Introduction
The safe disposal of high-level radioactive waste (HLW) is a challenge facing the development of nuclear energy (Long and Ewing, Reference Long and Ewing2004; Darda et al., Reference Darda, Gabbar, Damideh, Aboughaly and Hassen2021). Deep geological disposal is the internationally recognized and feasible method for HLW at present. Deep geological repositories for HLW are typically located several hundred to several thousand meters underground and are reinforced with multiple barriers to achieve long-term or permanent isolation of the waste from the environment (SKB, 2010; Wang, Reference Wang2010; Serie, Reference Serie2022). The host rock that surrounds a repository can form a natural barrier, and, together with the engineered barriers composed of buffer/backfill materials, the high-level waste storage container, and solidified state of the waste, this natural rock barrier helps to ensure the retention of radioactive waste. The buffer/backfill materials keep the waste tanks from coming into direct contact with the surrounding environment. For the construction of engineered barriers, in many countries, bentonite is selected as the preferred buffer/backfill material (Bucher and Müller-Vonmoos, Reference Bucher and Müller-Vonmoos1989; Villar and Lloret, Reference Villar and Lloret2008; Missana et al., Reference Missana, Alonso, Albarran, García-Gutiérrez and Cormenzana2011).
In addition to fractures naturally present in the host rock, fractures can be induced during repository construction. These fractures present possible channels through which groundwater can seep into the rock, causing clay colloids to form by erosion of bentonite by groundwater. Clay colloids form by erosion of bentonite by groundwater (Degueldre et al., Reference Degueldre, Pfeiffer, Alexander, Wernli and Bruetsch1996; Missana et al., Reference Missana, Alonso and Turrero2003; Baik et al., Reference Baik, Cho and Hahn2007; Albarran et al., Reference Albarran, Degueldre, Missana, Alonso, García-Gutiérrez and López2014). These colloids reduce the density of the bentonite in the tunnels of a disposal repository, threatening the long-term safety of the repository. The formation rate of bentonite colloids largely depends on groundwater qualities such as the acidity (Kretzschmar et al., Reference Kretzschmar, Holthoff and Sticher1998; Tombácz and Szekeres, Reference Tombácz and Szekeres2004), salinity (Swartzen-Allen and Matijevic, Reference Swartzen-Allen and Matijevic1976; Huynh and Chen, Reference Huynh and Chen2011; Huangfu et al., Reference Huangfu, Jiang, Ma, Liu and Yang2013), and colloid type (Missana et al., Reference Missana, Alonso, Fernández and García-Gutiérrez2018a). Zhu et al. (Reference Zhu, Fu, Yan, Li, Zhang, Wang and Chai2022) show that colloid formation is strongly inhibited in high-salinity and complex ionic environments rich in Na+. Xu et al. (Reference Xu, Pan, Sun and Wu2018) confirm the reversibility of the effects of pH and electrolytes on colloid formation, suggesting the dynamic effect of water on the colloids as the water chemistry changes. These external factors also affect the adsorption capacity of the colloids for radionuclides as the colloids migrate (Bouby et al., Reference Bouby, Geckeis, Lützenkirchen, Mihai and Schäfer2011; Zuo et al., Reference Zuo, Gao, Yang, Zhang, Chen, Li and Wu2017; Yin et al., Reference Yin, Pan, Liu, Wu, Li and Wu2018). Therefore, investigating the behavior of colloids in different aqueous environments and the effect of these environmental factors on the stability of colloids will help to gain a deeper understanding of the migration behavior of colloids and to evaluate the safety of disposal repositories.
Gaomiaozi (GMZ) bentonite is the buffer/backfill material proposed for the Beishan Geological Disposal Repository in China. GMZ bentonite is composed of montmorillonite (75.4%), and the secondary minerals quartz (11.7%), cristobalite (7.3%), feldspar (4.3%), kaolinite (0.8%), and calcite (0.5%) (S. Zhu et al., Reference Zhu, Wang, Zheng, Wang, Tian, Henderson and Yan2022). The montmorillonite has a particle diameter of 100–350 nm with a surface electric potential (ζ) of –30 to −50 mV (Plaschke et al., Reference Plaschke, Schäfer, Bundschuh, Ngo Manh, Knopp, Geckeis and Kim2001; Missana et al., Reference Missana, Alonso, Fernández and García-Gutiérrez2018b; Xian et al., Reference Xian, Zhou, Pan, Du, Chang, Hu and Liu2020; Wang et al., Reference Wang, Jiang, Yang, Cheng, Bao, Pan and Tuo2022). Studies on the stability of GMZ bentonite (Xian et al., Reference Xian, Zhou, Pan, Du, Chang, Hu and Liu2020; Xu et al., Reference Xu, Pan, Sun and Wu2018) have investigated the effects of the pH, ionic strength, and ion type on the aggregation kinetics of GMZ colloids. In addition, Pinnavaia et al. (Reference Pinnavaia, Rainey, Tzou and White1984) performed small-angle neutron scattering analysis on the layered structure and pore size of pillared clay and concluded that the stack thickness of these layered colloidal particles can be calculated using appropriate models. Ferrage et al. (Reference Ferrage, Hubert, Baronnet, Grauby, Tertre, Delville and Levitz2018) analyzed the small-angle neutron scattering spectra of the dehydrated structure (0W), monohydrated (1W), and bi-hydrated (2W) of vermiculite. They discovered that changes in the hydration structure led to a shift in the characteristic peaks in the high q region (q is a function of the scattering vector magnitude for the small-angle scattering), while changes in the power law index in the mid-q region indicate an anisotropic arrangement of the particles and particle defects. While these results help to understand the colloidal behavior of GMZ bentonite colloids, few studies have been performed on the in situ layered structure of water-borne bentonite colloids. Bentonite is a typical material with a layered structure. Given that bentonites in backfill will be in contact with water, it is important to understand the changes in its in situ layered structure in water as a result of environmental factors.
To better predict the migration behavior of nuclides on colloids in the repository environment, it is necessary to understand the swelling/collapsing behavior of GMZC which can generate additional preferential pathways for nuclides transfer (Bradford et al., Reference Bradford, Simunek, Bettahar, van Genuchten and Yates2003; Ferrage, Reference Ferrage2016). The structural properties of bentonite colloids in aqueous solutions were studied as a function of pH, cation concentration and charge. The stability and structure of GMZC were analyzed with emphasis. Small-angle X-ray scattering (SAXS) was used to reveal the structural changes of GMZC in different valence environments. The environmental behavior of GMZC is explained and used as a reference for safety assessment of HLW.
Materials and methods
Colloid preparation
The GMZ bentonite used in this study was obtained from Xinghe County, Inner Mongolia, China, and was supplied by the China Institute for Radiation Protection. The bulk bentonite sample was crushed into powder using an agate mortar and was passed through a 400-mesh sieve. The bentonite colloids were prepared by gravity as follows. GMZ bentonite powder (5 g) was dissolved in 500 mL of deionized water (resistivity ≥18.4 MΩ‧cm). The mixture was sonicated in a water bath for 2 h to ensure the thorough dispersion of the bentonite powder. The mixture was sealed and stored in a glass vessel for 3 days. The supernatant was separated and allowed to stand. The lower layer of residues was discarded. This process was repeated once every 3 days until no more large precipitates appeared in the bottom colloidal phase after more than 15 days (S. Zhu et al., Reference Zhu, Wang, Zheng, Wang, Tian, Henderson and Yan2022).
The colloids were further purified to eliminate the possible interference from dissolved salt ions introduced by colloids in the subsequent experimental steps. Colloids under 250 nm in size were extracted and used in the experiment. The colloids were dialyzed against deionized water for 3 days. After dialysis, the GMZC with a conductivity of below 20 μS cm−1 were stored in a glass vessel. Other reagents used for colloid preparation were analytical grade and did not require further purification.
Aggregate morphology
All the chemicals used in experiments were A.R. grade, supplied from Chengdu KeLong Chemical Co. Ltd., China without any further purification. Stock solutions of NaCl, KCl, MgCl2, and CaCl2 (0.5 mol L−1) prepared using 18.4 MΩ‧cm quality water were used to prepare the diluted solutions. Different proportions of stock solution were added to GMZ colloidal dispersions with various cationic concentrations, from 10−5 to 10−3 mol L−1. The prepared colloidal solutions were mixed in a shaker for 2 h and were left to stand for 3 days before the measurement was conducted. The pH of the colloids was adjusted (to pH 2 and pH 12) by adding different volumes of 0.1 and 0.5 mol L−1 NaOH and HNO3, and the pH and conductivity of the GMZ colloids were measured using a Thermo Fisher XL200 meter.
Colloid characterization
The morphology of the GMZ bentonite powder was revealed using a field-emission transmission electron microscope (Libra 200FE, Zeiss, Germany; magnification range: 80–106; operating voltage: 200 kV). The mineralogical characteristics of the colloid samples (dried at 60°C, oriented powders deposited on glass slides) were obtained by X-ray diffraction (XRD) (Ultima IV, Rigaku, Japan; Cu-Kα, λ = 0.154 nm). The colloidal particle size and zeta potential were measured using a multi-angle particle size and zeta potential analyzer (Omni, Brookhaven, USA; light source wavelength: λ = 640 nm). The hydrodynamic radius, R H, of the bentonite colloids was calculated using the Stokes-Einstein equation:
where kB is the Boltzmann constant (1.38064852×10−23 J K−1), T is the temperature, η is the viscosity of the solution, and D t is the diffusion coefficient.
An Anton-Paar SAXSpace (Austria, 40 kV, 50 mA, Cu-Kα, λ = 0.1542 nm) instrument was used to quantify the nanoscale colloidal structure. The scattered rays were collected on a Myshen2 detector (Detris, Switzerland). The colloidal samples were placed in a quartz capillary (1 mm inner diameter) and were measured for over 30 min to obtain the SAXS data. The raw data were normalized using SAXSquant (version 4.1.0.7505). The background scattering from the capillary and deionized water was removed.
The scattering intensity I(q) was measured as a function of the scattering vector magnitude (Guinier et al., Reference Guinier, Fournet, Walker and Yudowitch1979):
I and the interlayer space (d) were obtained from:
For randomly orientated thin plates or discs, for example clay colloids, the Guinier approximation at low Q values for the scattering intensity distribution is:
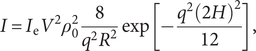 $$ {\displaystyle \begin{array}{c}I={I}_{\mathrm{e}}{V}^2{\rho}_0^2\frac{8}{q^2{R}^2}\exp \left[-\frac{q^2{(2H)}^2}{12}\right],\end{array}} $$
$$ {\displaystyle \begin{array}{c}I={I}_{\mathrm{e}}{V}^2{\rho}_0^2\frac{8}{q^2{R}^2}\exp \left[-\frac{q^2{(2H)}^2}{12}\right],\end{array}} $$
where I e is the intensity scattered by one electron, V is particle volume, ρ0 is mean particle density, and H is the thickness of the disc.
Results and Discussion
Characterization of GMZ bentonite colloids
The properties of the GMZC used in this study are presented in Table 1. The mass fraction showed the concentration of GMZ bentonite in water. It was determined in triplicate by evaporating the water from 80 mL aliquots of GMZC dispersions in an oven at 80°C for at least 4 days.
Table 1. Summary of data for the GMZ colloid parameters
The morphology and size of GMZ bentonite particles were determined by transmission electron microscopy (TEM); see Fig. 1a. A zoom-in of one edge (Fig. 1b) has some indentations and evidence of stacked layers at the upturned edge of the crystal. Dehydration of the sample during preparation for microscopy precluded an accurate determination of the layer number and interlayer space. Consequently, SAXS was used to characterize the layered structures of the GMZ and GMZC.
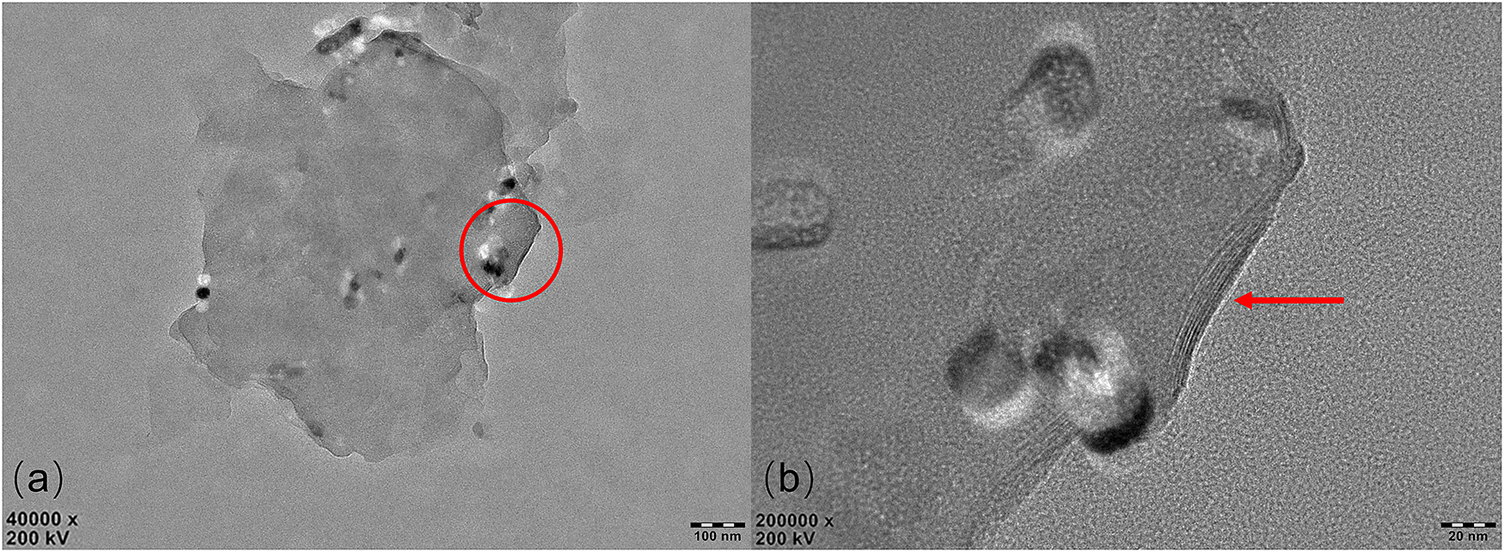
Figure 1. TEM images of GMZ bentonite: the particle (a) and details (b); the arrow shows the layered structure.
Change in hydration of GMZ bentonite
The effect of temperature on SAXS from GMZ bentonite at 25 and 300°C are shown in Fig. 2. Two characteristic peaks are present in the pattern of the GMZ at room temperature (25°C). The broad peak at ~4 nm−1 indicates overlapped peaks due to the scattering interference among the particles. This feature suggests the presence of non-uniform particle size or spacing. Further fitting of the broad peak yielded two smaller peaks.
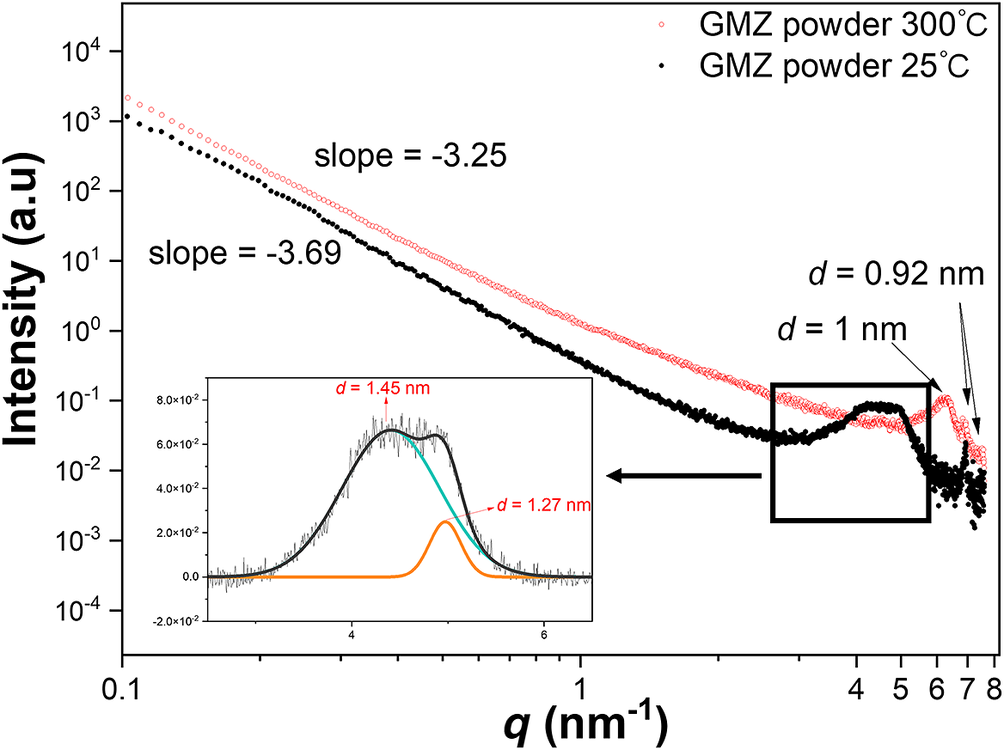
Figure 2. SAXS curves obtained from GMZ powders in 300°C (red open circles) and 25°C (black filled circles). The peak indicated that GMZ particles have a lamellar structure.
The d-spacings corresponding to peaks at 0.92, 1.27, and 1.45 nm (Fig. 2) are attributed to different interlayer hydration structures. The Na+ ions between the bentonite layers serve to balance the electric potential and exhibit strong water absorption. They combine with water molecules to form different hydration structures, giving rise to variations in the interlayer spacing. To confirm the origin of these two peaks in the GMZ spectrum at room temperature (25°C), GMZ powder was heated at 300°C (Hong et al., Reference Hong, Meng, Li, Yan, He and Fu2020). Compared with 25°C, the interlayer spacing of the GMZ is smaller at 300°C (Fig. 2). The high temperature evaporated the interlayer water (Ferrage et al., Reference Ferrage, Kirk, Cressey and Cuadros2007), and the interlayer of the GMZC was dehydrated and changed from a multi-hydration structure to a waterless interlayer structure. Based on this result, it is inferred that at 25°C, the three peaks in the GMZ powder spectrum correspond to three different interlayer hydration structures (Morida et al., Reference Morida, Fukushi, Sakuma and Tamura2023), i.e. the dehydrated (0W, d-space = 0.92 nm), monohydrated (1W, d-space = 1.27 nm), and bi-hydrated (2W, d-space = 1.46 nm). The GMZ powder spectrum contains two characteristic peaks at 300°C, corresponding to interlayer spacing values of 1.00 and 0.92 nm. The peak with d-spacing of 1.00 nm might correspond to the 0W structure formed via dehydration of the 2W structure, while the d-spacing of 0.92 nm corresponds to the spacing of the original GMZ powder without interlayer water or Na+ (Meunier, Reference Meunier2006; Brochard, Reference Brochard2021). The 001 peak of sodium bentonite typically indicates an interlayer spacing of 1.25 nm (Zhang et al., Reference Zhang, Miao, Bai, Yuan, Jia, Han and Su2014; Verma et al., Reference Verma, Semenkova, Krupskaya, Zakusin, Mohapatra, Romanchuk and Kalmykov2019), corresponding to the 1W hydration structure. From the peak shape information obtained via SAXS (Fig. 2), it was clear that the interlayer hydration state cannot be described by a single structure. The small spacing difference in the interlayer hydration structures (±0.3 nm) results in overlap of the characteristic peaks, making it difficult to accurately determine the various interlayer hydration structures of the GMZ powder via diffraction.
Small-angle scattering from upper and lower fractions of the dispersion (Fig. 3) indicated that the colloidal dispersion was not uniform. The scattering from the supernatant showed a broad indistinct peak, while the suspension of larger particles from the lower fraction of the dispersion exhibited prominent and characteristic peaks. The heavier solids collected from the colloid preparation procedure were dried at 40°C for 48 h and examined by SAXS (Fig. 4). The 1W hydration structure (d-space = 1.33 nm) was observed as the only characteristic peak of the precipitated GMZ. During the water absorption and swelling, the bentonite particles with a 1W hydration structure precipitated, while those with a tri-hydrated structure formed stable suspensions in the water. This difference may be caused by the interlayer defects in the bentonite as particles with severe defects do not swell fully and precipitate rapidly, even though swelling still occurs after water absorption. For the bentonite particles with a 0W structure, which have a low or no cation content (Brochard, Reference Brochard2021), their water absorption ability was weaker than that of the particles with 1W and 2W structures. Due to insufficient water absorption, these particles could not form stable suspensions in the water and precipitated.
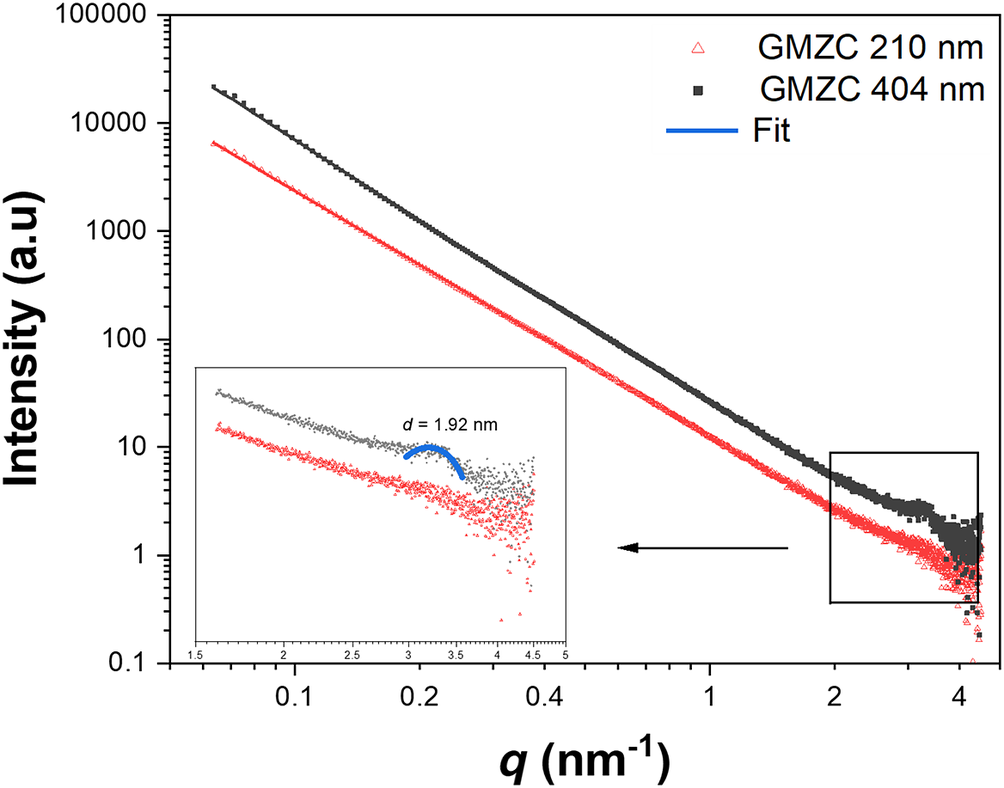
Figure 3. SAXS curves obtained from upper (210 nm) and lower (404 nm) fractions of the colloidal dispersion.
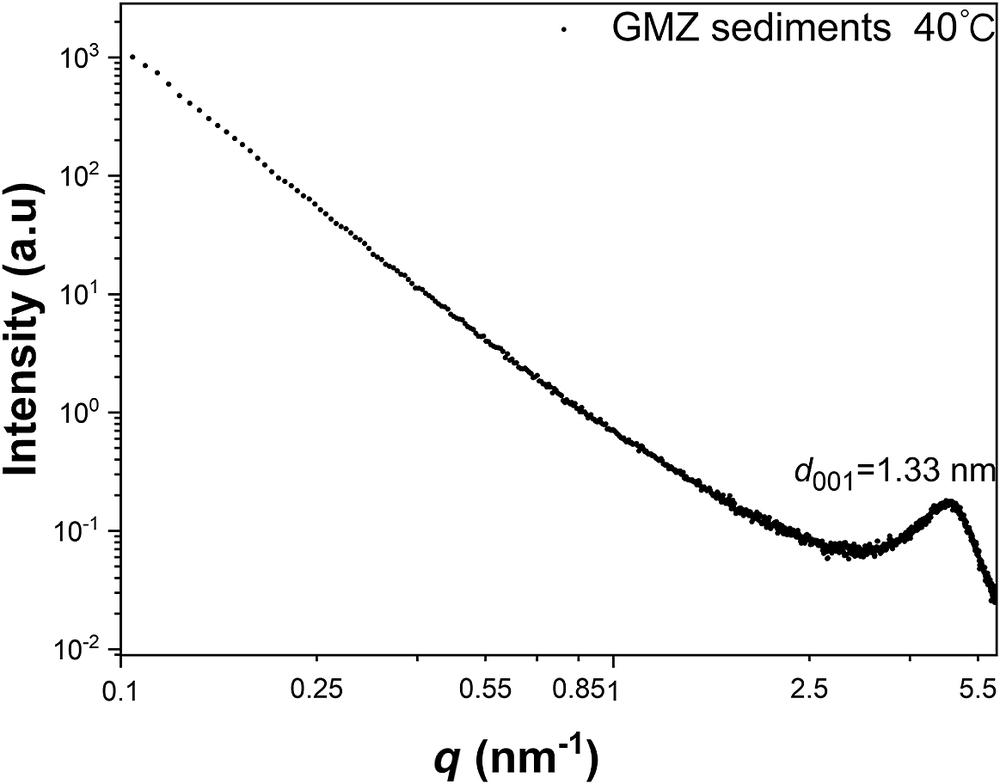
Figure 4. SAXS curves obtained from precipitates during the colloidal extraction process, dry at 40°C.
The stability of colloids at different pH values
To understand the effect of pH on the stability of the GMZC, the changes in the hydrodynamic diameter and zeta potential (ζ) of the colloids with pH were studied. In the acidic conditions (pH <5), the aggregation of the colloids was especially sensitive to salt concentration (Lagaly and Ziesmer, Reference Lagaly and Ziesmer2003). For this reason, no background electrolyte was added for these experiments. According to Fig. 5, the colloid size increased with decreasing pH, while ζ increased, indicating that the stability of the colloids decreased as the pH decreased. The faces and edges of the bentonite particles carry different charges: the face planar surfaces (silanol and aluminol sites) are at lower isoelectric points, while the edge surfaces (OH sites) are at higher isoelectric points (Svoboda, Reference Svoboda2013). Active sites on both planar and edge surfaces are easily affected by ions:
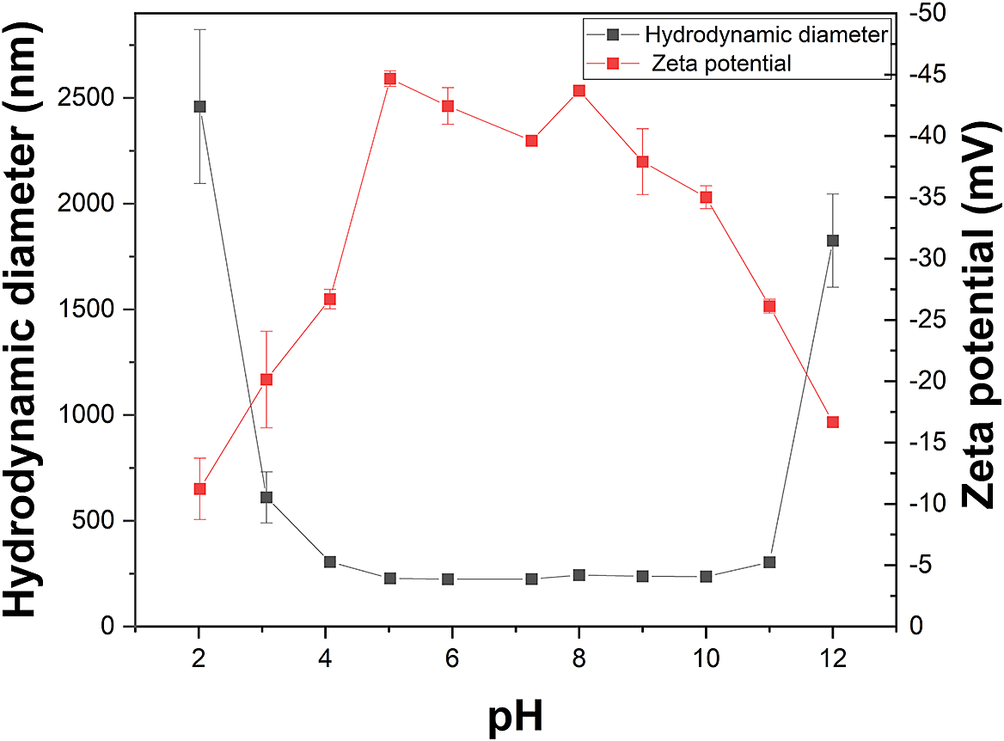
Figure 5. The pH dependence of GMZC size and zeta potential without background electrolyte.
As the H+ concentration was increased, the electrostatic effects became strong enough to trigger the attraction between the positively charged edges of the bentonite particles and the negatively charged faces, promoting the aggregation of colloidal particles and the transformation to a gel (Tombácz and Szekeres, Reference Tombácz and Szekeres2004). At pH <3, the colloidal solution exhibited a high ionic strength without adding background electrolyte. This reduced the attraction between face–face compared with edge–face (Lagaly and Ziesmer, Reference Lagaly and Ziesmer2003); however, the hydrodynamic diameter of the colloids significantly increased. At this time, the aggregation of the colloidal particles occurred through edge–face stacking.
In alkaline conditions, repulsion between layers was expected, as the surfaces of all the colloidal particles were negatively charged. While 7 ≤ pH ≤ 11 (Fig. 5), the hydrodynamic diameter of the colloidal particles did not change significantly. However, the hydrodynamic diameter of the colloidal particles increased at pH = 12, possibly due to the face-to-face aggregation of particles (Xian et al., Reference Xian, Zhou, Pan, Du, Chang, Hu and Liu2020).
Comparative analysis of the bentonite interlayer change with different cations
As the cation concentration increased, both hydrodynamic diameter and ζ of the particles gradually increased (Figs 6 and 7). For 10−4 mol L−1 Ca2+ and Mg2+ (the concentration refers to the concentration of groundwater in Beishan; Guo et al., Reference Guo, Wang, Yu, Liu and Zhong2005), considerable aggregation of the colloidal particles occurred, along with significant stratification in the colloids.
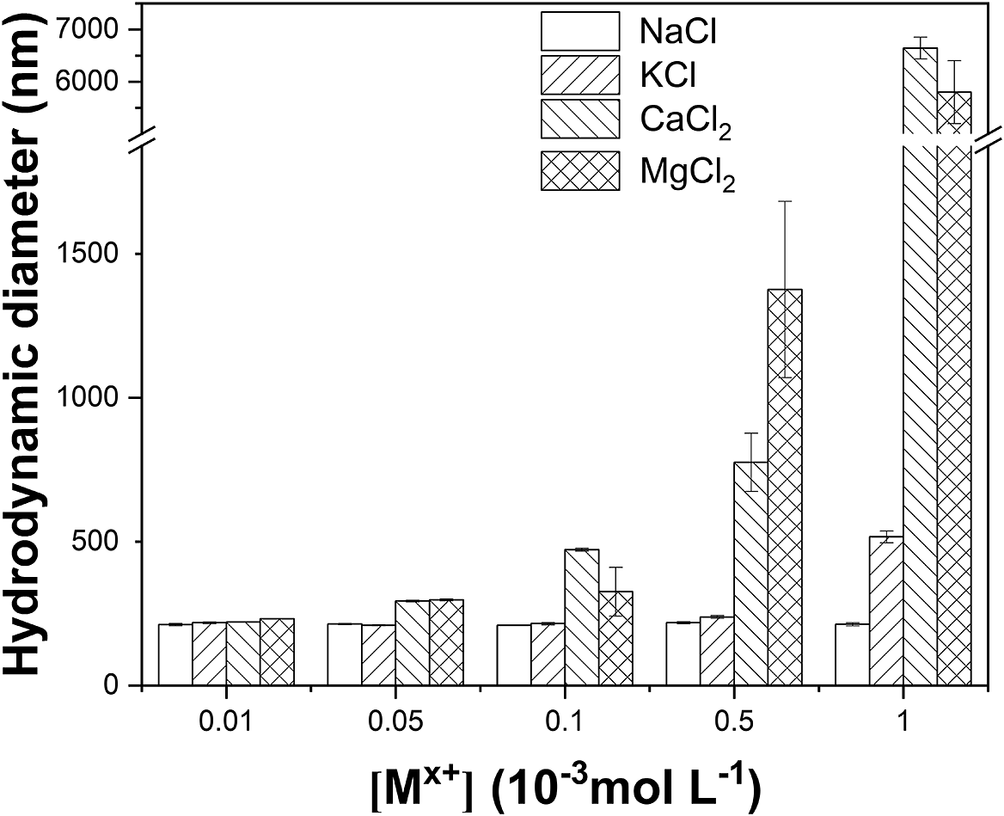
Figure 6. Effect of cations on the mean hydrodynamic diameter of GMZC at pH = 8.5±0.05.
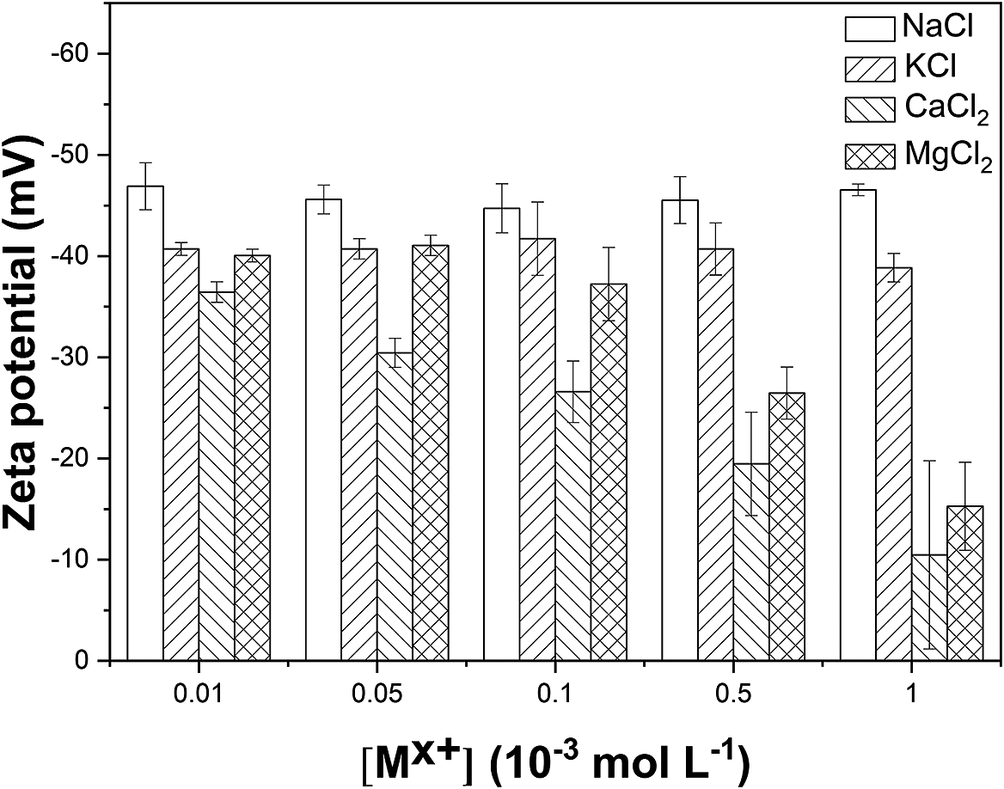
Figure 7. Effect of cations on the zeta potential of GMZC at pH = 8.5±0.05.
With the introduction of higher-valence ions, the critical coagulation concentration of the colloidal precipitation decreased. With respect to Deryagin-Landau-Verwey-Overbeek (DLVO) theory, bivalent cations induced a greater reduction in the thickness of the colloidal double electric layer facilitating aggregation of the colloidal particles. Moreover, the hydration radius and ionic radius of the cations significantly affected the aggregation of the colloidal particles. When the difference between the ionic radius and the hydration radius was small, the hydration shell was more easily detached as the ions bound to the colloidal particle surface, resulting in the formation of inner-sphere complexes between the ions and the particle surface (Xia et al., Reference Xia, Qi, Liu, Qi, Chen and Wiesner2017) and the ready aggregation of particles.
The powder XRD data (Fig. 8) showed that the main component of GMZ bentonite was montmorillonite, and the ions added during the experiment did not cause major structural change. For the sample with added Mg2+, the diffraction peak was shifted (from d 001 = 1.5 nm to d 001 = 1.54 nm) and the intensity of the signal was reduced. The result of adding Ca2+ was to broaden the peak. Broadening of a diffraction peak could be due to a number of factors including layers of different basal space and small particle size. Furthermore, the effect shown here might also be explained by the disorder of the silicate sheets, the coexistence of large-span thick layers and the formation of a gel-like structure (Wilson et al., Reference Wilson, Cuadros and Cressey2004). Due to these complications, the colloidal dispersions were examined by the small-angle scattering technique, with an emphasis on observing the effect of ion exchange on the interlayer hydration structure (Fig. 9).
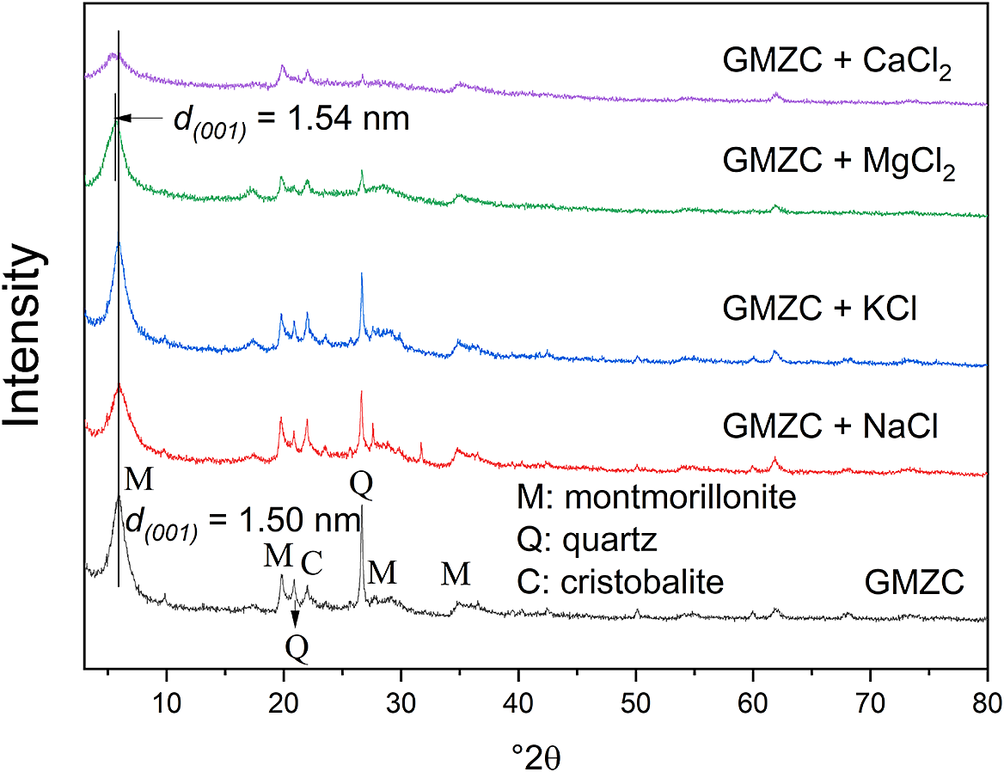
Figure 8. Comparison of XRD patterns of GMZ colloidal powders with different ions (c(Mx+) = 1×10–4 mol L–1).
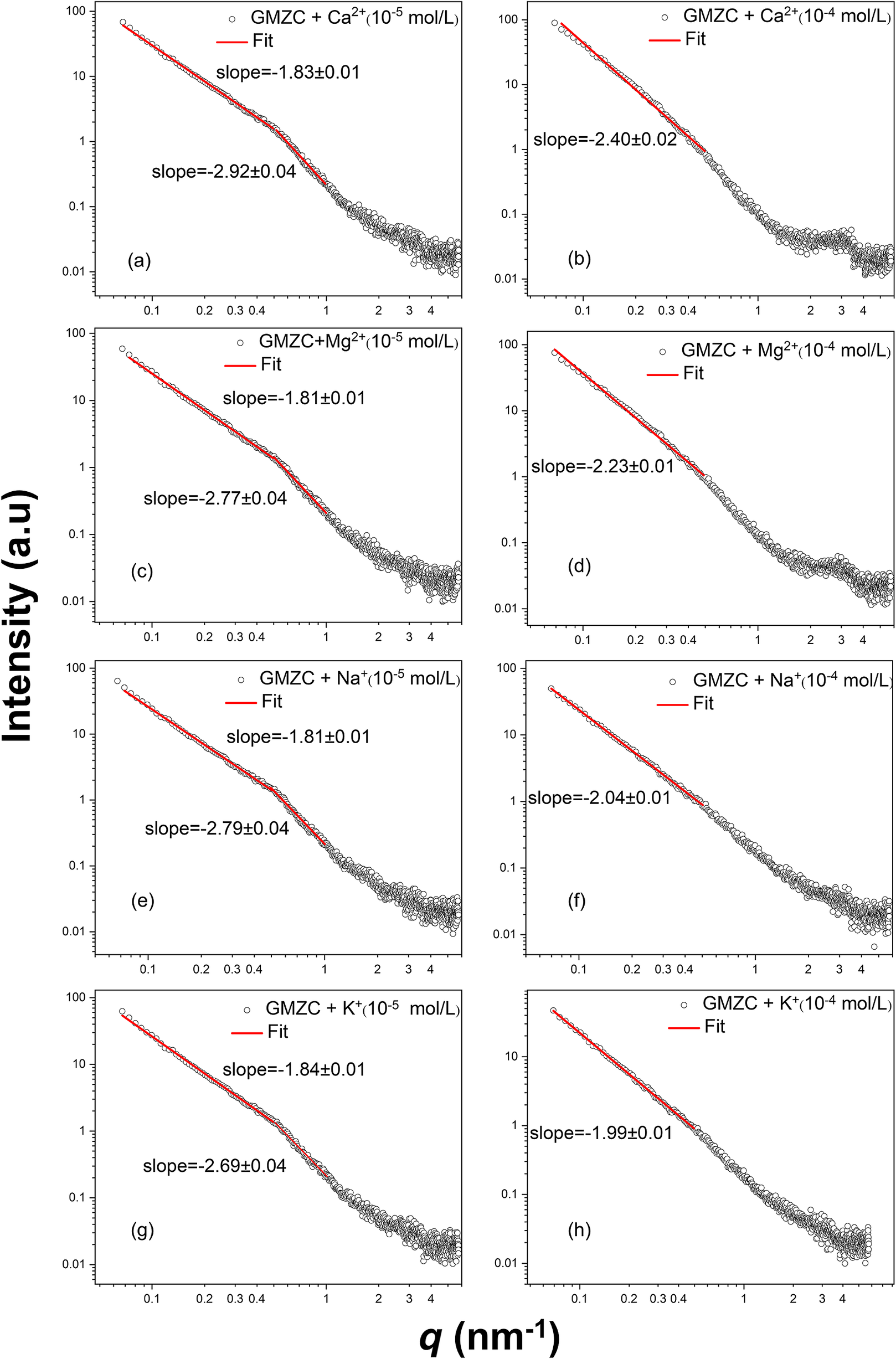
Figure 9. SAXS curves obtained from GMZC and chloride at different concentrations. The curves were fitted (lines) using a linear fitting model. The slope decreased, indicating aggregation of GMZ particles. (a) GMZC + Ca2+ 10–5 mol L–1; (b) GMZC + Ca2+ 10–4 mol L–1; (c) GMZC + Mg2+ 10–5 mol L–1; (d) GMZC + Mg 2+ 10–4 mol L–1; (e) GMZC + K+ 10–5 mol L–1; (f) GMZC + K+ 10–4 mol L–1; (g) GMZC + Na+ 10–5 mol L–1; (h) GMZC + Na+ 10–4 mol L–1.
The introduction of Ca2+ or Mg2+ to the GMZC dispersion resulted in the following changes to the SAXS curve: (i) the power law slope between 0.06 nm–1 ≤ q ≤ 0.5 nm–1 decreased for low cation concentrations; (ii) the intensity of the diffraction peak lessened, and the peak shape became indistinct. As the concentration of Ca2+/Mg2+ was raised, the power law slope decreased from –1.83/–1.81 to –2.40/–2.23, and the peak reappeared and became distinct.
These results suggest that at low concentrations, the interactions between the added divalent ions and the GMZC were dominated by ion exchange. That is, the Ca2+/Mg2+ entered the interlayer spaces of the GMZC and disrupted the initially uniform hydration structure, resulting in a non-uniform interlayer spacing and weaker peak shape. The presence of these ions led to an enhanced scattering signal at around q = 0.5 nm−1 and reduced the power law index (e.g. Ca2+ from –1.83 to –2.96). Neither ζ nor the hydrodynamic diameter changed significantly at this time. As the concentration was further increased, the cations that entered the interlayer reached saturation. The interlayer spaces became uniform again and the peak shape reappeared. The cations remaining outside the interlayer spaces bonded to the surface of the GMZC particles via electrostatic interaction, rapidly reducing their surface ζ and the electrostatic repulsion between the particles. This disrupted the stability of the system, and the particles aggregated, as demonstrated by increased power law slope.
Similarly, the addition of potassium/sodium ions decreased the power law slope and weakened the peak shape. Unlike the behavior observed for Ca2+/Mg2+, as the concentration of K+/Na+ ions increased, the power law did not change significantly and the peak shape remained indistinct. For the monovalent ions Na+ and K+, at the same electrolyte concentration, the K+-GMZC system showed a greater tendency to aggregate. Similar results were obtained by Wu-Quan et al. (Reference Wu-Quan, Jia-Hong, Lei, Xin-Min and Hang2017) for Na+/K+ in soil suspensions. However, as demonstrated by the presence of divalent cations, ion exchange was the dominant mechanism at low concentrations.
For Na+ and Ca2+ present in the alkaline condition (pH = 8.5), the electrostatic interaction among colloidal particles increased due to the presence of a surface charge. The relative dominance of Na+ also led to an increase in the electrochemical bilayer thickness at the colloidal particle surface, thereby reducing the tendency of the particles to aggregate compared with dispersions that contained solely Ca2+ (Jiang et al., Reference Jiang, Séquaris, Vereecken and Klumpp2012). The peaks reappeared after an excess of divalent cations was added (Fig. 9). For the mixed Na+-Ca2+ case, the divalent cation may not have been dominant in terms of the concentration, but it still exerted a significant influence on the colloidal layered structure because the divalent cations were more strongly attracted to the interlayer spaces. When the cations became saturated in these spaces, they induced the formation of a regular layered structure more readily due to their attraction to the layers. Compared with divalent cations, monovalent cations displayed limited attraction to the colloidal layers in the concentration range of this experiment.
Conclusions
The effect of dispersion pH 2–12 and the addition of Na+, K+, Mg2+, and Ca2+ (10–5 to 10–3 mol L–1) on the lamellar structure and agglomerate morphology of GMZ bentonite colloids (~200 nm) were investigated by TEM, dynamic light scattering, zeta potential measurements, and small-angle X-ray scattering. At acidic pH, these colloids showed aggregation by edge-to-face attraction, while in the alkaline condition aggregation was achieved by face-to-face aggregation. The cation type and concentration played significant roles in the layered structure and morphology of aggregated GMZ colloids. Divalent cations at the highest concentration studied (10–3 mol L–1), imparted order to the hydrated layered structure, even for dispersions that contained mixed M +-M 2+ ions, emphasizing the influence of Ca2+ or Mg2+ on stabilizing bentonite barriers in HLW sites. The stability of GMZ bentonite colloids is one of the important pre-requisites for evaluating the influence of colloids on nuclide migration, and findings from this work would improve the understanding of stability of colloids at nanoscale levels. Therefore, the interlayer hydration structure and aggregation morphology of colloids obtained in this work can provide a valuable reference for safety assessment of HLW repository.
Author contributions
Zhaomin Tan: Conceptualization, Investigation, Formal analysis, Methodology, Writing - original draft. Chengyun Fu: Writing - review & editing, Validation. Mark Julian Henderson: Supervision, Writing - review & editing. XuezhiDai: Writing - review & editing. Jianfeng Cheng: Formal analysis, Validation. Jingli Xie: Investigation, Formal analysis. Shan Zhu: Supervision, Formal analysis, Writing - review & editing. Minhao Yan: Supervision, Resources, Conceptualization, Project administration.
Acknowledgements
None.
Financial support
This study was funded by the Natural Science Foundation of Sichuan Province, China (nos. 2022NSFSC1228, 2022JDTD0017); CAEA Innovation Center for Geological Disposal of High-Level Radioactive Waste (no. CXJJ21102209); and the Key Laboratory Foundation for Neutron Physics of CAEP (grant no. 2019BB06).
Competing interest
The authors declare that they have no conflict of interest.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Code availability: not applicable.













