantification of both xanthophylls. The resulting calibration graph was linear within 0·01–0·22 μmol/l (r 2 = 0·9999). The LOD and LOQ were calculated on the basis of 500 mg liver/0·5 ml final volume and a CI of P < 0·10 and were as follows: LOD 24 pmol/g, LOQ 35 pmol/g. The LOQ allowed for quantification of 9 out of 10 xanthophyll concentrations in rat liver. The LOQ and LOD of retinol were calculated from the retinol calibration curve (0·47–93·74 μmol/l; r 2 = 1·000) on the basis of 500 mg liver/2 ml final volume and a CI of P < 0·05 (LOD 1·2 nmol/g, LOQ 1·8 nmol/g), allowing for quantification of all retinol concentrations in the rat livers. The extraction method was evaluated using commercial chicken livers spiked with aliquots of a β-cryptoxanthin standard solution (4 nmol/g). A small amount of β-cryptoxanthin already present in the livers was taken into account. The following recovery was calculated: 91·6 ± 5·1 % (n 3). As there was no blank rat plasma available, the extraction of xanthophylls from rat plasma was not evaluated. Accuracy was, however, reviewed by the peak area of β-carotene added as an internal standard to each plasma sample, revealing a high repeatability (sd 6·8 %; n 5).
The basic diet (NIH-31) used in this study was an open-formula, cereal-based rat diet. As it contained, among other things, corn, several xanthophylls were supposed to be constituents of the diet and consequently of rat blood and tissues. Our own HPLC analyses (Schlatterer & Breithaupt, Reference Schlatterer and Breithaupt2005) proved that lutein and zeaxanthin were the main xanthophylls, whereas β-cryptoxanthin formed a minor component (lutein 10·4 nmol/g (5·9 μg/g), zeaxanthin 4·1 nmol/g (2·3 μg/g), β-cryptoxanthin 1·5 nmol/g (0·8 μg/g; calculated from individual calibration graphs; three per group; Table 2). As specified by the supplier, the basic diet contained vitamin A in addition. HPLC analyses of saponified diet extracts (n 3) revealed a mean retinol concentration of 12·9 nmol/g (3·7 μg/g).
The average amount of blood obtained from rat hearts was 0·93 ± 0·34 ml (n 10). In samples obtained by extraction without previous addition of the internal standard, no carotenoids were found (below the LOD calculated for β-cryptoxanthin; 12 pmol/ml). Thus, plasma samples were not investigated further. The average weight of rat livers was 7·8 ± 0·7 g (n = 10). Using the 325 nm trace in HPLC analyses, liver extracts showed one peak corresponding to free retinol, as well as several peaks most probably related to different retinol esters (Gerlach et al. Reference Gerlach, Biesalski, Weiser, Haeussermann and Baessler1989, Majchrzak et al. Reference Majchrzak, Fabian and Elmadfa2006). This assumption is assisted by several observations: the UV–visible spectra were identical to that of free retinol (λmax 326 nm), and all peaks had longer retention times than the free form and disappeared after saponification. We did not, however, attempt to identify individual esters as only the total amount of retinol was under investigation.
In a previous rat feeding study using only β-cryptoxanthin (data not shown), we determined the amounts present in plasma, liver, spleen, lung, kidney and skin. The body fat was not analysed since it was highly difficult to obtain a large enough quantity for reliable analysis. Generally speaking, the highest concentrations were present in the livers. We therefore decided to analyse the β-cryptoxanthin concentration in the liver in this study.
Comparing the HPLC chromatograms of liver samples from rats belonging to the control and treatment groups showed an increase in concentration of β-cryptoxanthin in the treatment group (Fig. 3). At the retention time expected for α-cryptoxanthin (3), no peak appeared in either chromatogram of liver belonging to the treatment group (n 5). One compound (6) eluting in front of α-cryptoxanthin (3) was already present in liver extracts from the control group. Although the UV–visible spectrum pointed to a lutein-derived metabolite (λmax 421(sh)/445/474 nm), identification was not certain. As the UV–visible data do not correlate with those of the β-cryptoxanthin-(Z)-isomers already present in the supplement and because substance 6 was already present in liver extracts from the control group, we do not believe that 6 is a (Z)-isomer of β-cryptoxanthin.

Fig. 3 HPLC analyses (extended sections; 450 nm, diode array detector) of liver extracts of one rat from the control group (C) and the treatment group (T). Trace S shows a chromatogram obtained by spiking extract T with an aliquot of the diluted xanthophyll supplement. Peak assignment is as follows: 3, α-cryptoxanthin; 4, β-cryptoxanthin; 5, β-carotene (internal standard); 6, unidentified compound.
Further differences in the peak pattern of liver samples of the control and treatment groups were not found. Trace S (Fig. 3) shows a chromatogram obtained by spiking extract T (treatment group) with two drops of the diluted xanthophyll supplement (300 μl TBME–2 ml methanol; 1:1), resulting in the appearance of an α-cryptoxanthin peak (3) and in enhancement of the β-cryptoxanthin peak area (4). In addition, several peaks appeared corresponding to the Z-isomers of β-cryptoxanthin, already present in the xanthophyll supplement (Fig. 2 earlier). Since those peaks were not present in extracts from the treatment group, it was concluded that no in-vivo isomerisation had occurred.
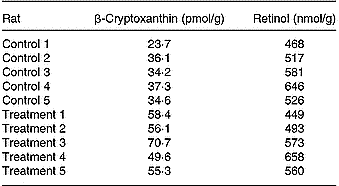
Quantitative analyses of β-cryptoxanthin and retinol in the livers of both groups showed the following results (Table 3). In the control group the average concentration was 33 ± 5 pmol/g, whereas in the treatment group the average concentration was 58 ± 7 pmol/g (five per group). Both data sets were statistically distinguishable; the significant statistical level was set to P < 0·01, applying one- and two-sided F and t tests, and all data sets were normally distributed (R/s test). The retinol concentration found in the two groups (control group 548 ± 61 nmol/g, treatment group 547 ± 72 nmol/g; five per group) was not statistically distinguishable (P < 0·01).
Table 3 Concentrations of β-cryptoxanthin (pmol/g) and retinol (nmol/g) reached in the individual livers of rats belonging to the control or treatment groups (five per group) after 23 d feeding
Discussion
The main focus of this study was to obtain basic data on the absorption efficiency of α- and β-cryptoxanthin. Therefore, a corn-oil-based xanthophyll supplement was designed comprising both xanthophylls in low concentrations. Taking into account a mean consumption of 15 g of the basic NIH-31-diet, the daily intake of β-cryptoxanthin is estimated to be 23 nmol. Applying 0·5 ml of the xanthophyll supplement to rats in the treatment group resulted in an additional daily intake of 17·3 nmol β-cryptoxanthin per rat. Based on the assumption that all xanthophylls were absorbed and that there was a linear relationship between the amount ingested and the liver response, application of the xanthophyll supplement theoretically results in a near-doubling of the β-cryptoxanthin concentration. Quantitative results from this study indeed showed an enhancement of the β-cryptoxanthin concentration in livers from the treatment group compared with those from the control group (mean values 58 pmol/g and 33 pmol/g, respectively), being near to the expected level. This suggested that β-cryptoxanthin was efficiently absorbed even from a minimally processed formulation and was stored in the liver. This observation is in accordance with the fact that no xanthophylls were found in the plasma. As the level of retinol in the basic diet was rather high (a mean consumption of 15 g basic diet resulting in a calculated daily intake of 194 nmol), it was not possible to detect minute changes in the liver retinol concentration caused by an enzymatic cleavage of β-cryptoxanthin to form vitamin A.
As the xanthophyll supplement additionally comprised α-cryptoxanthin (an ingestion of 9·2 nmol/d), one consequence should be appearance of an α-cryptoxanthin peak in the liver extracts. However, α-cryptoxanthin was not present in any liver sample. Consequently, the xanthophyll may be not absorbed or may be readily metabolised to vitamin A or apocarotenals; alternatively, it may be stored and accumulated in other tissues in the rat. It is in fact not possible to answer the question of what happened to α-cryptoxanthin within in the rat body.
Further clinical trials, particularly with human participants, have to prove whether the absorption efficiency of xanthophylls decreases in the order lutein to zeaxanthin to β-cryptoxanthin to α-cryptoxanthin, using similar formulations. As a high absorption rate is a prerequisite for developing health benefits from xanthophyll, the results will have a direct impact on the selection of xanthophylls used in human dietary supplements.
Acknowledgements
We thank M. T. Lortia Gómez and G. K. Ramírez Padilla for feeding the animals. We also express our sincere thanks to V. Andrade (Faculdat de Medicina, Universidad Autónoma de Querétaro) for housing the rats and for helpful assistance during the study.








