One of the most common CHDs is bicuspid aortic valve (with a prevalence of 0.5–2%). It can occur as an isolated valvular defect or in association with other CHDs and is defined as a complete fusion of two aortic valve cusps instead of three morphologically intact cusps.
There are three types of bicuspid aortic valve, characterised by the fusion of the cusps and the cusp from which the coronary arteries arise: right and left fusion, right and noncoronary fusion, and left and noncoronary fusion. Reference Sievers and Schmidtke1 The calcification of the aortic valve cusps in bicuspid aortic valve causes stenosis and increases the risk of infective endocarditis. In addition, bicuspid aortic valve is associated with congenital abnormalities of the aorta, such as coarctation of the aorta and interrupted aortic arch, which are combined with a ventricular septal defect.
Congenital abnormalities of the aorta, such as coarctation of the aorta and interrupted aortic arch, associated with ventricular septal defect, often require immediate surgical repair in the neonatal period. The surgical treatment of aortic arch obstruction with ventricular septal defect as a one-stage repair enables almost normal cardiac development. This challenging operation, however, carries a substantial risk of perioperative mortality and morbidity. Reference Haas, Goldberg, Ohye, Mosca and Bove2–Reference Ng’walali, Ohtsu and Tsunenari7 Furthermore, temporary pulmonary artery banding can cause malformation of the right ventricle and the pulmonary artery. Reference Norwood, Lang, Castaneda and Hougen4
The technical challenge of primary repair and various concerns regarding management of cardiopulmonary bypass have led many centres to favour primary one-stage correction, despite the absence of randomised studies comparing both approaches for aortic arch obstruction with ventricular septal defect. Reference Haas, Goldberg, Ohye, Mosca and Bove2,Reference Norwood, Lang, Castaneda and Hougen4,Reference Tlaskal, Chaloupecky and Marek8 Improved operative techniques, including the perfusion strategy, have clearly been influential for this approach.
We retrospectively evaluated the association of bicuspid aortic valves in early and late contemporary operation outcomes, including reoperation and transcatheter reintervention rates, after one-stage correction for interrupted aortic arch with ventricular septal defect or for aortic coarctation with hypoplastic aortic arch with ventricular septal defect from November, 1999 to June, 2016 from a single high-volume centre.
Materials and methods
Study population
This study received approval by the Institutional Review Board of Leipzig University. We performed a retrospective analysis of the cardiac surgery database at our institution and reviewed 74 consecutive patients (35 boys, 47% and 39 girls, 53%) with interrupted aortic arch (n = 41, 55%) or aortic coarctation with hypoplastic aortic arch (n = 33, 45%) with ventricular septal defect who underwent early one-stage correction at our institution from November, 1999 to June, 2016. Median age was 7 ± 29 days (range, 2–150, IQR: 11); median weight was 3.3 ± 0.7 kg (range, 1.5–6.0, IQR: 0.70), with 21 (28%) patients <3.0 kg. The median aortic valve annulus diameter was 6.0 ± 1.2 mm. The classification of interrupted aortic arch was type A in 19 patients, type B in 21 (28%) patients (B1: n = 9, 12%, B2: n = 12, 16%), and type C in one patient. Considering all patients with coarctation of the aorta, the median diameter of the aortic arch was 2.5 mm, and the median diameter of the ascending aorta was 7 mm. Distal hypoplastic aortic arch was present in 24 (32%) patients and proximal hypoplastic aortic arch in 9 (12.2%) patients, and coarctation of the aorta-associated anomalies in these patients included a large ventricular septal defect in all, common arterial trunk in 7 (9%), aortopulmonary window in 4 (5%), severe left ventricular outflow tract obstruction in 3 (4%), atrioventricular septal defect in 2 (3%), transposition of the great arteries in 2 (3%), and double outlet right ventricle in 1 (1%) patient. Furthermore, a second atrial septum defect or persistent foramen ovale was present in 67 (91%) patients; patent ductus arteriosus was present in all patients. DiGeorge syndrome (CATCH 22) was present in 11 (15%) patients. In addition, five (7%) patients were premature neonates.
Bicuspid aortic valve was examined at least twice before operation using transthoracic echocardiography. The examination was performed under mild sedation, while spontaneous respiration was maintained. Diagnosis of bicuspid aortic valve was based on the demonstration of two cusps and two commissures during short-axis visualisation. Supportive features included cusp redundancy, eccentric valve closure, and a single coaptation line between the cusps during diastole.
Pre-operative management included improving lower body perfusion, avoiding acidosis, infusing prostaglandin, and mechanically ventilating with inotropic support for pre-operative stabilisation in case necessary. The perioperative course and follow-up with physical examination, echocardiogram, and cardiological evaluation were obtained. A total of 48 (65%) patients who were suspected for reintervention by echocardiogram received post-operative evaluation using cardiac catheterisation (n = 39, 53%) or cardiac magnetic resonance imaging (n = 9, 12%). To determine neurological outcomes, physical examination, head ultrasound and, if necessary, computer tomography were performed.
Definitions
The aortic arch consists of the transverse arch and isthmus. The transverse arch is the part of the aorta between the brachiocephalic artery and the left subclavian artery. The isthmus is the part of the arch between the left subclavian artery and the ductus (or ligamentum arteriosus) arteriosus.
Hypoplastic aortic arch was defined as an aortic arch diameter less than 0.5 times the diameter of the ascending aorta as measured in the echocardiographic report or evaluated subjectively by the operating surgeon at the time of operation. Reference van Heurn, Wong and Spiegelhalter10 Reinterventions included conventional reoperation and catheter reintervention after primary correction, excluding rethoracotomy for bleeding, wound debridement, mechanical circulatory support, or pacemaker implantation. Functional status was described according to the NYHA classification, and follow-up was determined from recent hospital visits or from written correspondence with the patient’s community cardiologist or general practitioner.
Surgical techniques
Surgery was performed via a median sternotomy. The superior and inferior vena cava were cannulated with metal-tip 10F to 12F cannulas (Stöckert, Germany), and the ascending aorta with an angulated 2–2.6-mm cannula (Stöckert). In all patients with interrupted aortic arch, additional temporary cannulation of the patent ductus arteriosus (2.0-mm arterial cannula) was used for systemic perfusion during cooling. All patients had a left atrial vent inserted through the right upper pulmonary vein and a cardioplegic cannula placed in the aortic root for selective coronary perfusion. Patients were cooled to 24°C blood temperature (range, 18–33°C), during which time further dissection of the aortic arch, head vessels, and descending aorta was performed. Topical cooling of the head was utilised in all patients.
After clamping of the ascending and descending aorta, the left carotid and left subclavian arteries were snared, and low-flow antegrade cerebral perfusion via the innominate artery (the arterial cannula was turned into the innominate artery and snared proximally) and low-flow coronary perfusion through the cardioplegic cannula, connected to the aortic cannula were maintained. The antegrade cerebral perfusion strategy was equivalent for all patients. Near infrared spectroscopy was used to guide the flow in the innominate artery. There was no circulatory arrest, and the extracorporeal circulation perfusion was kept at 20% of normal flow. The patent ductus arteriosus was ligated and resected carefully. The aortic arch was reconstructed using a direct anastomosis of the descending to the ascending aorta in all 40 patients with interrupted aortic arch, Reference Trusler and Izukawa9 and an extended end-to-side anastomosis of the descending aorta to the proximal aortic arch was done, following the blind closure of the distal aortic arch behind the left subclavian artery in all 33 (45%) patients presenting with aortic coarctation with hypoplastic aortic arch. Aortic arch reconstruction was performed in beating heart fashion during cooling. After arch reconstruction, the head vessels and the descending aorta were reopened and full flow extracorporeal circulation reinstituted; a single-shot of 30 mL/kg St. Thomas crystalloid solution was infused into the aortic root. The ventricular septal defect was closed using an autologous pericardial patch and interrupted 5–0 polypropylene sutures, buttressed with Teflon pledgets. We did our primary anastomosis without a patch in all interrupted aortic arch patients; a transatrial approach was used in 68 (92%) patients and a transventricular approach in 6 (8%) patients.
A low-volume prime extracorporeal circulation circuit (total priming volume 210 mL) was used. The reservoir and the pump were placed directly beside the surgeon. The prime solution consisted of 120 mL packed red cells, 80 mL 5% human albumin, 3 mL/kg 15% mannitol, 5 mL sodium bicarbonate, 100 IU/kg heparin, and 20 mg/kg steroids (Urbason; Aventis Pharma Deutschland GmbH, Bad Soden, Germany). During the entire operation, the haematocrit was maintained above 30%. Haemofiltration was used in all patients during perfusion.
Statistical analysis
We performed statistical analysis using SPSS Windows 20 software (SPSS Inc., Chicago, IL, USA). All demographic data are expressed as mean ± standard deviation or median with range. We used the Kaplan–Meier method to assess freedom from percutaneous or surgical cardiac intervention during follow-up. Risk factors for reintervention were identified using Log-Rank-test. The Cox proportional hazards model was used for univariable and multivariable analysis to identify predictors of reintervention. We used a stepwise forward multiple analysis with variables that were univariable significant to identify the risk factors. A p value of less than 0.05 was considered statistically significant. For variable selection, the SPSS BSTEP procedure was used. With this backward selection algorithm, a variable is removed if the significance level is greater than 0.15.
Results
Baseline characteristics and operative data are summarised in Table 1. Primary one-stage surgical correction for aortic arch obstruction and ventricular septal defect patch closure was achieved in all patients. Early mortality was 1.3%, one premature neonate died in the hospital with extracorporeal membrane oxygenation after coarctation of the aorta plus ventricular septal defect repair. There was no further mortality. All patients were discharged in a stable and improved condition.
Table 1. Patient characteristics and operative data
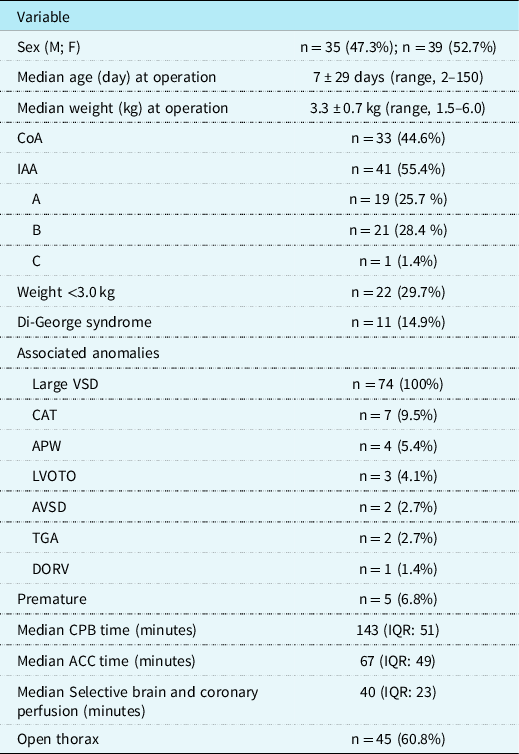
Surgical correction of aortic arch obstruction and ventricular septal defect patch closure was performed in all patients. In addition, two (3%) patients received an arterial switch operation, 4 (5%) patients had a closure of an aortopulmonary window, 7 (9%) patients had a correction for persistent truncus arteriosus with right ventricular outflow tract implantation of a valve conduit, and 1 (1%) patient had a patch redirection for double-outlet right ventricle. In 1 (1%) patient with COA-HyAA, acute aortic dissection evolved intraoperatively; aortic repair and pulmonary artery banding were subsequently performed on this patient, with definitive repair successfully performed 6 months later.
The median bypass time was 143 minutes (IQR: 51), median aortic cross-clamp time was 67 minutes (IQR: 49), and median selective brain and coronary perfusion duration was 40 minutes (IQR: 23). Weaning from bypass was uneventful in all patients using moderate dopamine (3 g · kg−1 · minute−1) and dobutamine support (4–8 g · kg−1 · minute−1) and milrinon 50 μg/kg in cardiopulmonary bypass before discontinuation of extracorporeal circulation (Sanofi, Paris, France). All patients received volume-controlled ventilation with 20-parts per million nitric oxide due to severe pulmonary hypertension, common in these patients. Delayed chest closure was performed in 45 patients after 2.3 ± 1.2 days.
The median hospital stay was 13 (IQR: 12). Post-operative complications were low cardiac output with extracorporeal membrane oxygenation implantation (n = 2, 3%), wound infection (n = 2, 3%), pneumonia (n = 1, 1%), and renal failure requiring peritoneal dialysis (n = 1, 1%). Post-operatively, 68 (92%) patients were in sinus rhythm and 5 (7%) were in atrioventricular rhythm; 1 (1%) patient developed atrioventricular block III post-operatively and required a pacemaker during his hospital stay. All other patients were in sinus rhythm at discharge.
During follow-up, all patients underwent cerebral ultrasonography examinations. Three patients, two with hydrocephalus, developed neurological complications in form of cerebral bleeding in late follow-up and received CT scanning. On ultrasonography examinations, the remaining 71 (96%) patients showed no bleeding complications, oedema, or hydrocephalus in comparison with routine pre-operative cerebral ultrasonography examinations. The Doppler echocardiogram indicated normal cerebral flow pattern in all surgical patients. One patient developed left bronchial compression, with no evidence for vocal cord paralysis. Echocardiography revealed good left and right ventricular function in all patients post-operatively. A small residual ventricular septal defect was present in 10 patients (13.5%). Only 1 (1%) patient underwent reoperative residual ventricular septal defect closure.
Median follow-up was 5.7 years (IQR: 10.48 years). The median follow-up calculated with the reverse Kaplan–Meier method was 10.441 (IQR: 7.18) (Fig 1). There was no mortality during follow-up. All patients developed normally up to the most recent outpatient examination, with normal growth and no neurologic symptoms. Echocardiography revealed good ventricular function in all patients at last follow-up.
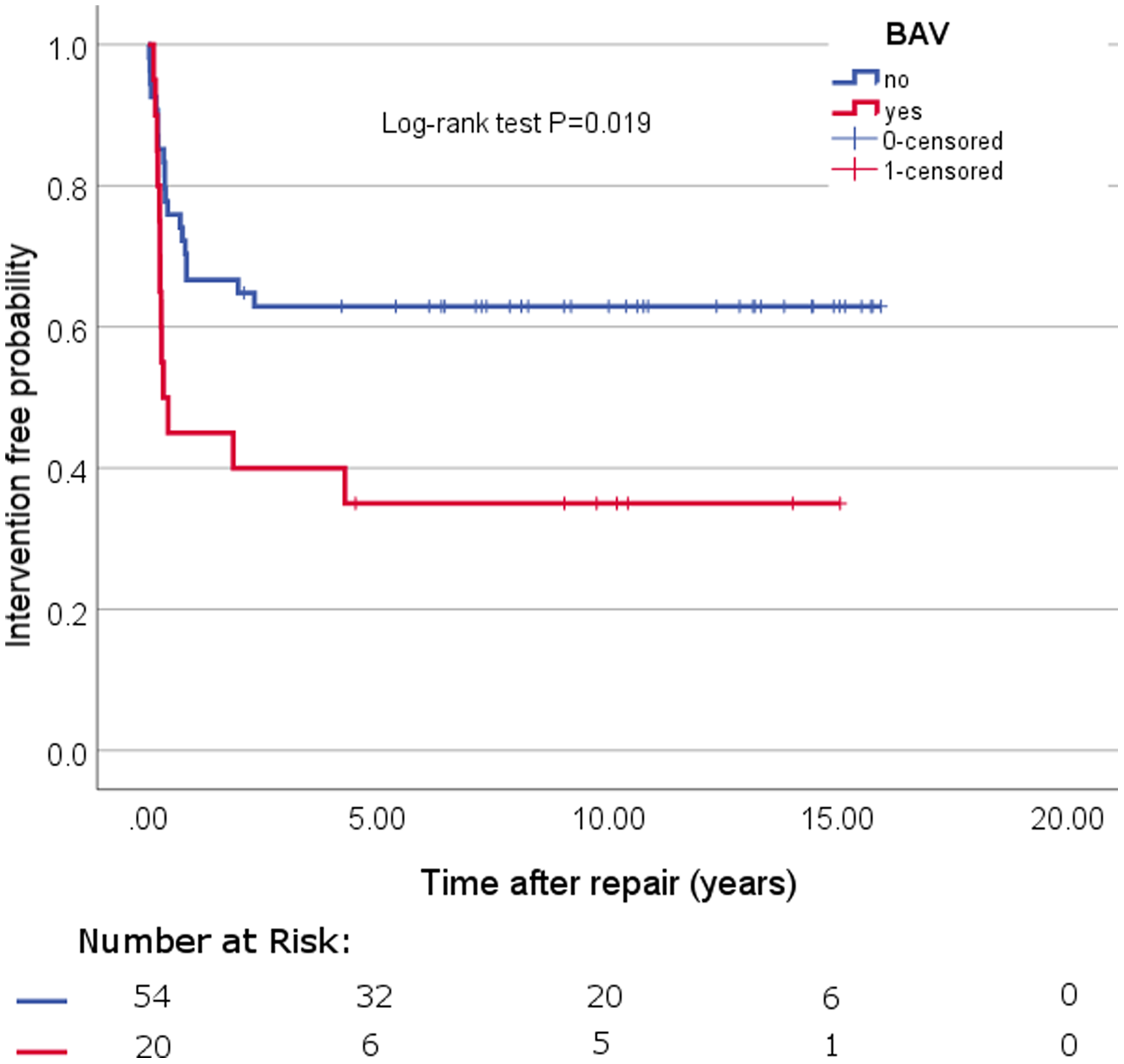
Figure 1. Kaplan–Meier reintervention curve.
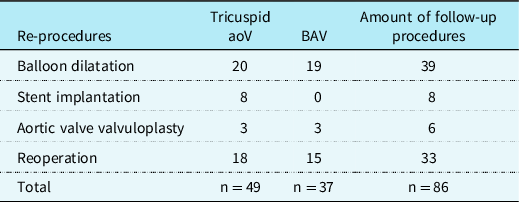
Reinterventions occurred in 36 (49%) patients. The mean overall survival time of all patients was 9.094 years (SE .890). The median time to reinterventions of those patients with an actual reintervention was 0.29 years (IQR:0.52) (Fig 1). Ten (13%) patients underwent reintervention due to left ventricular outflow tract obstruction, 14 (19%) patients due to aortic arch stenosis, and 12 (16%) patients because of both. The incidences for left ventricular outflow tract obstruction was 26% and for recoarctation 32%. Balloon angioplasties were required in 18 (24%) patients, reoperations in 4 (5%) patients, and both in 14 (19%) patients (Table 2). A total of 86 follow-up procedures were required in these 36 (49%) patients: aortic valve valvulopasty (n = 6, 8%), stent implantation (n = 8, 11%), balloon dilatation (n = 39), and reoperation (n = 33, 45%) (Table 3).
Table 2. Frequency of patients with reintervention
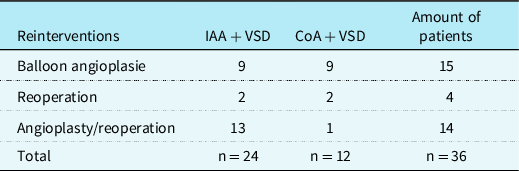
Table 3. Frequency of reprocedures
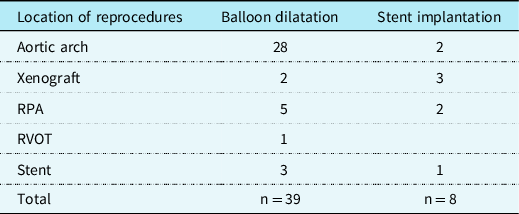
Aortic valve valvulopasty was required in 5 (7%) patients. One (1%) of the patients with interrupted aortic arch type B2 and bicuspid aortic valve had a second valvuloplasty after a short time. Three (4%) patients had stents placed in the right ventricle-pulmonary artery conduit, 2 (3%) patients in the aortic arch, and 2 (3%) in the pulmonary artery. In 1 (1%) patient, the stent was implanted inside another stent located in the aortic arch. Balloon dilatation was done in the aortic arch (n = 28), the pulmonary arteries (n = 5), the right ventricle pulmonary artery conduit (n = 2), the right ventricle outflow track (n = 1), and the aortic arch stent (n = 3) (Table 4).
Table 4. The location and frequency of reprocedures
Reoperations were summarised in Table 5. There were 2 (3%) patients with IAA type B; each underwent four reoperations. In addition, 2 (3%) patients with interrupted aortic arch type A and B underwent three reoperations, separately. All patients developing normally with persistent improvement in haemodynamic function up to their most recent follow-up and had no obvious neurologic impairment in routine examinations. The hospital survival for reintervention was 100%, with no late deaths.
Table 5. Frequency of reoperations
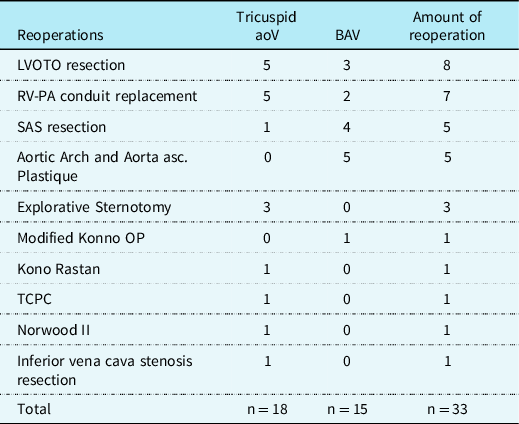
RV-PA, right ventricle pulmonary artery; SAS, subaortic stenosis; TCPC, total cavopulmonary connection.
Twenty (27%) patients had bicuspid aortic valve, and the remaining 54 (73%) had a tricuspid aortic valve. Patients with a combination of interrupted aortic arch and bicuspid aortic valve were 11 and all of them underwent reintervention. Patients with a combination of coarctation with hypoplastic aortic arch and bicuspid aortic valve were nine and only five patients underwent reintervention (Fig 2). The median bicuspid aortic valve annulus diameter was 5.0 ± 0.33 mm (range, 3.0–7.0). A potential risk factor for reintervention after interrupted aortic arch and coarctation of the aorta with ventricular septal defect repair was bicuspid aortic valve (p = 0.019, Chi2 (1) = 5.457). In addition, multivariable Cox analyses confirm that bicuspid aortic valve (HR = 0.381, p = .016), and interrupted aortic arch (HR = 0.412, p = 0.043) were predictors of late reintervention.
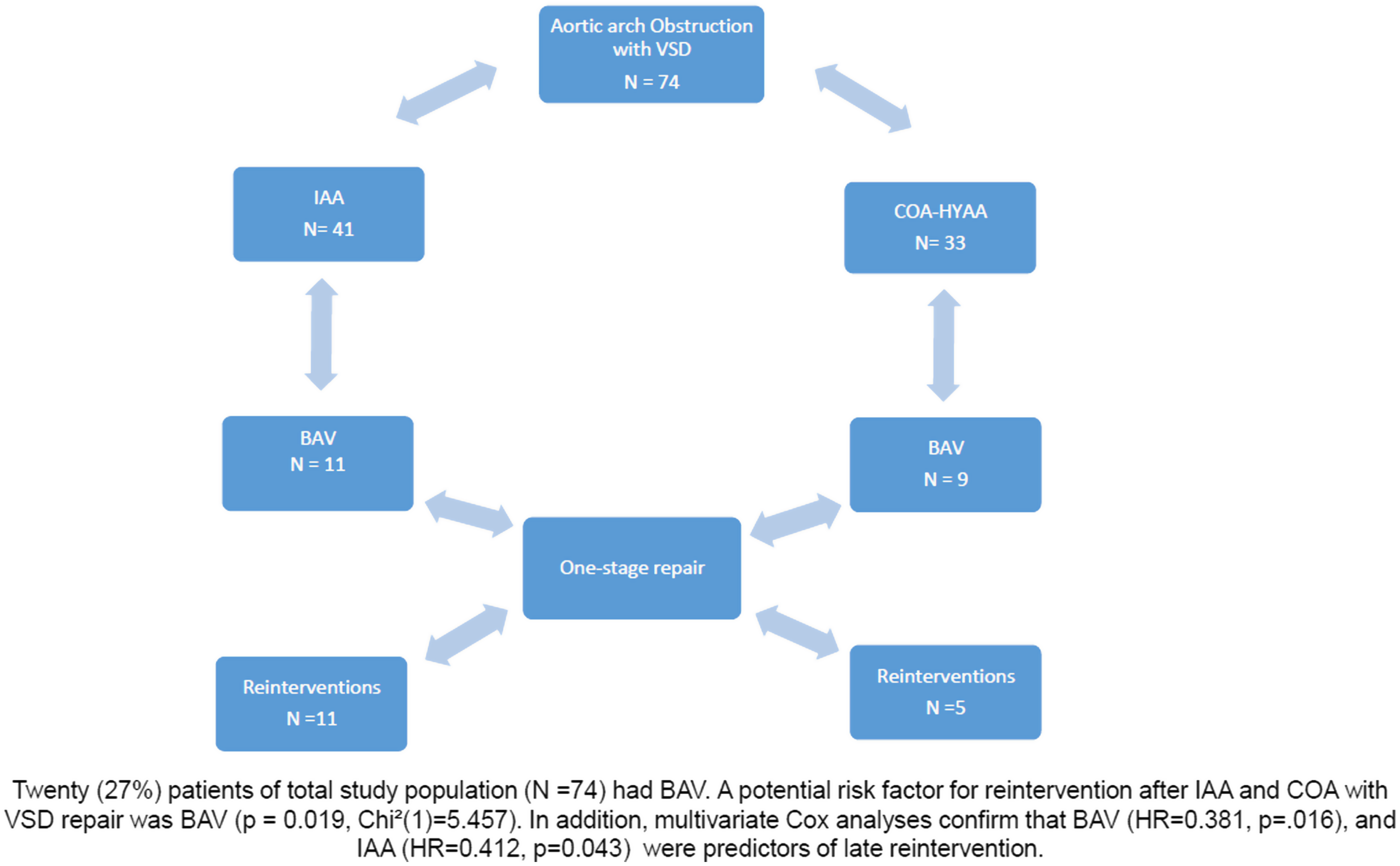
Figure 2. Study design.
Discussion
In this retrospective analysis focusing on primary repair of aortic arch obstruction associated with ventricular septal defect, we found that one-stage correction could be performed with excellent results, even in preterm neonates and newborns with very low birth weight. These patients had severe pathologies presenting with a variety of clinical findings, such as residual blood flow, anomalies of the head vessels, and varying diameters of the ascending aorta and related coronary blood flow, closely related to the clinical presentation. The size, number, and location of the septal defects, in particular, are important for the further therapeutic planning.
Furthermore, we focus in this study on the association of bicuspid aortic valve for reintervention in mid- to long-term outcomes after one-stage correction for patients with interrupted aortic arch and coarctation of the aorta with ventricular septal defect with a median follow-up 5.7 years (IQR: 10.48). We achieved an acceptable surgical result under our treatment selection criteria and show bicuspid aortic valve to be a potential risk factor for valve-related morbidity after interrupted aortic arch and coarctation of the aortawith ventricular septal defect repair, even in infants and young children. This observation with the bicuspid aortic valve dysfunction is comparable to the results in other studies. Reference Li, Li and Fan15–Reference Sugimoto, Ota and Miyakoshi17 The main difference is mainly due to the larger patient population with ages younger than demonstrated in the previous reports. Reference Li, Li and Fan15–Reference Sugimoto, Ota and Miyakoshi17 In addition, all patients in our study underwent single-stage repair of interrupted aortic arch and coarctation of the aorta with ventricular septal defect.
Aortic arch obstruction presents at different levels of the aorta, ranging from severe stenosis to complete interruption. In this study, we analysed results from both patients with interrupted aortic arch and patients presenting with coarctation with hypoplastic aortic arch. Similar aspects of pathology as well as comparable operative repair strategies justify this combined approach of analysis. Generally, patients with aortic arch obstruction with ventricular septal defect are in poor clinical condition. They usually present with an open persistent ductus arteriosus. However, restrictive patent ductus arteriosus can cause a severe condition requiring prostaglandin therapy, inotropic support, and ventilator support to stabilise the haemodynamic and metabolic clinical condition.
Despite improvements in surgical techniques and perioperative management, the treatment of interrupted aortic arch and coarctation with hypoplastic aortic arch with ventricular septal defect remains a major surgical challenge. All patients require surgical therapy, and primary correction has developed as the gold standard. According to results from 1989 up to the present, the one-stage correction strategy has an excellent functional outcome in preterm and newborn babies, regardless of birth weight, although residual morbidity remains as high as 10–15%. Reference Haas, Goldberg, Ohye, Mosca and Bove2–Reference Norwood, Lang, Castaneda and Hougen4,Reference Ishino, Kawada, Irie, Kino and Sano11 There is a very low but persistent risk of mortality, clearly dependent on the patient’s clinical condition. As reported earlier, one patient required a staged repair owing to an acutely evolving aortic dissection. Reference Walther, Kiefer, Dahnert and Kostelka12 In conclusion, primary correction of aortic arch obstruction with ventricular septal defect is a challenging operation at any age.
Optimal perioperative and post-operative management are paramount in achieving a successful outcome. Reference Kostelka, Walther and Geerdts13 During recent years, the perioperative strategies of extracorporeal circulation and new techniques for selective cerebral perfusion have improved and decreased the risk of perioperative neurologic damage. In all patients, we applied selective coronary perfusion during aortic arch reconstruction. This novel technique reduced the duration of cardiac arrest, advantageous in patients with very complex intracardiac anatomy in whom a longer cross-clamp duration is inevitable.
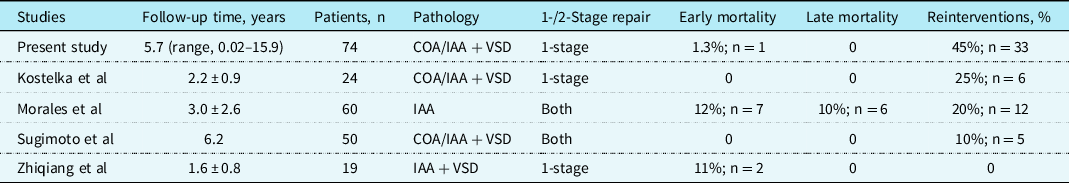
Selective cerebral perfusion is the standard approach for all our patients and can be achieved quite easily by turning the aortic cannula directly into the innominate artery. Reference Uemura, Yagihara, Kawahira, Yoshikawa and Kitamura14 In addition, it can be used in most intracardiac corrections requiring concomitant aortic arch reconstruction. Extracorporeal perfusion was established via a small circuit located directly beside the surgeon, requiring a very low amount of priming volume. Routine perioperative hemofiltration was used in all patients and allowed us to achieve a negative fluid balance in all patients immediately post-extracorporeal membrane oxygenation. Our primary correction of aortic arch obstructions with ventricular septal defect reduced significantly the postoperative morbidity (only 1 (1%) patient with renal failure and need for peritoneal dialysis) and resulted in fewer cases of post-operative neurologic deficit as compared to data from other cohorts (Table 6). Reference Li, Li and Fan15–Reference Sugimoto, Ota and Miyakoshi17 All children had no obvious neurologic impairment in routine examinations at last follow-up.
Table 6. Literature review of series that included patients with aortic arch obstruction associated with VSD
The prophylactic inhaled nitric oxide is a powerful therapeutic used in neonatology. Its use is evidenced-based for term and near-term infants with persistent pulmonary hypertension; however it is frequently used off-label both in term and preterm babies, decreasing pulmonary vascular resistence leading to diminished extrapulmonary shunt and also with microselective effect improving ventilation/perfusion matching. In addition, inhaled nitric oxide is a pulmonary vasodialator improving oxygenation. All of this lessen the need for extracorporeal membrane oxygenation post-operatively. This could be supported by our clinical results with minimal use of extracorporeal membrane oxygenation post-operatively. The strategy pursued seems to corroborate our opinion to have this regime as a standard adjunct medical treatment.
In present cohort, the rate of overall reintervention (n = 36, 49%) was quite high compared to other series, especially for arch restenosis. Reference Li, Li and Fan15–Reference Sugimoto, Ota and Miyakoshi17 This can be related with surgical exposure in this challenging operation. The improvement of surgical exposure could help to do the operation much faster and safer. Therefore, we will modify the perfusion protocol in the future to improve the exposure. A total of 86 follow-up procedures were required in these 36 (49%) patients. More than 30 of these procedures were done in only 4 (5%) patients. This high reintervention rate depends on several factors influencing the post-operative outcomes. Optimal exposure is paramount to succeeding in these challenging operations. Controversy remains among experts whether the limited space provides ideal conditions to reconstruct the aortic arch. However, we believe beating heart arch reconstruction not to be an impairment in terms of optimal exposure and technical results for the arch. Aortic arch restenosis is defined as a stenosis ≥20 mmHg. Many of our patients who received a reintervention of the arch had a restenosis of less than 20 mmHg. We identified a relatively low threshold used by our cardiologists for reinterventions as a possible underlying reason for our high reintervention rates. The Congenital Heart Surgeons Society recommends using a patch for interrupted aortic arch and arch advancement. Reference Uzzaman, Khan and Davies18 We did our primary anastomosis without a patch in all interrupted aortic arch patients as we believe that the use of a patch to have several disadvantages, such as stenosis, thickening, and atrophy.
Pre-operative left ventricular outflow tract obstruction is a well-recognized risk factor and has consistently been associated with a need for reintervention in interrupted aortic arch or CoA/VSD. Reference Salem, Starnes and Wells19 The present study contains a low prevalence of patients with pre-operative left ventricular outflow tract obstruction when compared with most prior series and failed to demonstrate significance as a risk factor for late reintervention. Furthermore, echocardiographic diagnosis of bicuspid aortic valve remains quite challenging, particularly in the newborn. We are aware of the significant limitations of echocardiography differentiating bicuspid aortic valve from mono cusp valves, quite common in neonates, and, more rarely, from tricuspid AV. Unfortunately, not all OR reports documented the morphology of the valve, so it may be the case that not all pre-operatively diagnosed bicuspid aortic valve were proven bicuspid in the operative setting. We were not able to perform other form of valve diagnostics in the clinical approach. Other clinical reports such as Sugimoto et al Reference Sugimoto, Ota and Miyakoshi17 likewise performed their bicuspid diagnostic via echocardiography. This point is an important issue to be addressed in future studies. However, echo was the only imaging performed in most patients, having a recognised false positive ratio of 20% or greater. We presume this limitation not to affected our findings, due to the large number or echo diagnosed bicuspid aortic valve patient population who required reintervention. Only four patients with bicuspid aortic valve are free of reintervention over the follow-up period.
Bicuspid aortic valve appear more frequently in patients with interrupted aortic arch and coarctation of the aorta than in the normal population, with an incidence varying from 25 to 85%. Reference Niaz, Poterucha and Johnson20–Reference Tortora, Wischmeijer and Berretta21 In the present patient cohort, 20 of 74 patients (27%) had bicuspid aortic valve, similar to earlier reports. The clinical symptoms of bicuspid aortic valve disease varies from severe valve disease in childhood to asymptomatic valve or thoracic aortic disease in adulthood. The development of symptoms in bicuspid aortic valve population occurs often in older age. Reference Siu and Silversides22 According to previous studies, bicuspid aortic valve shows to be a risk factor for valve-related complications, such as AR, in the interrupted aortic arch or coarctation of the aorta population. Reference Boodhwani, de Kerchove and Glineur23 In the present study, we confirm this statement of bicuspid aortic valve being a potential risk factor for reintervention after interrupted aortic arch and COA with ventricular septal defect repair (p = 0.019, Chi2 (1) = 5.457). In addition, multivariable Cox analyses confirm bicuspid aortic valve (HR = 0.381, p = 0.016), and interrupted aortic arch (HR = 0.412, p = 0.043) to be predictors of late reintervention. Morales and his colleagues show in their study bicuspid aortic valve to be one of the risk factors for death in 60 interrupted aortic arch patients and also for 50% of the late deaths. Reference Morales, Scully and Braud16 They concluded that a bicuspid aortic valve become stenotic in the follow-up period and therefore is associated with a higher late morbidity and thus mortality in interrupted aortic arch patient population.
Median follow-up was 5.7 years (IQR: 10.48). However, the long-term outcomes of these patients remain unclear. Many of our patients will need reinterventions in the future. Close check on these patients is mandatory for these patients over the long-term period to investigate on the aortic valve function, also important in patients who have no valve-related problems during the post-operative period.
Conclusion
Bicuspid aortic valve is one of the most common congenital cardiac anomalies and is often seen, combined with a ventricular septal defect, in patients with an interrupted aortic arch or coarctation of the aorta. The primary repair in newborns with aortic arch obstruction associated with ventricular septal defect can be performed with excellent operative survival and functional outcomes even in neonates with very low birth weight. However, the management of the complex pathology remains a great surgical challenge that requires a highly experienced team. Bicuspid aortic valve was a significant risk factor for valve-related reintervention after one-stage repair for interrupted aortic arch or aortic coarctation with hypoplastic aortic arch with ventricular septal defect for the reason that a bicuspid aortic valve become stenotic and therefore is it associated with higher late morbidity and thus mortality. Although, particularly neonates with bicuspid aortic valve will require reintervention in the future. Regular lifelong cardiac follow-up is therefore recommended.
Acknowledgements
First of all I would like to thank Prof. Dr. med. J. Vázquez-Jiménez for his support and excellent supervision. Without his support, it would not have been possible to accomplish my manuscript. In addition, I would like to thank Prof. Dr. med. M. Kostelka, Prof. Dr. med. F. Bakthiary and Prof. Dr. med. I. Dähnert for their support.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Key question
What is the association of bicuspid aortic valve on long-term outcome following one-stage repair of aortic arch obstruction associated with ventricular septal defect?
Key finding
Bicuspid aortic valve is a significant risk factor for valve-related reintervention after one-stage repair for aortic arch obstruction with ventricular septal defect.
Take-home message
The one-stage repair of aortic arch obstruction with ventricular septal defect affords excellent surgical results. Particularly neonates with bicuspid aortic valve will require reintervention in the future.











