Fe, an essential trace element, is required for a wide spectrum of biological functions. Women in the reproductive age group in low-income countries are particularly at risk for Fe deficiency and anaemia. Up to 85 % of women in the reproductive age group in rural India have Hb levels < 120 g/l(Reference Toteja, Singh and Dhillon1), potentially resulting in adverse pregnancy outcomes, reduced productivity at work and impaired physical capabilities(Reference Sen and Kanani2, Reference Agarwal, Agarwal and Sharma3). Biofortification of commonly eaten foods with Fe is a practical approach to intervention in this target group(Reference Stein, Meenakshi and Qaim4, Reference Thankachan, Muthayya and Walczyk5). In a cohort of non-pregnant, non-lactating women aged 18–35 years in south India, mean dietary Fe intake was 9·5 mg/d predominantly from consumption of cereals, pulses and vegetables, but only 2·8 % of non-haem Fe was bioavailable(Reference Thankachan, Muthayya and Walczyk5) because of the presence of phytates that inhibit duodenal Fe absorption. Fe is absorbed primarily from the duodenum. Recent studies suggest that Fe is also absorbed from the proximal colon, with such absorption being enhanced by the presence of easily fermentable carbohydrates such as inulin(Reference Scholz-Ahrens and Schrezenmeir6, Reference Scholz-Ahrens and Schrezenmeir7). Inulin and other prebiotic carbohydrates stimulate the growth of bacteria that produce SCFA such as propionic acid, which in turn enhances mineral uptake(Reference Macfarlane, Steed and Macfarlane8). Rats chronically fed carbohydrates, which results in propionic acid fermentation, show an accumulation of Fe in their caeca(Reference Levrat, Remesy and Demigne9).
We hypothesised that the faecal flora of young women with Fe deficiency may be deficient in certain groups of bacteria that produce SCFA, and we undertook to investigate this by conducting a case–control study in south India.
Methods
The participants were young women aged between 18 and 25 years residing in a student hostel in Vellore. After focus group discussions with the students and their teachers, eligible individuals were invited to participate. Hb was estimated using an automated counter, and interested individuals with Hb levels ≤ 100 or ≥ 120 g/l were enrolled into the study. Participants were excluded if they had consumed antibiotics within the last 1 month, or if they had a history of menstrual disturbance. All participants maintained a detailed diet diary for 1 week after initial training in its use. The diaries were reviewed daily by an investigator (J. H.) in order to ensure that the dietary intake was adequately recorded. Food quantities were measured according to a standard set of cups and spoons, and the daily intake of the relevant nutrients was calculated from standard tables for the composition of Indian foods(Reference Gopalan, Rama Sastri and Balasubramanian10). The socioeconomic status of the participant's family was assessed using a questionnaire standardised for the Indian rural population(Reference Kumar, Shekhar and Kumar11).
Samples of venous blood were taken for the estimation of Hb and Fe. Each participant provided a sample of freshly passed stool in the morning, and this was transported to the laboratory on ice and stored in aliquots at − 80°C until DNA extraction. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the institutional review board of the Christian Medical College. Informed written consent was obtained from the participants.
Faecal DNA was extracted using QiaAmp stool DNA minikits (Qiagen, Hilden, Germany). Faecal bacteria were analysed by quantitative PCR using primers targeted at 16S ribosomal DNA of Eubacterium rectale, Clostridium leptum group, Bifidobacterium genus, Bacteroides–Prevotella–Porphyromonas group and Lactobacillus acidophilus group. The specificities of these primers and the PCR conditions have been reported earlier(Reference Balamurugan, Janardhan and George12). The L. acidophilus group primers detected Lactobacillus acidophilus, Lactobacillus amylovorus, Lactobacillus amylolyticus, Lactobacillus crispatus, Lactobacillus gasseri and Lactobacillus johnsonii. The bacterial groups chosen for study represent numerically important constituent classes of faecal bacteria found in this age group. Lactobacilli, though much less numerous, are widely considered to be beneficial to human health in many ways. All of them significantly impact bacterial fermentation of carbohydrates in the colon. Real-time PCR was done in a Chromo4 system (Bio-Rad Laboratories, Hercules, CA, USA) using SYBR Green master mix (Eurogentec, Liege, Belgium) as we have described earlier(Reference Balamurugan, Janardhan and George12). Conserved 16S ribosomal DNA sequences found in all bacteria (‘universal’ sequences conserved for domain bacteria) were amplified simultaneously using appropriate primers, and they served as the denominator, against which quantitative amplification of the other 16S ribosomal DNA amplicons was reported. DNA copy was expressed not as an absolute copy number but as a ‘relative difference’, i.e. the cycle threshold at which DNA for each target was detected relative to the cycle threshold at which ‘universal bacterial’ DNA was detected upon amplification(Reference Balamurugan, Janardhan and George12). The relative difference was automatically calculated using the Opticon 3.1 software available with the Chromo4 when the universal amplicon was set as the reference. The reference amplicon was assigned a value of 1, and target bacterial groups were expressed as decimal values in proportion to this.
Statistics
Values were expressed as median (range or interquartile range). Significance of differences between groups was assessed using unpaired t test (with logarithmic transformation in case of bacteria). A two-tailed P value < 0·05 was considered statistically significant.
Results
Of the 120 individuals screened after the focus group discussion, nine had Hb levels < 100 g/l, twenty-two had Hb levels of 100–120 g/l and the rest had Hb levels>120 g/l. Thirty-four participants were included in the study, eight participants with Hb < 100 g/l and twenty-six participants with Hb>120 g/l. As shown in Table 1, the participants were matched for socioeconomic status and for height, weight and BMI. Hb and serum Fe levels were lower and serum Fe-binding capacity was higher in the anaemic participants than in their normal peers (Table 1). The dietary intakes of energy, carbohydrate, fibre and Fe were not significantly different (Table 2) between the two groups. Intakes of milk and milk products were low in both the groups, but were not significantly different.
Table 1 Demographic characteristics of study participants
(Median values with their ranges)
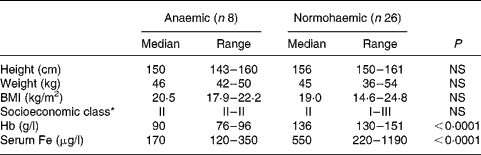
* Socioeconomic class (I, upper class; II, upper middle class; III, middle class) was assigned from a composite score based on educational achievement, occupation and family income.
Table 2 Dietary intakes of study participants
(Median values and ranges)
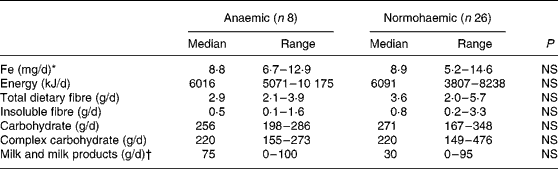
* Median intake of haem Fe was nil in both the groups. Only one participant in the anaemic group and three participants in the reference group ate any meat or fish.
† Only one individual in the anaemic group and two individuals in the reference group consumed fermented milk products.
Faecal levels of Bifidobacterium genus, Bacteroides–Prevotella group, E. rectale and C. leptum were similar between the two groups (Fig. 1). However, faecal levels of L. acidophilus group bacteria were significantly lower in anaemic women (median 6·60 × 10− 8, interquartile range 1·2 × 10− 8–2·1 × 10− 6) than in the normal group (2·95 × 10− 6, 3·7 × 10− 7–2·1 × 10− 5; P = 0·006) (Fig. 2).

Fig. 1 Quantitative PCR of (a) Bifidobacterium genus, (b) Bacteroides–Prevotella–Porphyromonas, (c) Eubacterium rectale and (d) Clostridium leptum from faeces of anaemic and normohaemic young women, normalised to amplification of universal 16S ribosomal DNA. Bars depict median and interquartile range. None of the differences was statistically significant.

Fig. 2 Quantitative PCR of Lactobacillus acidophilus group from faeces of anaemic and normohaemic young women, normalised to amplification of universal 16S ribosomal DNA. Bars depict median and interquartile range. P = 0·001 by unpaired t test after logarithmic transformation.
Discussion
The present study identified a significant reduction in the faecal population of lactobacilli in young women with Fe deficiency and anaemia in south India. Other major classes of bacteria in the stool were not significantly altered. Dietary ingestion of energy, total and insoluble dietary fibre, and milk products was similar in individuals with and without anaemia.
Mean dietary Fe intake in either group (8·8–8·9 mg/d) was in the range expected for this population, and was almost exclusively non-haem Fe(Reference Thankachan, Muthayya and Walczyk5). Certain lactobacilli have a growth requirement for Fe under specific environmental conditions(Reference Elli, Zink and Rytz13). While it is possible that reduced faecal Lactobacillus numbers were secondary to low luminal Fe levels in the Fe-deficient women, there were no differences in Fe intake between the two study groups. It is therefore reasonable to speculate on other explanations for the isochronous occurrence of reduced Lactobacillus numbers and Fe-deficiency anaemia in these young women, and to consider the role of the colon in Fe absorption.
Approximately 10 % of ingested Fe is absorbed in health, mainly or solely from the duodenum, aided by the presence of several Fe transporters in enterocytes. Evidence from experimental animals suggests that Fe may also be absorbed from the proximal colon. In pigs, the colon contributes to approximately 12 % of Fe absorption(Reference Blachier, Vaugelade and Robert14). The Fe transporters, divalent metal transporter 1 and ferroportin, are expressed in the colon of animals and man(Reference Blachier, Vaugelade and Robert14–Reference Brookes, Hughes and Turner17), and expression may be increased in Fe deficiency states(Reference Takeuchi, Bjarnason and Laftah15, Reference Johnston, Johnson and Marks16). In the proximal colon, the absorption of Fe and other divalent cations is enhanced by SCFA, which are produced by bacterial fermentation in the colon. Non-digestible disaccharides increased caecal SCFA pools and prevented Fe-deficiency anaemia in gastrectomised rats(Reference Asvarujanon, Ishizuka and Hara18, Reference Shiga, Nishimukai and Tomita19). Inulin, non-digestible in the small bowel, increased Fe retention in rats(Reference Delzenne, Aertssens and Verplaetse20, Reference Ohta, Ohtsuki and Baba21). Inulin also up-regulated mRNA expression for divalent metal transporter 1 and ferroportin, and increased faecal levels of Lactobacillus and Bifidobacterium species in pig colon(Reference Tako, Glahn and Welch22). In the present study, dietary fibre intake (soluble and insoluble) was similar in anaemic and normal women. However, the dietary intake of specific non-digestible disaccharides and oligosaccharides was not quantified in the present study. Lactobacilli contribute to colonic fermentation(Reference Meimandipour, Shuhaimi and Hair-Bejo23), a process that reduces the inhibitory effect of phytate on Fe absorption(Reference Lopez, Coudray and Bellanger24). L. acidophilus has been shown to increase Fe uptake into Caco-2 cells in vitro by reducing total soluble phenols in the digesta(Reference Laparra, Glahn and Miller25). These variables (SCFA, phytate and phenols) were not investigated in the present study, and any comments pertaining to them must remain speculative.
In conclusion, the present study documents an interesting association between Fe deficiency and reduced faecal lactobacilli in young women in India. Understanding the relationship between Fe status and faecal lactobacilli has the potential to lead to alternative strategies to combat Fe deficiency in this population.
Acknowledgements
The authors have no conflicts of interest to declare. The study was supported by a research grant from the Christian Medical College, Vellore. R. B. was supported by a Senior Research Fellowship from the Indian Council of Medical Research. R. R. M. was partially supported by a Summer Fellowship of the Indian Academy of Sciences. The department received support through grant no. LSI-141/2002 (Funds for Infrastructure in Science and Technology) from the Department of Science and Technology, Government of India. R. B., R. R. M. and B. S. R. were responsible for conception of the study; R. R. M. was responsible for recruitment and follow-up of participants; R. B. and S. C. were responsible for the molecular analyses; H. J. and R. S. D. were responsible for dietary survey; B. S. R. was responsible for funding; and R. B. and B. S. R. were responsible for writing the manuscript.






