Introduction
The aim of the present paper is to analyse the risk of developing dementia and, more specifically, to explore whether socio-economic status (SES) could moderate the effect of a well-known genetic risk factor: the apolipoprotein E (APOE) ε4 allele. Special attention is paid to the question of whether such potential modifications do in fact vary between the sexes. Our point of departure is that the risk of developing dementia seems to be unevenly distributed in the population. Individuals with low SES are at greater risk of developing different forms of this disease, and the same applies to individuals carrying the APOE ε4 allele (Blennow, de Leon and Zetterberg Reference Blennow, de Leon and Zetterberg2006; Scheltens et al. Reference Scheltens, Blennow, Breteler, de Strooper, Frisoni, Salloway and Van der Flier2016). Additionally, in large parts of the world, dementia seems to disproportionally affect women, especially among the oldest old (Winblad et al. Reference Winblad, Amouyel, Andrieu, Ballard, Brayne, Brodaty, Cedazo-Minguez, Dubois, Edvardsson, Feldman, Fratiglioni, Frisoni, Gauthier, Georges, Graff, Iqbal, Jessen, Johansson, Jonsson, Kivipelto, Knapp, Mangialasche, Melis, Nordberg, Rikkert, Qiu, Sakmar, Scheltens, Schneider, Sperling, Tjernberg, Waldemar, Wimo and Zetterberg2016). However, knowledge of whether, and how, social inequities interact with individual genetic predispositions in the development of dementia is still limited.
The multi-factorial nature of dementia
Dementia is the umbrella term for a range of disorders characterised by cognitive decline. Alzheimer's disease (AD) accounts for 50–70 per cent of all cases and thus represents the most frequent form (Blennow, de Leon and Zetterberg Reference Blennow, de Leon and Zetterberg2006; Winblad et al. Reference Winblad, Amouyel, Andrieu, Ballard, Brayne, Brodaty, Cedazo-Minguez, Dubois, Edvardsson, Feldman, Fratiglioni, Frisoni, Gauthier, Georges, Graff, Iqbal, Jessen, Johansson, Jonsson, Kivipelto, Knapp, Mangialasche, Melis, Nordberg, Rikkert, Qiu, Sakmar, Scheltens, Schneider, Sperling, Tjernberg, Waldemar, Wimo and Zetterberg2016). It is followed by vascular dementia (VaD), for which the corresponding figure is approximately 20 per cent (Rizzi, Rosset and Roriz-Cruz Reference Rizzi, Rosset and Roriz-Cruz2014). In 2016, Alzheimer's Disease International reported that the number of people suffering from such disorders worldwide is over 47 million, and with increasing longevity this number is expected to triple by 2050. This means that the societal economic burden of dementia, estimated at US $818 billion globally in 2016, will continue to grow (Prince et al. Reference Prince, Comas-Herrera, Knapp, Guerchet and Karagiannidou2016). By extension, this implies that the search for treatments, and not least protective factors, is of the utmost importance.
Genetic risk in dementia
What is common to all dementia sub-types, except for the rare, familial and autosomal dominant form of AD, is that the causes of disease are heterogeneous (Blennow and Wallin Reference Blennow and Wallin1992; Cacabelos et al. Reference Cacabelos, Martínez, Fernández-Novoa, Carril, Lombardi, Carrera, Corzo, Tellado, Leszek, McKay and Takeda2012; Livingston et al. Reference Livingston, Sommerlad, Orgeta, Costafreda, Huntley, Ames, Ballard, Banerjee, Burns and Cohen-Mansfield2017; Verghese, Castellano and Holtzman Reference Verghese, Castellano and Holtzman2011; Whalley, Dick and McNeill Reference Whalley, Dick and McNeill2006). The major genetic risk factor for AD is the APOE ε4 allele, which has been reported to increase disease risk by between three and 15 times (Blennow, de Leon and Zetterberg Reference Blennow, de Leon and Zetterberg2006; Scheltens et al. Reference Scheltens, Blennow, Breteler, de Strooper, Frisoni, Salloway and Van der Flier2016). It has also recently been suggested that APOE ε4 could be associated, albeit not as strongly, with other forms of dementia such as vascular dementia and dementia with Lewy bodies (Keogh et al. Reference Keogh, Kurzawa-Akanbi, Griffin, Douroudis, Ayers, Hussein, Hudson, Pyle, Cordell, Attems, McKeith, O'Brien, Burn, Morris, Thomas and Chinnery2016; Liu et al. Reference Liu, Li, Liu, Deng, Zhu, Li and He2012; Rohn Reference Rohn2014; Tsuang et al. Reference Tsuang, Leverenz, Lopez, Hamilton, Bennett, Schneider, Buchman, Larson, Crane, Kaye, Kramer, Woltjer, Kukull, Nelson, Jicha, Neltner, Galasko, Masliah, Trojanowski, Schellenberg, Yearout, Huston, Fritts-Penniman, Mata, Wan, Edwards, Montine and Zabetian2013). Even though there seems to be no APOE ε4 allelic association with sex, employment grade or education, which implies that the corresponding genetic risk of developing dementia is evenly distributed in the population (Borenstein Graves et al. Reference Borenstein Graves, Mortimer, Bowen, McCormick, McCurry, Schellenberg and Larson2001; Moceri et al. Reference Moceri, Kukull, Emanuel, Van Belle and Larson2000; Zhao et al. Reference Zhao, Brunner, Kumari, Singh-Manoux, Hawe, Talmud, Marmot and Humphries2005), there is still a possibility that the effect of the gene variant is unevenly distributed. In fact, not all people who carry this gene variant develop the disease (Blennow, de Leon and Zetterberg Reference Blennow, de Leon and Zetterberg2006; Rohn Reference Rohn2014; Scheltens et al. Reference Scheltens, Blennow, Breteler, de Strooper, Frisoni, Salloway and Van der Flier2016; Tsuang et al. Reference Tsuang, Leverenz, Lopez, Hamilton, Bennett, Schneider, Buchman, Larson, Crane, Kaye, Kramer, Woltjer, Kukull, Nelson, Jicha, Neltner, Galasko, Masliah, Trojanowski, Schellenberg, Yearout, Huston, Fritts-Penniman, Mata, Wan, Edwards, Montine and Zabetian2013; Verghese et al. Reference Verghese, Castellano and Holtzman2011), which raises questions regarding what factors outside the genome could potentially increase or decrease disease susceptibility. This is particularly pertinent given recent findings suggesting that elimination of some of the most influential modifiable risk factors could reduce dementia incidence by approximately 30–35 per cent (de Bruijn et al. Reference de Bruijn, Bos, Portegies, Hofman, Franco, Koudstaal and Ikram2015; Livingston et al. Reference Livingston, Sommerlad, Orgeta, Costafreda, Huntley, Ames, Ballard, Banerjee, Burns and Cohen-Mansfield2017).
Socio-economic differences in dementia
Like most diseases, dementia has a social gradient. In general terms, the notion of there being a social gradient in health is the result of the vast body of research on health inequities conducted during the past decades. Interestingly, much (if not all) of this research seems to point in the same direction, namely towards the fact that poor social and economic conditions have a negative impact on health and that the risk of premature death increases with decreasing SES (Halleröd and Gustafsson Reference Halleröd and Gustafsson2011; Marmot Reference Marmot2004; Marmot and Brunner Reference Marmot and Brunner2005; Wilkinson and Marmot Reference Wilkinson and Marmot2003). As regards dementia, and AD in particular, low educational attainment is one of the most thoroughly investigated variables (Meng and D'Arcy Reference Meng and D'Arcy2013; Ngandu et al. Reference Ngandu, von Strauss, Helkala, Winblad, Nissinen, Tuomilehto, Soininen and Kivipelto2007; Qiu, Xu and Fratiglioni Reference Qiu, Xu and Fratiglioni2010; Wang et al. Reference Wang, Gustafson, Kivipelto, Pedersen, Skoog, Windblad and Fratiglioni2012). In fact, recent figures from the Lancet Commissions suggest that elimination of the risk factor ‘no education beyond primary’ could prevent as much as 8 per cent of new dementia cases (Livingston et al. Reference Livingston, Sommerlad, Orgeta, Costafreda, Huntley, Ames, Ballard, Banerjee, Burns and Cohen-Mansfield2017). Furthermore, a meta-analysis of several cohort studies reported a pooled estimate of increase in AD risk equal to almost 60 per cent between individuals with the lowest level of educational attainment and those with the highest (Caamaño-Isorna et al. Reference Caamaño-Isorna, Corral, Montes-Martínez and Takkouche2006). In relation to the potentially protective ability of high occupational class, previous analyses are fewer and have shown inconsistent results. While some studies indicate that education and occupational class may separately reduce the risk of dementia (Qiu et al. Reference Qiu, Karp, von Strauss, Winblad, Fratiglioni and Bellander2003; Sattler et al. Reference Sattler, Toro, Schoenknecht and Schroeder2012), others imply that the effect of the latter is diminished when education is added to the model (Evans et al. Reference Evans, Hebert, Beckett, Scherr, Albert, Chown, Pilgrim and Taylor1997; Karp et al. Reference Karp, Kareholt, Qiu, Bellander, Winblad and Fratiglioni2004). While the exact cognitive and neuronal mechanisms underlying the associations between SES and dementia have not yet been fully established, it has been suggested that the mental stimulation inherent in, for example, education and other related activities, creates a ‘cognitive reserve’ by improving resilience and compensatory abilities in the neuronal networks (Stern Reference Stern2002, Reference Stern2012). For instance, previous findings suggest that high occupational task complexity could reduce the risk of dementia (Andel et al. Reference Andel, Crowe, Pedersen, Mortimer, Crimmins, Johansson and Gatz2005; Dekhtyar et al. Reference Dekhtyar, Wang, Scott, Goodman, Koupil and Herlitz2015; Karp et al. Reference Karp, Andel, Parker, Wang, Winblad and Fratiglioni2009; Kröger et al. Reference Kröger, Andel, Lindsay, Benounissa, Verreault and Laurin2008; Then et al. Reference Then, Luck, Luppa, Thinschmidt, Deckert, Nieuwenhuijsen, Seidler and Riedel-Heller2014). In this context, however, we would like to stress that other identified risk factors for dementia – such as poor social network, depression, stress, lack of physical activity, high blood pressure, obesity, alcohol abuse and smoking (Fratiglioni, Paillard-Borg and Winblad Reference Fratiglioni, Paillard-Borg and Winblad2004; Fratiglioni et al. Reference Fratiglioni, Wang, Ericsson, Maytan and Winblad2000; Johansson et al. Reference Johansson, Guo, Waern, Ostling, Gustafson, Bengtsson and Skoog2010, Reference Johansson, Guo, Hällström, Norton, Waern, Ostling, Bengtsson and Skoog2013; Kivipelto et al. Reference Kivipelto, Rovio, Ngandu, Kåreholt, Eskelinen, Winblad, Hachinski, Cedazo-Minguez, Soininen, Tuomilehto and Nissinen2008) – are also partly related to SES (Marmot Reference Marmot2004). In other words, educational attainment and/or occupational social class are likely to impact on lifestyle behaviours and hence give rise to what could be referred to as an accumulation of (dis)advantage (Dannefer Reference Dannefer2003). Previous studies focusing specifically on gene–SES interactions in relation to dementia are scarce and point in somewhat different directions. For example, it has been suggested that while higher education reduces the risk of developing dementia among both APOE ε4 carriers and non-carriers, there are no multiplicative interaction effects between APOE ε4 allelic status and education (Meng and D'Arcy Reference Meng and D'Arcy2013). In contrast, Wang et al. (Reference Wang, Gustafson, Kivipelto, Pedersen, Skoog, Windblad and Fratiglioni2012) proposed that education could reduce by half the risk of dementia among APOE ε4 carriers and, hence, that it modifies the effect of the gene variant. In relation to previous studies, and to the inconsistencies outlined above, we argue that more research targeting the possibility of interaction effects between APOE ε4 and different socio-economic indicators is needed. Additionally, the question of whether occupational class and education have separate effects on dementia, or could instead be used interchangeably, remains to be fully explored.
Differences in prevalence between men and women
In large parts of the world, AD and other dementias are more common among women than among men, especially for the oldest old (Alzheimer's Association 2016; Winblad et al. Reference Winblad, Amouyel, Andrieu, Ballard, Brayne, Brodaty, Cedazo-Minguez, Dubois, Edvardsson, Feldman, Fratiglioni, Frisoni, Gauthier, Georges, Graff, Iqbal, Jessen, Johansson, Jonsson, Kivipelto, Knapp, Mangialasche, Melis, Nordberg, Rikkert, Qiu, Sakmar, Scheltens, Schneider, Sperling, Tjernberg, Waldemar, Wimo and Zetterberg2016). The prevailing view has been that since women generally live longer than men, and age is a major risk factor, differences in dementia prevalence are primarily attributable to differences in life expectancy. Although the research on alternative explanations is still limited, it has been suggested that variation in other biological and social factors could also contribute to these differences (Alzheimer's Association 2016). For instance, the association between APOE ε4 and AD is thought to be more pronounced in women (Altmann et al. Reference Altmann, Tian, Henderson and Greicius2014; Farrer et al. Reference Farrer, Cupples, Haines, Hyman, Kukull, Mayeux, Myers, Pericak-Vance, Risch and Van Duijn1997), and some argue that potential gene–sex interactions have been largely overlooked in previous studies (Altmann et al. Reference Altmann, Tian, Henderson and Greicius2014; Mazure and Swendsen Reference Mazure and Swendsen2016). Moreover, for other risk factors such as low education and/or low occupational attainment, substantial inequities have existed between the sexes in previous generations. In Sweden, for example, the employment rate among men between 40 and 44 years of age was 96.6 per cent in 1970, while the corresponding figure for women in the same age group was 69.5 per cent (Statistics Sweden 1973). Hence, it is plausible that sex differences in the prevalence of dementia are also partly attributable to structures of social inequity (Mazure and Swendsen Reference Mazure and Swendsen2016).
The different dimensions of SES
In studies concerned with social inequity in health, different measures of SES, such as income, education and occupational class, are often used interchangeably. Given that these measures are highly correlated and ‘reflect overlapping resources in terms of social standing’ (Torssander and Erikson Reference Torssander and Erikson2010: 465), this may be considered justified to some degree. However, it has also been argued that these axes, along which social stratification plays out, may at least partly be linked to health via different mechanisms. Hence, and despite the interrelations between various dimensions of SES, different indicators are occasionally needed to grasp the complex pathways through which health inequities arise (Lahelma et al. Reference Lahelma, Martikainen, Laaksonen and Aittomäki2004; Torssander and Erikson Reference Torssander and Erikson2010). On the basis of this discussion, and given that previous research on dementia and SES has produced somewhat varying results, we argue that an elaboration of both the concept of occupational class and the relationship between education and social class is appropriate at this stage.
At any given point, there is a strong causal impact of parents’ occupational class, i.e. class background, on children's educational choices (Bihagen Reference Bihagen2007; Bourdieu Reference Bourdieu1984; Erikson and Goldthorpe Reference Erikson and Goldthorpe1992a; Erikson and Jonsson Reference Erikson and Jonsson1996; Halleröd and Gustafsson Reference Halleröd and Gustafsson2011; Nordlander Reference Nordlander2015). Hence, educational attainment typically provides information about living conditions during childhood, the capacity to manage education, as well as acquired knowledge and abilities. Once an individual leaves the educational system, educational attainment is also a strong predictor of labour market position and hence of occupational class. Almost 100 years ago, Max Weber concluded that ‘a class situation is one in which there is a shared typical probability of procuring goods, gaining a position in life and finding inner satisfactions’ (Weber [1922] Reference Weber1978: 302). Since then, it has repeatedly been shown that occupational class structures the distribution of what Weber called ‘life chances’ in practically all societies. Again, different processes through which this occurs can be distinguished. For example, occupational class is thought to affect three, partly distinct, aspects of life: economic conditions (which result in systematic differences in consumption), class-specific behavioural differences (often referred to as class culture) and exposure to different working conditions (Bihagen Reference Bihagen2000; Bihagen and Halleröd Reference Bihagen and Halleröd2000). Based on the brief discussion above, we draw the following conclusions. First, measures of education and occupational class encompass, at least to some degree, all of the social class aspects mentioned above. Consequently, in empirical analyses where SES is used as a control variable, the choice between education, occupational class or any other indicator can be dealt with in a pragmatic manner. Nevertheless, if we wish to understand the social mechanisms (Hedström Reference Hedström2005) that link SES to a specific outcome, in this case dementia, we need to pay attention to the fact that different indicators might relate differently to different SES dimensions. While it may well be that these dimensions are linked in an indefinable interaction pattern, which in praxis means that the corresponding indicators are inseparable, this remains an empirical question. For that reason, we use both education and occupational class as predictors of dementia. Subsequently, education is identified as the main indicator of upbringing conditions, capacity to manage educational requirements and acquired abilities, whereas occupational class is considered to capture the long-term consequences of economic and working conditions as well as class culture.
Methods
Participants
The study sample is derived from the H70 Birth Cohort Study and the Prospective Population Study on Women (PPSW) in Gothenburg, Sweden, which were merged to become one for the year 2000 examination (baseline in the present study). All participants were sampled from the Swedish population register and systematically selected on the basis of birth dates. Both persons living in private households and persons in residential care were included (Karlsson et al. Reference Karlsson, Klenfeldt, Sigström, Waern, Östling, Gustafson and Skoog2009). The present analyses are based on a sample of 580 individuals, 229 men and 351 women, all born in 1930 and living in Sweden on 1 September 2000 (Table 1). Of these, all men and 99 women (28.2%) were recruited to the studies in 2000. The remaining 252 women (71.8%) had previously been part of the PPSW (Karlsson et al. Reference Karlsson, Klenfeldt, Sigström, Waern, Östling, Gustafson and Skoog2009). Follow-up examinations were carried out in 2005–06 (N = 443) and in 2009–10 (N = 368). A more detailed description of the baseline sample can be found elsewhere (Karlsson et al. Reference Karlsson, Klenfeldt, Sigström, Waern, Östling, Gustafson and Skoog2009, Reference Karlsson, Sigström, Waern, Östling, Gustafson and Skoog2010). Informed consent was acquired from all participants or their relatives, and the studies were approved by the regional Ethical Review Board for medical research in Gothenburg (Skoog et al. Reference Skoog, Waern, Duberstein, Blennow, Zetterberg, Börjesson-Hanson, Östling, Guo, Kern, Gustafson, Gudmundsson, Marlow and Kern2015).
Table 1. Characteristics of the study population
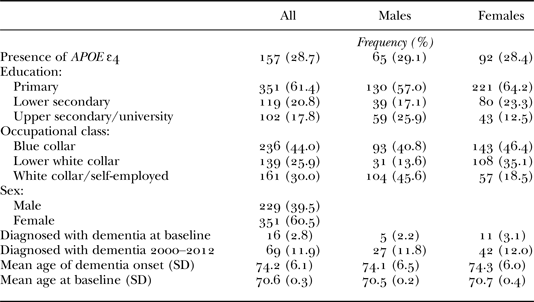
Notes: N = 580. SD: standard deviation.
Neuropsychiatric examinations, diagnoses and genotyping
The clinical examination was conducted at an outpatient department or in the participant's home. It included comprehensive social, functional, physical, neuropsychiatric and neuropsychological examinations, as well as an interview with a close informant. The semi-structured neuropsychiatric examinations were performed by trained psychiatric research nurses and comprised ratings of common symptoms and signs of dementia (e.g. assessments of memory, orientation, general knowledge, apraxia, visuospatial function, understanding proverbs, following commands, naming ability and language). A more detailed description of the procedures can be found elsewhere (Guo et al. Reference Guo, Waern, Sjögren, Lissner, Bengtsson, Björkelund, Östling, Gustafson and Skoog2007; Skoog et al. Reference Skoog, Nilsson, Palmertz, Andreasson and Svanborg1993). Semi-structured interviews with a close informant were also performed and included questions regarding changes in behaviour and intellectual function, psychiatric symptoms, activities of daily living and, in cases of dementia, age of onset and disease course (Karlsson et al. Reference Karlsson, Klenfeldt, Sigström, Waern, Östling, Gustafson and Skoog2009; Skoog et al. Reference Skoog, Waern, Duberstein, Blennow, Zetterberg, Börjesson-Hanson, Östling, Guo, Kern, Gustafson, Gudmundsson, Marlow and Kern2015). Dementia was diagnosed by geriatric psychiatrists according to the Diagnostic and Statistical Manual of Mental Disorders, 3rd edition revised (DSM-III-R; American Psychiatric Association 1987). The diagnoses were based on symptoms rated during the neuropsychiatric examinations as well as on information from the close informant interviews, as previously described in detail (Guo et al. Reference Guo, Waern, Sjögren, Lissner, Bengtsson, Björkelund, Östling, Gustafson and Skoog2007; Skoog et al. Reference Skoog, Nilsson, Palmertz, Andreasson and Svanborg1993). For individuals lost to follow-up, incident dementia cases (until 2012) were diagnosed on the basis of information from medical records, evaluated by geriatric psychiatrists, or from the Swedish Hospital Discharge Register (Guo et al. Reference Guo, Waern, Sjögren, Lissner, Bengtsson, Björkelund, Östling, Gustafson and Skoog2007). Information on age of onset was gathered either from the Swedish Hospital Discharge Register, the neuropsychiatric examinations or the close informant interviews. Blood samples were collected and the SNPs (single nucleotide polymorphisms) rs7412 and rs429358 in APOE (gene map locus 19q13.2) were genotyped using the KASPar® PCR SNP genotyping system (LGC Genomics, Hoddesdon, UK) or by mini-sequencing, as previously described in detail (Blennow et al. Reference Blennow, Ricksten, Prince, Brookes, Emahazion, Wasslavik, Bogdanovic, Andreasen, Båtsman and Marcusson2000). Genotype data for these two SNPs were used to define ε2, ε3 and ε4 alleles. Since ε4 is the only allelic variant clearly associated with an increased risk of AD, the statistical analyses focused on this variant. Information on APOE ε4 allelic status is available for 547 of the 580 individuals in the present sample (94.3%). Among women, 92 individuals carry the ε4 allele (28.4%). For men, the corresponding number is 65 (29.1%) (Table 1).
Dependent variable – time
We employ Cox proportional hazards regression and the continuous component of the dependent variable measures years-at-risk for dementia starting from age 65. The reason for choosing age 65 as the starting point is twofold. First, dementia chiefly affects people aged 65 years or older and hence it makes theoretical sense to let this be the starting point (Blennow, de Leon and Zetterberg Reference Blennow, de Leon and Zetterberg2006; Livingston et al. Reference Livingston, Sommerlad, Orgeta, Costafreda, Huntley, Ames, Ballard, Banerjee, Burns and Cohen-Mansfield2017). Second, by doing so, individuals who had been diagnosed with dementia prior to the baseline examinations, i.e. before age 70, could be included in the analysis. The dichotomous component of the dependent variable indicates whether or not an individual had developed dementia during the period up to 2012. For reasons related to availability and sample size, we do not distinguish between dementia sub-types in the subsequent analyses.
Independent variables
Information on occupation and educational attainment was obtained through interviews at baseline and/or in conjunction with the follow-up examinations. All respondents were asked to specify their main occupation throughout life. Among men, information on lifetime principal occupation is available for 228 individuals (99.6%). With regards to women, the corresponding information is available for 345 (98.3%) individuals. Among these, 37 (10.7%) stated that they had primarily engaged in domestic work during working age, and they were hence excluded from the subsequent analyses (Table 1). The remaining responses were coded in accordance with the Swedish SEI standards for socio-economic classification (Statistics Sweden 1982), which has many commonalities with the Eriksson–Goldthorpe scheme (Goldthorpe Reference Goldthorpe2007) and is often claimed to build on a neo-Weberian approach to class analysis (Breen Reference Breen and Olin Wright2005). Based on the initial classifications, three aggregated socio-economic groups were specified. Blue collar corresponds to manual workers (un-skilled, semi-skilled and skilled). Lower white collar corresponds to assistant, non-manual employees, with or without subordinates, in occupations that require a maximum of three years of post-comprehensive schooling. The final category, white collar and self-employed, includes intermediate/higher non-manual workers and professionals in occupations that require three to six years of post-comprehensive education, as well as upper-level executives, self-employed and farmers. The education variable is mainly constructed from responses gathered in conjunction with the baseline examination in 2000–01. All respondents were asked to specify the level/type of their educational attainment, and for those who did not, information was, if available, obtained from the follow-up examinations. Of the 580 individuals examined, information on educational attainment is available for 344 women (98.0%) and 228 men (99.6%). For reasons related to data availability, the present variable is based on a categorical survey question (not on years spent in education) and has three values. Primary corresponds to elementary school/vocational school. Lower secondary refers to girls’ school (preparatory, vocational or theoretical education that constituted a continuation of elementary school)/junior secondary school/folk high school. Secondary/university corresponds to high school/university. In the following cases, i.e. where response alternatives overlap in the questionnaire, ‘high school/grammar school’ was coded as secondary/university and ‘grammar school/junior secondary school/folk high school’ as lower secondary. For both occupational class and educational attainment, substantial differences exist between men and women in the present sample as well. For example, as regards education, 25.9 per cent of the males have completed a secondary/university education, but only 12 per cent of the females. Likewise, the percentage of men employed in upper white-collar professions is 45.6 per cent in our sample, while the corresponding figure for women is 18.5 per cent (Table 1). Presumably, this reflects the general segregation of the labour market during the period of time when these individuals were active in the workforce (see Statistics Sweden 1973).
Statistical analyses
In order to analyse the timing of dementia onset, we apply Cox regression, which focuses on ‘whether and when an event takes place’ (Guo Reference Guo2010: 3). One advantage of Cox regression compared to ordinary regression techniques is that it accommodates right-censored cases, i.e. individuals who never suffer the event of interest, in this case dementia, but still contribute survival time (Flynn Reference Flynn2012; Guo Reference Guo2010). The values of the beta-coefficients are estimated through Partial Likelihood Estimation (Allison Reference Allison2014), and we use the Efron method for handling coterminous events (ties) (Box-Steffensmeier and Jones Reference Box-Steffensmeier and Jones2004). For the present analyses, a post-estimation test based on Schoenfeld residuals was conducted for all models in order to assess the proportionality of hazards, that is, ‘that the effect of each variable (on the log of the hazard) is the same at all points in time’ (Allison Reference Allison2014: 43). The tests showed no signs of violations. In total, six interaction models, three for men and three for women, were computed: (a) occupational class × APOE ε4, (b) education × APOE ε4 and (c) occupational class × APOE ε4, adjusted for education. Hence, we were able to test both whether the potential effect of APOE ε4 differs between individuals in different SES groups and, by extension, whether these effects are in turn dependent on sex. While this is advantageous, including interaction terms makes interpretation somewhat more complicated (StataCorp 2013). First and foremost, multiplicative interaction models differ from linear-additive regression models in the sense that the coefficient of any constitutive term X cannot be interpreted as an unconditional marginal effect. Instead, it only indicates the effect of a one-unit change in X on Y when the conditioning variable is zero (Brambor, Clark and Golder Reference Brambor, Clark and Golder2006). Second, because it is required that all constitutive terms be added when we estimate a multiplicative interaction model, multicollinearity is likely to occur, which could ultimately inflate the standard errors. As noted above, however, we are seldom interested in the significance/non-significance of the model parameters per se, but rather in the effect of X on Y for relevant values of Z. Taken together, the specific features of interaction models described above imply that it is not possible to draw any substantial conclusions based solely on the estimates shown in the traditional results table (Brambor, Clark and Golder Reference Brambor, Clark and Golder2006). Thus, in the following sections, we focus on comparing predictions for combinations of empirically meaningful variable values. In order to use the predicted confidence intervals to test hypotheses about differences between groups, we compute confidence intervals corresponding to a confidence level of 0.839. Confidence intervals constructed using this level overlap in 95 per cent of all cases when they are based on samples drawn from the same random variable. When confidence intervals are constructed using a confidence level of 0.95, the confidence intervals of two samples drawn from the same random variables overlap in 0.994 of cases. Rejecting the hypothesis that there is a difference between two measures using such confidence intervals is hence too conservative, and the rate of Type II errors would be 0.994 instead of the desired 0.95. Thus, in order to achieve a Type II error rate of 0.05, one has to use α = 0.84 when constructing the confidence intervals (MacGregor-Fors and Payton Reference MacGregor-Fors and Payton2013; Payton, Greenstone and Schenker Reference Payton, Greenstone and Schenker2003).
Results
The main effects for all independent variables were examined using bivariate Cox regression (Table 2). With regards to APOE ε4, significant associations with dementia onset were observed among both men (hazard ratio (HR) = 2.53) and women (HR = 2.54). The estimate for sex indicates a negative, albeit non-significant, effect (HR = 0.90). For both SES indicators (education and occupational class) the estimates suggest, in general, that high SES lowers the risk of dementia (HR values < 1). Even though these effects were found to be non-significant, possibly due to the relatively high prevalence of low education and/or the small number of dementia cases in the present data-set (Wang et al. Reference Wang, Gustafson, Kivipelto, Pedersen, Skoog, Windblad and Fratiglioni2012), the size of the estimates must also be taken into consideration when interpreting the results (Ziliak and McCloskey Reference Ziliak and McCloskey2008). For instance, the effects of lower/upper secondary education (both sexes) are quite sizeable (HR values = 0.72–0.74), which would imply a hazard reduction of nearly 30 per cent compared to the primary education group. More importantly, the absence of significant main effects does not rule out the possibility that the effect of APOE ε4 varies across different SES groups, which is the principal focus of the present paper. Consequently, a total of six interaction models (described in detail above) were computed (Table 3). With reference to the discussion on interpreting such models, the point estimates will not be discussed in further detail here. For example, based on Model I, it can only be concluded that APOE ε4 has a positive and significant effect when the conditioning variable (occupational class) is equal to zero, i.e. among blue-collar workers (Brambor, Clark and Golder Reference Brambor, Clark and Golder2006). Therefore, in the subsequent sections, we focus on comparing graphically illustrated predictions for combinations of different variable values.
Table 2. Main effects: Cox proportional hazards regression (estimated effects on time to dementia onset)
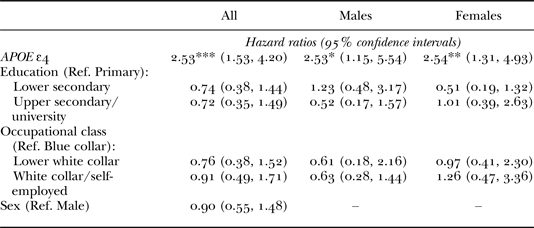
Note: Ref.: reference category.
Significance levels: * p < 0.05, ** p < 0.01, *** p < 0.001.
Table 3. Interaction models: Cox proportional hazards regression (estimated effects on time to dementia onset)

Notes: 1. Occupational class × APOE ε4. 2. Education × APOE ε4. 3. Occupational class × APOE ε4, adjusted for education. PH: proportional hazards. df: degrees of freedom.
Significance levels: * p < 0.05, ** p < 0.01, *** p < 0.001, ns: not significant.
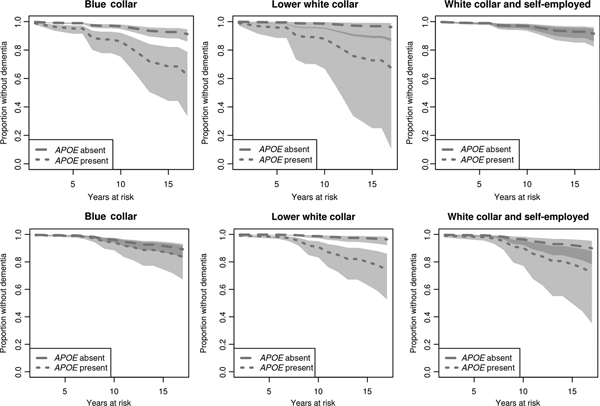
Among male, blue-collar workers, there is a clear difference in ‘survival’ (henceforth referred to as disease onset) between APOE ε4 carriers and non-carriers (Figure 1, upper row). A similar tendency, albeit not statistically significant, can be observed among lower white-collar workers. The corresponding difference among white-collar/self-employed males is, however, substantively small and far from statistically significant. Regarding the corresponding predictions for women, a different picture emerges (Figure 1, lower row). Among blue-collar workers, there is no significant difference in disease onset between APOE ε4 carriers and non-carriers. The same applies to women in upper white-collar work. In addition, we observe a significant difference in disease onset between carriers and non-carriers among lower white-collar workers.
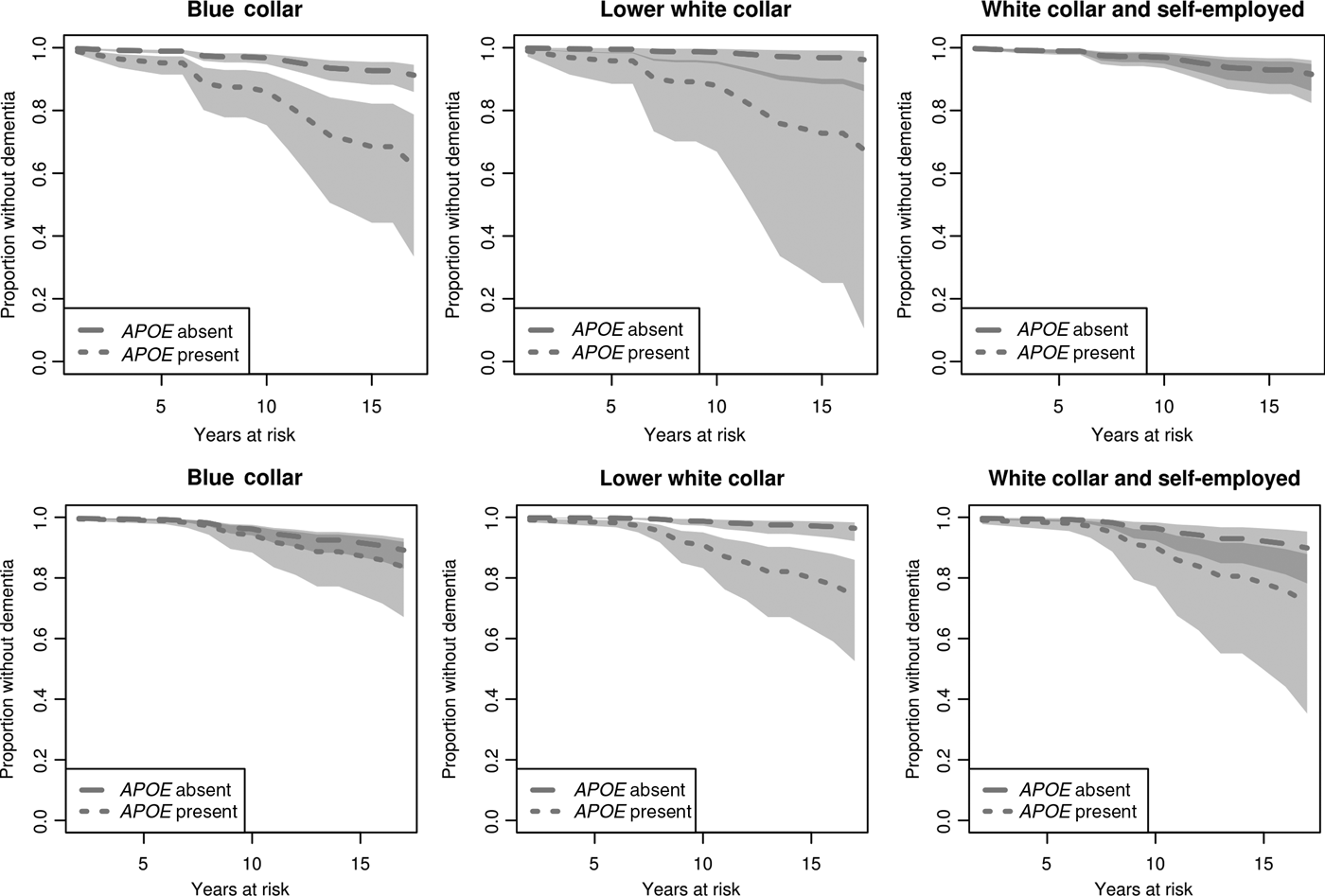
Figure 1. Estimated effects of APOE ε4 and occupational class on time to dementia onset for males (upper row) and females (lower row). Survival curves based on predicted hazard rates. Light grey area: non-overlapping confidence intervals; dark grey area: overlapping confidence intervals.
Concerning men in different educational groups, the results imply that for male APOE ε4 carriers with a primary or lower secondary education, dementia occurs earlier than it does among men who do not carry the allele but have the same level of education (Figure 2, upper row). The difference is statistically significant among men with primary education, but not statistically significant among those with lower secondary education. There is no difference between APOE ε4 carriers and non-carriers among men with secondary/university education. Again, the associations look different and are less coherent among women (Figure 2, lower row). The time to disease onset seems to be shorter among women with the lowest and the highest level of education. Independent of educational attainment, women who carry the APOE ε4 allele develop dementia earlier than those who do not. The difference reaches borderline statistical significance among women with primary education and secondary/university education. The magnitude of the estimated difference among those with lower secondary education is similar to that in the former group, albeit not statistically significant.
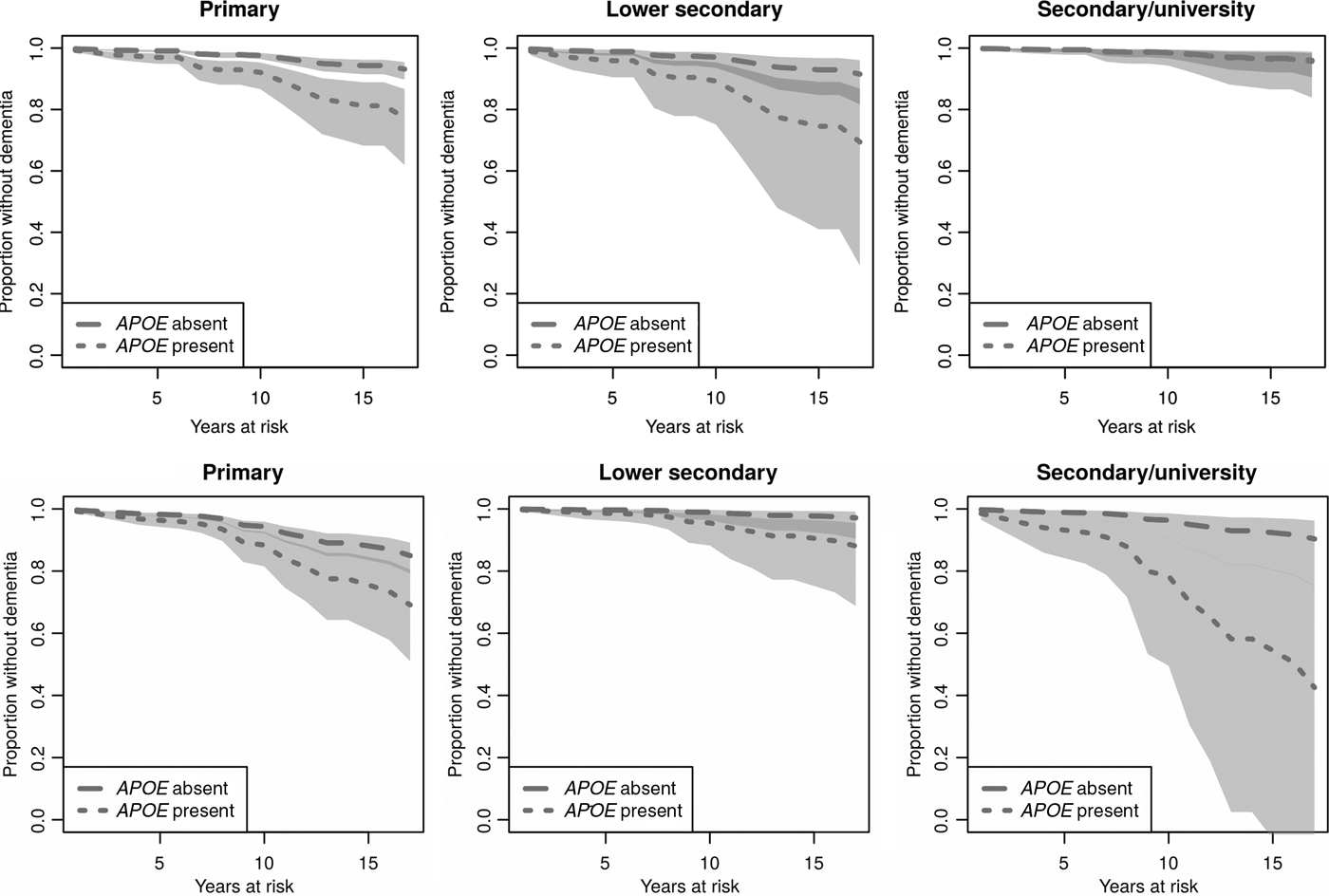
Figure 2. Estimated effects of APOE ε4 and education on time to dementia onset for males (upper row) and females (lower row). Survival curves based on predicted hazard rates. Light grey area: non-overlapping confidence intervals; dark grey area: overlapping confidence intervals.
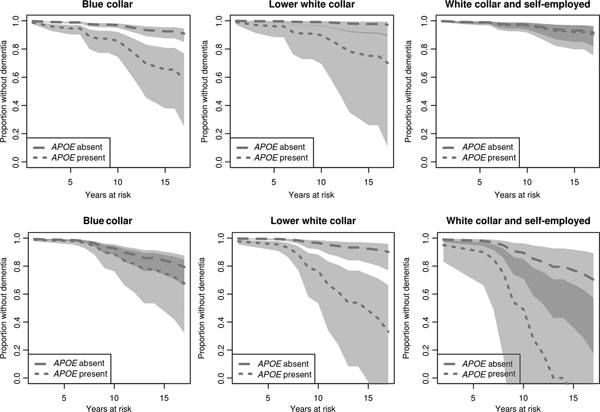
Lastly, we estimate disease onset for men and women in different occupational groups, but adjust for education (Figure 3). In the present figure, the predicted disease onset for APOE ε4 carriers and non-carriers in all occupational groups is computed with education set to its reference value, i.e. primary education. We note that for males, the difference in onset between APOE ε4 carriers and non-carriers in the blue-collar group remains significant (Figure 3, upper row). When education is instead set to lower or upper secondary, the tendency of a difference remains but is no longer significant (graphs not shown here but can be requested from the author). Also among women, the results in the adjusted model are similar to those in the unadjusted model when education is set to its reference value, i.e. primary education (Figure 3, lower row). When education is instead set to lower or upper secondary, there is no longer a significant difference in onset between carriers and non-carriers in the lower white-collar group (graphs not shown here but can be requested from the author).
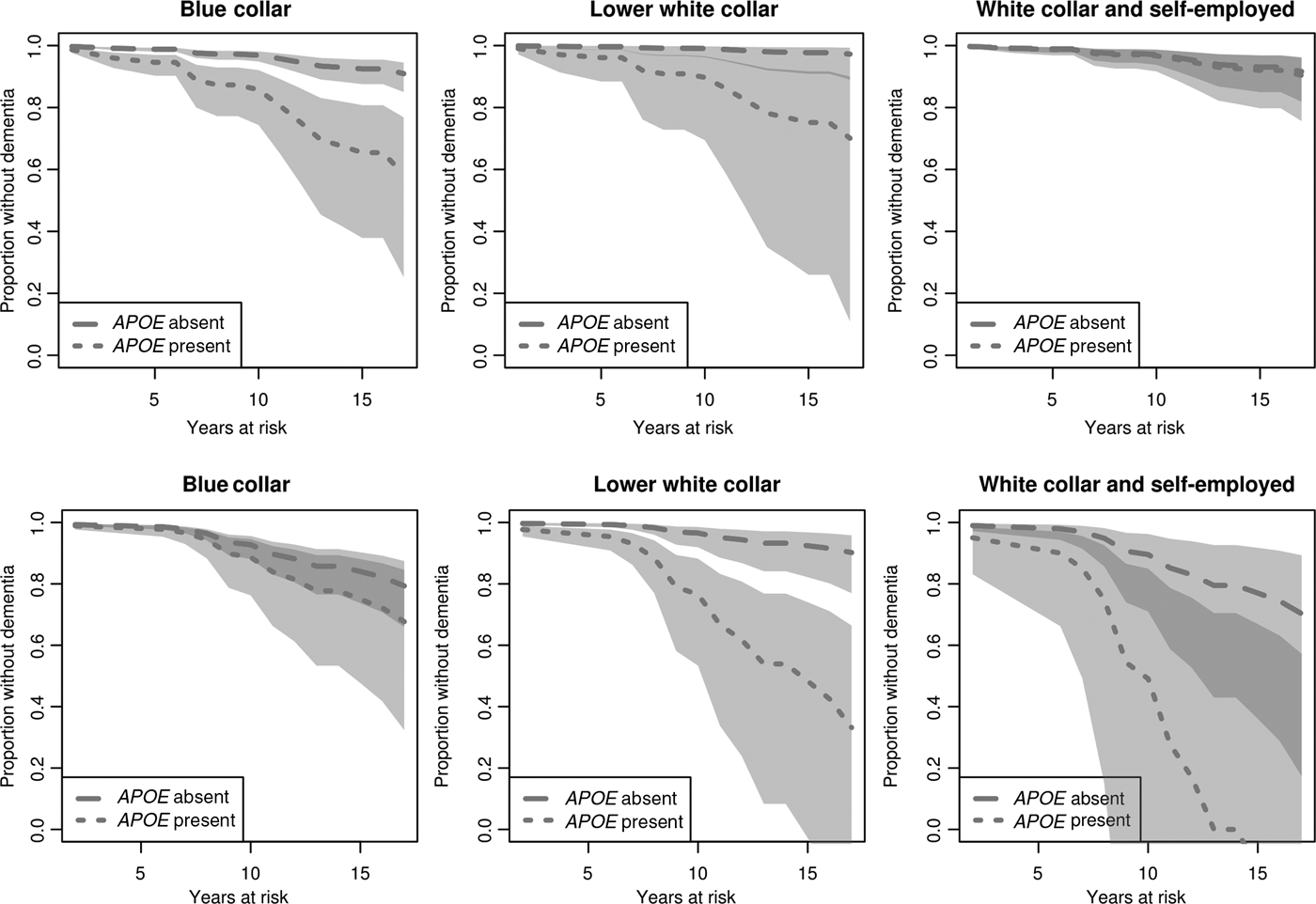
Figure 3. Estimated effects of APOE ε4 and occupational class on time to dementia onset for males (upper row) and females (lower row), adjusted for education. Survival curves based on predicted hazard rates (education set to ‘primary’). Light grey area: non-overlapping confidence intervals; dark grey area: overlapping confidence intervals.
Discussion
In the present study, we explored and identified SES-related modifications of the association between APOE ε4 and the timing of dementia onset, as well as differences between men and women in this respect. Our overall results imply that SES modifies the effect of APOE ε4 among men, whereas among women, high SES does not seem to exhibit the same ‘compensatory ability’.
A fundamental feature of SES, regardless of whether it is measured as occupational class or as educational level, is that it indicates a hierarchical position that, as stated by Weber almost a century ago, affects individuals’ ‘life chances’. In simple terms, this suggests that the higher one's education and/or the higher one's occupational position, the better one's life chances. The unequal distribution of life chances further implies fundamental differences in the accumulation of either advantage or disadvantage over the lifecourse (Dannefer Reference Dannefer2003). For example, many risk factors for dementia, such as lack of physical activity, stress, high blood pressure, obesity, alcohol abuse and smoking, are known to be more common among the less affluent and hence constitute potential pathways between SES and dementia (Fratiglioni, Paillard-Borg and Winblad Reference Fratiglioni, Paillard-Borg and Winblad2004; Kivipelto et al. Reference Kivipelto, Rovio, Ngandu, Kåreholt, Eskelinen, Winblad, Hachinski, Cedazo-Minguez, Soininen, Tuomilehto and Nissinen2008; Marmot Reference Marmot2004; Wang et al. Reference Wang, Gustafson, Kivipelto, Pedersen, Skoog, Windblad and Fratiglioni2012). Consequently, we hypothesised that individuals found in more disadvantageous socio-economic positions would develop dementia earlier than their high SES counterparts and that low SES, in combination with the known genetic risk factor APOE ε4, would entail an additional risk increase. Further, it was assumed that differences might exist between men and women in this regard.
By including a gene–SES interaction term in all models, we were able to test the hypotheses outlined above. In contrast to some previous findings (Meng and D'Arcy Reference Meng and D'Arcy2013; Ngandu et al. Reference Ngandu, von Strauss, Helkala, Winblad, Nissinen, Tuomilehto, Soininen and Kivipelto2007), yet in line with others (Wang et al. Reference Wang, Gustafson, Kivipelto, Pedersen, Skoog, Windblad and Fratiglioni2012), multiplicative interaction effects were detected in relation to both occupational class and education. By extension, these results imply that SES could in fact moderate the effect of APOE ε4, albeit primarily among men. For example, among males who had primarily held blue-collar positions throughout life, there was a significant difference in the timing of dementia onset between APOE ε4 carriers and non-carriers. A similar, yet non-significant, difference was found also among lower white-collar workers but not in the highest occupational group. By extension, this suggests that among upper white-collar workers, the APOE ε4 does not imply a risk increase in relation to age of onset. As regards education, a similar pattern was observed. Among men with primary education, there was a substantial and significant difference in disease onset between APOE ε4 carriers and non-carriers. In contrast, no such difference was revealed among males with secondary/university education. In summary, what our results suggest is that, among men, the increased susceptibility to dementia among APOE ε4 carriers is in fact SES-dependent, as it is only detectable among individuals with lower SES, i.e. with a primary-level education and/or in blue-collar occupations. Thus, the disadvantages accumulated by individuals with low SES could potentially ‘trigger’ the genetic predisposition to dementia or, conversely, the advantages accompanying high SES are likely to compensate for the increased genetic risk implied by the APOE ε4 allele.
For women, the results are less straightforward. We found, as expected, a clear tendency indicating that women who carry the APOE ε4 allele develop dementia earlier in life than those who do not. However, the risk distribution among women was not as clearly related to high/low SES and not actually possible to understand as a consequence of position in the social hierarchy. For instance, women who spend most of their working life in white-collar occupations, and who do not carry the APOE ε4 allele, develop dementia later in life. Yet, among white-collar women who carry the APOE ε4 allele, the time to disease onset was shorter and on a par with that of women in blue-collar occupations. Additionally, among blue-collar women, the time to dementia onset did not differ depending on whether or not the ε4 allele was present. To summarise, if high SES seems to postpone dementia onset among male APOE ε4 carriers, this does not appear to be the case among women. These findings need to be the focus of further investigation. There is, of course, a possibility that women and men tend to react differently when exposed to the systematic differences in the living conditions captured by SES. However, we consider such a conclusion far-fetched. Within sociology, there has been a long-standing debate on how to measure class position at the household level. In the early stages of this discussion, an empirically derived position was that if both husband and wife were present in the household, the household's class position ought to be based on the husband's occupational class (Erikson and Goldthorpe Reference Erikson and Goldthorpe1992b; Hellevik Reference Hellevik1988). The reason for this position was simply that men, compared to women, are generally more strongly attached to the labour market and usually earn more money. This position has been much discussed and criticised and, in fact, has become increasingly obsolete, as women, in general, and Swedish women, in particular, have strengthened their labour market position. Thus, already in the 1980s, it was suggested that household class should instead be based on the highest occupational class position, regardless of whether the husband or wife held it (Erikson Reference Erikson1984). We will not discuss this at length here, but only conclude that for a cohort born in 1930 there were large systematic differences in men's and women's labour market/educational trajectories. Consequently, women's own occupational class or educational level might not fully capture their actual socio-economic position. By extension, this could explain why the ‘compensatory ability’ of higher SES appears to be weaker among women than among men. Alternatively, it could be that the conditions accompanying any given SES position differ between the sexes, i.e. that the advantages accompanying higher SES positions are greater for men than for women. For example, in 2009, the Swedish National Board of Health and Welfare reported that while, for example, smoking, sleep disturbances and anxiety problems are generally more common among blue-collar workers than among white-collar workers, there are large variations between men and women within all occupational classes. In fact, health-related problems or risk factors such as those mentioned above are more common among women than among men within practically all SES groups (Danielsson and Berlin Reference Danielsson, Berlin, Danielsson, Berlin, Heimerson and Talbäck2009). Thus, our conclusion at this stage is not that social conditions are less important for women, but rather that our standard SES indicators are less well designed to capture SES differences among women, especially in older cohorts.
Although different SES indicators, such as education and occupational class, are largely overlapping and causally related, they also tap into different aspects of SES (Lahelma et al. Reference Lahelma, Martikainen, Laaksonen and Aittomäki2004; Torssander and Erikson Reference Torssander and Erikson2010). As regards education, it primarily picks up conditions during upbringing as well as acquired knowledge and abilities. Educational attainment is, of course, highly important for labour market opportunities, but once we add occupational class to the equation, class is more likely to capture the long-term impact of work and labour market conditions as well as systematic differences in economic conditions generated by the labour market. When we analyse the impact of occupational class but adjust for education (set to ‘primary’), the results in the adjusted models are, for both sexes, largely similar to those in the unadjusted models. For example, among men, the difference in onset between APOE ε4 carriers and non-carriers in the lowest occupational group remains significant. When education is instead set to lower or upper secondary, the same pattern is generally observed, even though the differences are no longer significant. Therefore, the non-significance could possibly be attributed to the loss of statistical power, rather than to the multicollinearity, that occurs when we add yet another variable to the model. While this might imply that education and occupational social class impact on the timing of dementia onset through different pathways (Lahelma et al. Reference Lahelma, Martikainen, Laaksonen and Aittomäki2004; Sattler et al. Reference Sattler, Toro, Schoenknecht and Schroeder2012; Torssander and Erikson Reference Torssander and Erikson2010), the empirical evidence presented here is not sufficient to allow us to definitely draw that conclusion. This possibility does underscore, however, the importance of designing studies that include comprehensive information about different SES components and have a sample size that is large enough to allow detailed studies of relevant sub-groups.
Naturally, the present study has a number of limitations that need to be taken into consideration. First, a general issue in studies targeting health inequities in older cohorts is non-random mortality selection (Dupre Reference Dupre2007, Reference Dupre2008; Willson, Shuey and Elder Reference Willson, Shuey and Elder2007). In short, the concept refers to the fact that the least affluent tend to experience higher mortality rates at young ages, which leaves a more ‘robust’ and, hence, biased sample of survivors. Similar changes might also occur as a result of genetic differences. For example, it has been proven that carriers of the APOE ε4 allele are more at risk of developing other conditions besides dementia, e.g. cardiovascular diseases, which means that they risk dying at younger ages (Jofre-Monseny, Minihane and Rimbach Reference Jofre-Monseny, Minihane and Rimbach2008; Skoog et al. Reference Skoog, Waern, Duberstein, Blennow, Zetterberg, Börjesson-Hanson, Östling, Guo, Kern, Gustafson, Gudmundsson, Marlow and Kern2015). Still, analyses conducted on a more ‘robust’ or ‘healthier’ sample would result in under-estimation, rather than over-estimation, of associations. Second, our sample is relatively small and our data on dementia are limited to a period of 17 years. Hence, the sample does not include information on individuals above age 82, which implies that the number of dementia cases is still limited. While one strength in this respect is that we employ Cox regression instead of a cross-sectional analytical technique, we encourage future studies to continue exploring gene–SES interactions in relation to dementia. Finally, due to lack of information, we do not distinguish between dementia sub-types in this study. This is a significant limitation in the sense that APOE ε4 is generally considered to be a major risk factor for AD, whereas for other dementia sub-types, associations are less well established. However, it should be emphasised that AD is the most common form of dementia, accounting for approximately 50–70 per cent of all cases. In addition, recent studies suggest that the ε4 allele could also be associated, albeit not as strongly, with other forms of dementia such as vascular dementia, which is the second most common form (Keogh et al. Reference Keogh, Kurzawa-Akanbi, Griffin, Douroudis, Ayers, Hussein, Hudson, Pyle, Cordell, Attems, McKeith, O'Brien, Burn, Morris, Thomas and Chinnery2016; Liu et al. Reference Liu, Li, Liu, Deng, Zhu, Li and He2012; Rohn Reference Rohn2014; Tsuang et al. Reference Tsuang, Leverenz, Lopez, Hamilton, Bennett, Schneider, Buchman, Larson, Crane, Kaye, Kramer, Woltjer, Kukull, Nelson, Jicha, Neltner, Galasko, Masliah, Trojanowski, Schellenberg, Yearout, Huston, Fritts-Penniman, Mata, Wan, Edwards, Montine and Zabetian2013).
Conclusions
To sum up, we conclude that the interaction effect between SES and APOE ε4 on dementia onset is manifested differently among men and women. High SES seems to buffer the effect of APOE ε4 among men, whereas among women, high SES does not seem to exhibit the same ‘compensatory ability’. These findings underscore the long-term impact of social inequity on health, as well as the importance of considering potential interactions between social and genetic risk factors if we are to understand better the complex aetiology of dementia and other multi-factorial diseases. Further research on the mechanisms through which social inequities in dementia arise is still needed, and future studies ought to explore more closely how historical differences in socio-economic trajectories between the sexes could affect health during old age.
Acknowledgements
This study was funded by: The Stena Foundation, the Swedish Research Council (2015-02830, 2013-8717), the Swedish Research Council for Health, Working Life and Welfare (2004-0145, 2006-0596, 2008-1111, 2010-0870, 2013-1202, 2013-2300, 2013-2496), the Alzheimer's Association Zenith Award (ZEN-01-3151), the Alzheimer's Association Stephanie B. Overstreet Scholars (IIRG-00-2159), Sahlgrenska University Hospital (ALF), Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse, Eivind och Elsa K:son Sylvans stiftelse, Stiftelsen Söderström-Königska Sjukhemmet, Stiftelsen för Gamla Tjänarinnor, Handlanden Hjalmar Svenssons Forskningsfond, Stiftelsen Professor Bror Gadelius’ Minnesfond, the Swedish Brain Foundation (Hjärnfonden), the Swedish Alzheimer Foundation (Alzheimerfonden), the Torsten Söderberg Foundation and the Swedish Society of Medicine. None of the funding sources were involved in the design of the study, the collection, analysis and interpretation of data, or writing the manuscript. HZ is a Wallenberg Academy Fellow and KB holds the Torsten Söderberg Professorship in Medicine. Informed consent was acquired from all participants or their relatives. The regional Ethical Review Board for medical research in Gothenburg, Sweden approved the H70/PPSW examination waves in 2000 (reference Ö402-99), 2004 (reference T453 04) and 2009 (reference 075-09). Informed consent was acquired from all participants or their relatives. CH was responsible for the conception and design of the study, processed the data and carried out the statistical analysis (together with HE) as well as drafted and finalised the manuscript. BH, HE, IS and MMF supervised the first author and participated in the conception and design of the study, the interpretation of data and revision of the manuscript. MMF contributed specifically in the sample selection process. BH contributed specifically in the drafting of the manuscript. IS was responsible for the acquisition of data and assessed information obtained from the neuropsychiatric examinations in order to diagnose dementia. KB, AZ and HZ were responsible for the genotyping procedures and/or the interpretation of genetic analyses. All authors reviewed and revised the manuscript as well as provided useful comments and insights in relation to their specific field of expertise. All authors have approved the final paper. The authors declare that they have no conflicting interests to report.