Introduction
Nitric oxide (NO), a highly reactive, membrane-permeable free radical, is a widespread intracellular and intercellular messenger with a broad spectrum of regulatory functions in many physiological processes. In the past 20 years NO has been reported to be involved in various key physiological processes of plants, including seed germination, leaf senescence, ethylene emission, stomatal closure, and various plant responses to biotic and abiotic stresses, such as salinity, drought and ultraviolet (UV)-B-radiation (Neill et al., Reference Neill, Desikan and Hancock2003; Delledonne, Reference Delledonne2005). It has been reported that molecules such as NO can enhance germination and/or break dormancy in seeds (Beligni and Lamattina, Reference Beligni and Lamattina2000; Beligni et al., Reference Beligni, Fath, Bethke, Lamattina and Jones2002; Neill et al., 2003; Simontacchi et al., Reference Simontacchi, Jasid and Puntarulo2004; Zhang et al., Reference Zhang, Shen, Zhang and Xu2005; Bethke et al., 2007) and significantly impact plant growth and development (Lamattina et al., Reference Lamattina, Garcia-Mata, Graziano and Pagnussat2003; Neill et al., 2003; del Rio et al., Reference del Rio, Corpas and Barroso2004). Gouvea et al. (Reference Gouvea, Souza, Magalhaes and Martins1997) reported that exogenously applied sodium nitroprusside (SNP), as an NO donor, can enhance elongation growth in maize root segments, and application of methylene blue (MB), which inhibits NO production and/or NO action in plants, could reverse these responses.
Gould et al. (Reference Gould, Lamotte, Klinguer, Pugin and Wendehenne2003) reported that heat, osmotic and salinity stresses induced a rapid increase in NO production in tobacco cells. Furthermore, application of SNP alleviated the toxic effects caused by heat stress in weed calluses by decreasing hydrogen peroxide (H2O2) and malondialdehyde (MDA) contents and increasing the activities of superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and peroxidase (POD) (Song et al., Reference Song, Ding, Zhao, Sun and Zhang2006). The positive effect of NO on alleviation of oxidative stress was also found after plant exposure to cold (Zhao et al., Reference Zhao, Chen, Zhang and Zhang2009) and drought (Mata and Lamattina, Reference Mata and Lamattina2001).
Like many other crop plants of tropical or subtropical origin, cultivated tomato (Solanum lycopersicon Mill.) is sensitive to chilling as well as freezing temperatures. Temperatures below 10°C inhibit growth, and those under 6°C may cause irreparable damage (Geisenberg and Stewart, Reference Geisenberg, Stewart, Atherton and Rudich1986). Since the particular climatic conditions of the Mediterranean permit off-season production, tomato is often sown in winter or in early spring in cold greenhouses, plastic tunnels and seedbeds, or directly in the field. In early sowing, periods with suboptimal temperatures close to the minimum germination temperatures are frequently encountered. In these conditions, seedling emergence may be reduced, delayed and spread over time (Leskovar and Sims, Reference Leskovar and Sims1987).
Treatments to enhance seed vigour have been proven to be very effective in achieving rapid and uniform seed germination. The priming treatment may constitute a useful tool in overcoming the problems described above and, thus, assuring a high probability of successful establishment for each seed planted. Since some tomato genotypes are more sensitive to cold than others, it is possible that priming could make these genotypes germinate as fast as the cold-tolerant ones. Furthermore, it could also improve the performance of the cold-tolerant ones (Dahal et al., Reference Dahal, Bradford and Jones1990).
Chen and Arora (Reference Chen and Arora2012) proposed a model illustrating the cellular physiology of priming-induced stress tolerance, which is likely achieved via two strategies. First, seed priming sets in motion germination-related activities, such as respiration (Corbineau et al., Reference Corbineau, Ozbincol, Vinel, Come, Black, Bradford and Vazuez-Ramos2000), endosperm weakening (Dahal et al., Reference Dahal, Bradford and Jones1990), embryo enlargement and transcription and translation (Schwember and Bradford, Reference Schwember and Bradford2010), that facilitate the transition of quiescent dry seeds to germination and improve germination potential. Second, priming imposes an abiotic stress on seeds that represses radicle protrusion but stimulates stress responses, e.g. the accumulation of late embryogenesis abundant (LEA) proteins (Capron et al., Reference Capron, Corbineau, Dacher, Job, Côme and Job2000), potentially inducing cross-tolerance. Together, these two strategies constitute a ‘priming memory’ in seeds, which can be recruited upon a subsequent stress exposure to mediate greater stress-tolerance of germinating primed seeds (Chen and Arora, Reference Chen and Arora2012). Seed priming accelerates germination and improves seedling uniformity in many crops (Cayuela et al., Reference Cayuela, Pérez-Alfocea, Caro and Bolarín1996; Benamar et al., Reference Benamar, Tallon and Macherel2003; Farooq et al., Reference Farooq, Basra and Hafeez2006), especially where they are grown under unfavourable environmental conditions (Dahal et al., Reference Dahal, Bradford and Jones1990; Tiryaki et al., Reference Tiryaki, Kizilsimsex and Kaplan2009; Amooaghaie, Reference Amooaghaie2011).
Currently, the major focus of priming research is on optimizing protocols to improve germination performance. However, only a few biochemical studies have investigated enzymatic antioxidant activities during and after priming (Bailly, Reference Bailly2004; Amooaghaie et al., Reference Amooaghaie, Nikzad and Shareghi2010; Chen et al., 2010b; Amooaghaie, Reference Amooaghaie2011). Even fewer attempts have been made to understand the cellular and molecular mechanisms and signalling pathways of stress tolerance induced by priming (Chen and Arora, Reference Chen and Arora2012).
The objective of the present study was to investigate the effects of exogenous SNP and osmopriming on germination, seedling vigour and suboptimal temperature tolerance of the two tomato genotypes Falcato and Cherry. Furthermore, a possible signalling role of NO in priming-induced responses was investigated.
Materials and methods
Plant material and treatments
Seeds of two tomato genotypes (Falcato, Cherry), were used to investigate the effects of priming on seed germination, seedling vigour and low-temperature tolerance. Seed treatments included ospriming in polyethylene glycol solution (PEG 6000; Shanghai Chemical Reagent Co. Ltd, Shanghai, China) at 300 g l− 1, 200 μM sodium nitroprusside (SNP) as NO donor (Merck, Darmstadt, Germany), 100 μM methylene blue (MB; Fluka, Shanghi, China), 200 μM SNP +100 μM MB, PEG +100 μM MB, and distilled water as control (Con). For osmopriming, seeds were primed with continuous aeration. After 24 h at 25°C, the seeds were removed, rinsed in distilled water, wiped free of water and air-dried at 25°C for 24 h. For SNP or MB treatments, seeds were presoaked for 1 d in these solutions. As controls, seeds were soaked in distilled water for 1 d and then transferred to Petri dishes immediately.
Laboratory seed germination tests were conducted in incubators in 9-cm Petri dishes (50 seeds each per plate and three replicates for each treatment) on a layer of filter paper moistened with distilled water. Seeds were incubated in controlled-growth chambers at 10, 15, 20 and 25°C. Seed germination was counted daily and counts continued until no further germination occurred for three consecutive days. Seeds were considered germinated when radicles had protruded for more than 2 mm. Germination rate (GR) and germination index (GI) were calculated as described by the Association of Official Seed Analysts (AOSA) (1983), using the following formulae:
in which Gt is the number of germinated seeds in t days; Dt is the number of corresponding germination days; n is the cumulative number of germinated seeds in t days.
Root and shoot length were measured 7 d after the time of radicle protrusion. The vigour index was determined by the following equation:
Biochemical analysis
All biochemical parameters (amylase activity, sugar content and NO content) were measured 12 h after incubation before any radicle protrusion took place.
Measurement of α- and β- amylase and soluble sugars
Alpha- and β-amylase activities of seeds at different temperatures were determined using the method of Bernfeld (Reference Bernfeld1955), based on the reduction of free maltose, resulting from the enzymatic hydrolysis of starch, by 3,5-dinitrosalicylic acid, resulting in the formation of orange-coloured 3-amino-5-nitrosalicylic acid, which was determined colorimetrically at 540 nm. The activity of both enzymes was expressed as μmoles maltose (g seed)− 1. Soluble sugars were analysed by the anthrone method (Fales, Reference Fales1951).
NO measurement
NO production was determined according to the method described by Zhou et al. (Reference Zhou, Guo, Xing and Huang2005) with slight modifications. Samples were ground in a mortar and pestle in 3 ml of 50 mM cold acetic acid buffer (pH = 3.6, containing 4% zinc diacetate). The homogenates were centrifuged at 10,000 g for 15 min at 4°C. The supernatant was collected. The pellet was washed with 1 ml of extraction buffer and centrifuged as before. The two supernatants were combined and 0.1 g of charcoal was added. After vortexing and filtration, the filtrate was collected. A mixture of 1 ml of filtrate and 1 ml of Greiss reagent was incubated at room temperature for 30 min. Another identical filtrate which was pretreated with 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt (cPTIO), a specific NO scavenger, for 15 min, was used as a control. Absorbance was assayed at 540 nm. NO content was calculated by comparison with a standard curve of NaNO2.
Statistical analysis
Seed germination and seedling development experiments were performed as a factorial experiment with completely randomized design with three replicates. Data significance was assessed by analysis of variance and the differences between treatment means were compared by Duncan's multiple range test.
Results
Seed germination and seedling vigour
The significant interaction of genotype × temperature is determined by the different responses of genotypes at low temperatures. In control treatments, germination percentage (GP) at 25°C was similar for the two genotypes (100% for both) but at 10°C the difference was significant (31.0% for Falcato and 50.3% for Cherry). GP was not affected significantly at 20°C in Cherry (91.6%) as compared with 25°C, but it was significantly reduced at 15 and 10°C, to 78 and 50.3%, respectively (P< 0.05). In contrast, in Falcato, GP was reduced significantly at 20, 15 and 10°C to 75, 56 and 31%, respectively (P< 0.05). A similar trend was observed for GI and GR in both genotypes in response to temperature reduction (Fig. 1). However, both priming and SNP pretreatment were able to partly alleviate low-temperature reduced seed germination, which was consistent with the responses of other germination parameters, such as GI (Fig. 1).

Figure 1 The effects of osmopriming (PEG) and sodium nitroprusside (SNP) and methylene blue (MB) treatments on germination percentage, germination index and germination rate of two tomato genotypes at various temperatures.
A similar trend was observed for root and shoot length of the seedlings and, consequently, the vigour index (VI) in both genotypes in response to temperature reduction. Osmopriming and SNP were effective in promoting greater root and shoot length of seedlings and VI than the control at 10°C, especially for Falcato (Fig. 2). Although GP, GI, GR, root and shoot length and VI were higher in Cherry than Falcato in all treatments and at all temperatues, osmopriming and SNP improved these parameters to a larger extent in Falcato than Cherry (Figs 1 and 2). For example, at 10°C, SNP increased shoot and root length of Falcato seedlings by 0.65 and 1.1 cm, respectively, but in Cherry seedlings by 0.4 and 0.6 cm respectively (Fig. 2).

Figure 2 The effects of osmopriming (PEG) and sodium nitroprusside (SNP) and methylene blue (MB) treatments on shoot and root length of seedling and vigour index of two tomato genotypes at various temperatures.
Alleviation of tomato seed germination and seedling growth inhibition as conferred by osmopriming or SNP pretreatment, could be reversed by the combination of osmopriming, or SNP, plus MB, a blocker of NO action (Figs 1 and 2).
Sugar content and amylase activity
Both α- and β-amylase activities and sugar contents declined significantly in germinating tomato seeds under low-temperature stress as compared to the control (P< 0.05, Fig. 3). In contrast, both osmopriming and SNP pretreatments brought about an obvious increase in α- and β-amylase activities and these responses were accompanied by an accumulation of soluble sugars, especially at 10°C (Fig. 3). Application of MB to the osmopriming and SNP solutions reversed these responses.
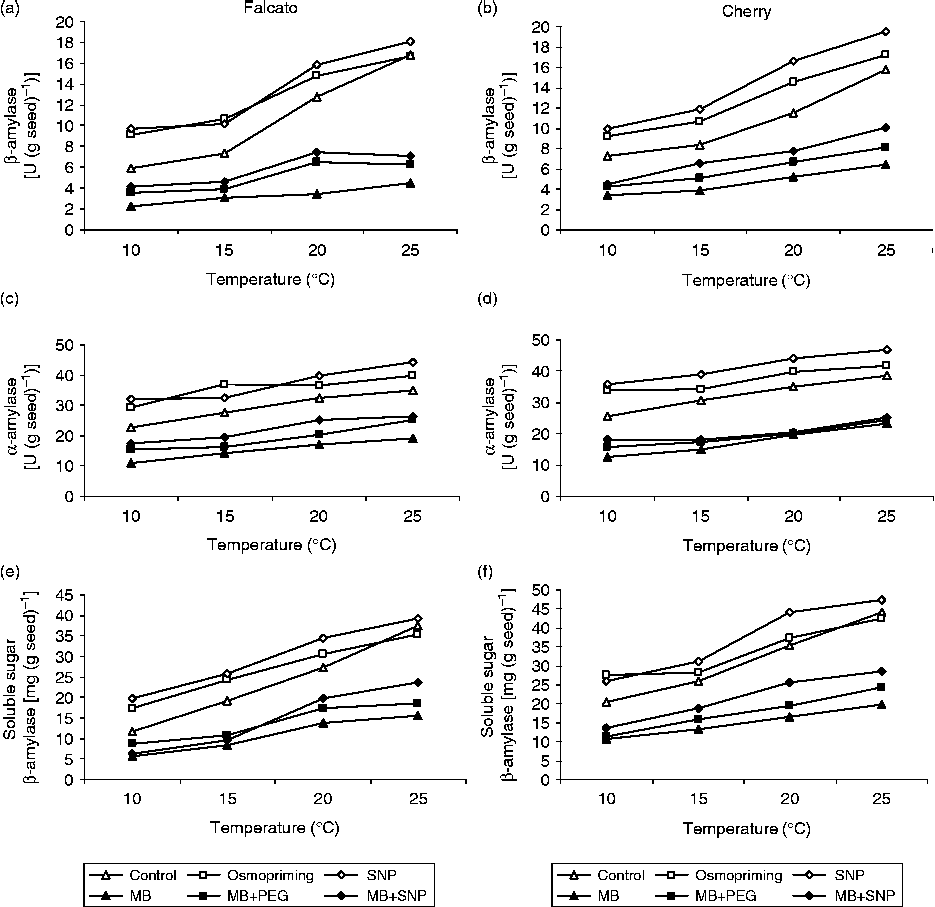
Figure 3 The effects of osmopriming (PEG) and sodium nitroprusside (SNP) and methylene blue (MB) treatments on α- and β-amylase activities and soluble sugar content in seeds of two tomato genotypes at various temperatures.
NO production driven by priming and SNP pretreatments
The results above demonstrated that priming-induced responses were sensitive to the NO scavenger. To further confirm whether above priming-driven responses were related to NO signalling we determined NO contents of the seeds. Low-temperature treatment (10°C) enhanced NO levels moderately (Fig. 4), whereas osmopriming and SNP pretreatment produced a very strong increase in NO content that was significantly higher than that of the sample under low-temperature stress alone. When MB was added to the osmopriming or SNP solutions, the rate of NO generation was dramatically inhibited, reaching the basal level of low-temperature stress alone. Interestingly, we also noticed that MB pretreatment alone significantly resulted in the decrease of NO production, in comparison with low-temperature stress alone and even compared with the control sample at 25°C (P< 0.05), suggesting that endogenous NO might be involved in tomato seed germination under both normal and low-temperature stressed conditions. Osmopriming and SNP increased NO content more in Falcato than Cherry (Fig. 4), although higher NO content was observed in Cherry in all treatments.

Figure 4 The effects of osmopriming (Prim) and sodium nitroprusside (SNP) and methylene blue (MB) treatments on NO content in two tomato genotypes at various temperatures. Con, control.
Discussion
Most commercial genotypes of tomato (Solanum lycopersicum Mill.), are sensitive to low germination temperatures. Germination temperatures range from a minimum of 8–10°C to a maximum of 35–38°C with an optimum between 20 and 25°C (Geisenberg and Stewart, Reference Geisenberg, Stewart, Atherton and Rudich1986), which was consistent with our data showing an optimum temperature of 25°C. With reducing the temperature to 10°C, the controls showed a concomitant reduction in GP, GI and VI, as well as in α- and β-amylase activities, and sugar content in both genotypes. Falcato proved to be more sensitive to low temperature than Cherry. The differences between the two genotypes were likely due to their differing genetic background.
Priming and SNP induced earlier and more uniform germination and stronger seedlings at all temperatures, as indicated by higher GP, GI and VI, but their effect on these parameters was more pronounced at suboptimal temperatures. Both SNP and osmopriming treatments increased amylase activities and levels of soluble sugar in seeds. These sugars would be readily utilized to support the energy and carbon metabolism that are operational during seed imbibition. SNP and osmopriming appeared to stimulate seed germination via a similar biochemical process. Both treatments enhanced amylase activities and seeds contained more soluble sugars, being able to more readily support metabolic activities and consequently resulting in a higher rate of emergence, which correlated with α-amylase activity and GI. Enhancement of amylase activity and sugar content by SNP (Zhang et al., Reference Zhang, Shen, Zhang and Xu2005; Xu et al., Reference Xu, Sh., Lou, Zhao, Gao, Dong, Jiang, Shen, Huang and Wang2011) and priming (Farooq et al., Reference Farooq, Basra and Hafeez2006) have been reported by other authors.
Correa-Aragunde et al. (Reference Correa-Aragunde, Graziano, Chevalier and Lamattina2006) have demonstrated that NO modulates the expression of cell-cycle regulatory genes in tomato and plays a central role in determining lateral root development, allowing further understanding of the components that control plant cell differentiation and proliferation. Therefore, the NO-induced increase in seedling growth under suboptimal temperature conditions may have been due to an increase in cell division and/or cell enlargement, although these phenomena were not examined in the present study. Similarly, differential display during maize germination has detected an earlier expression in primed germinating seeds (as compared to unprimed) of ZmAA9-24, a gene encoding a protein believed to regulate cell expansion and elongation (Cruz-Garcia et al., Reference Cruz-Garcia, Gomez, Zuniga, Plasencia and Vazquez-Ramos2003). Our data showed that no difference was observed in seed germination between control and osmopriming and SNP pretreatments at 25°C, but osmopriming and SNP increased GI, GR and VI. Probably, seeds can produce just adequate amounts of NO required for germination at 25°C and these treatments probably shortened the time to achieve the required NO levels for seed germination and subsequent seedling growth. It has been reported that exogenously applied NO can enhance germination or break seed dormancy (Beligni and Lamattina, Reference Beligni and Lamattina2000), and when no dormancy breakage is required, greater germination rates have been observed by supplementation with an NO donor (Kopyra and Gwóźdź, Reference Kopyra and Gwóźdź2003).
Under low-temperature conditions, SNP and osmopriming resulted in the enhancement of seedling growth of both tomato genotypes, but the enhancement in growth of the low-temperature-sensitive genotype (Falcato) was higher than that of Cherry. Wu et al. (Reference Wu, Zhu, Zhang, Ding and Zhang2011) reported that SNP-induced enhancement of growth of a salt-sensitive tomato genotype (Hufan2496) was higher than that of a salt-tolerant genotype (Hufan1480). In addition, external NO could act as a regulator of antioxidant intervention strategy, in preventing oxidative stress in response to cadmium stress in barley seedlings in a genotype-dependent pattern (Chen et al., Reference Chen, Wang, Sun, Cai, Mao, Zhang, Vincze and Wu2010a). On the other hand, suboptimal temperatures increased the NO content of the controls. Enhancement of endogenous NO content has been reported for plants under cold (Zhao et al., Reference Zhao, Chen, Zhang and Zhang2009), heat (Song et al., Reference Song, Ding, Zhao, Sun and Zhang2006) and salinity (Xu et al., Reference Xu, Sh., Lou, Zhao, Gao, Dong, Jiang, Shen, Huang and Wang2011) stresses. NO acts as an important signal in responses against abiotic stress. Therefore, under stress conditions, higher levels of NO are required for the maintenance of cell homeostasis, and once NO is endogenously generated or gets inside the cell from an exogenous source, it enhances stress tolerance (Neill et al., Reference Neill, Desikan and Hancock2003; Delledonne, Reference Delledonne2005). Our results suggest that SNP- and osmopriming-induced suboptimal temperature tolerance in seeds is associated with higher NO contents. Similar roles of SNP have been suggested for lettuce seeds in the darkness (Beligni and Lamattina, Reference Beligni and Lamattina2000), and in salinity-stressed (Zheng et al., Reference Zhang, Shen, Zhang and Xu2009) and copper-stressed (Hu et al., Reference Hu, Hu, Li, Zhang and Zhang2007) wheat seeds. Furthermore, SNP- and osmopriming-mediated responses appeared to be NO dependent, as they were blocked by treatment with MB, a scavenger of NO. The MB effect on reduction of NO content and reversal of beneficial effects of SNP on salt tolerance has also been reported for maize (Zhang et al., Reference Zhang, Wang, Liu, Zhang, Wei and Zhang2006) and wheat (Xie et al., Reference Xie, Ling, Han, Liu, Zheng, Huang, Yuan, He, Hu, Fang, Shen, Yang and Shen2008) seedlings. The beneficial effects of seed priming on germination under stress conditions have been observed in tomato (Dahal et al., Reference Dahal, Bradford and Jones1990; Cavallaro et al., Reference Cavallaro, Mauromical and Di Vincenzo1994), spinach (Chen et al., Reference Chen, Arora and Arora2010b) and white clover (Tiryaki et al., Reference Tiryaki, Kizilsimsex and Kaplan2009). However, to our knowledge, the present paper is the first report on the effect of osmopriming on NO content of seeds and blocking of the priming responses by MB. Our results highlight the probability of a role of NO signalling in priming responses.
Two mechanisms may explain the protective action of NO against oxidative damage. First, NO might act on reactive oxygen species (ROS) directly, to form peroxynitrite which is less toxic, thus limiting cellular damage (Martinez et al., Reference Martinez, Mascio, Bonini, Agusto, Briviba and Sies2000) and, second, it might act as a signal in activating ROS-scavenging enzyme activities under abiotic stress (Neill et al., Reference Neill, Desikan and Hancock2003; Delledonne, Reference Delledonne2005; Zhou et al., Reference Zhou, Guo, Xing and Huang2005). NO plays an important role in resistance to salt, drought, temperature (high and low), UV-B and heavy metal stress (Lamattina et al., Reference Lamattina, Garcia-Mata, Graziano and Pagnussat2003). Song et al. (Reference Song, Ding, Zhao, Sun and Zhang2006) found that application of SNP and S-nitrose-N-acetylpenicillamine, both NO donors, dramatically alleviated heat-stress-induced ion leakage, growth suppression and decrease of cell viability in callus of reed under heat stress, and enhanced the activities of SOD, CAT, APX and POD. Similarly, several authors have reported that priming enhanced accumulation of non-enzymatic compounds and activities of detoxifying enzymes (e.g. SOD, APX, CAT) and improved membrane integrity under stress conditions (Sivritepe, Reference Sivritepe2008; Amooaghaie et al., Reference Amooaghaie, Nikzad and Shareghi2010; Chen et al., Reference Chen, Arora and Arora2010b; Amooaghaie, Reference Amooaghaie2011; Sun et al., Reference Sun, Lin, Wang, Wu and Wang2011). These findings suggest that enzymatic and metabolic activities that are the basis of priming-induced seed invigoration and low-temperature tolerance, are probably promoted via NO signalling.
Taken together, our results suggest that endogenous NO plays a key role in priming-mediated responses, although we have not explored the potential source of NO driven by priming. Therefore, further investigations should address this question.
Acknowledgements
This study was supported by research assistance of Shahrekord University, Iran. The authors are grateful to Dr Henk Hilhorst for editing the manuscript and corrections to the English language.






