High blood pressure (BP) is a global public health problem(Reference Kearney, Whelton and Reynolds1). BP is strongly associated with age-specific mortality rates from stroke, IHD and other vascular causes, even at the lower levels of the distribution (down to 115/75 mmHg)(Reference Lewington, Clarke and Qizilbash2). It has been estimated that an average reduction in BP of 2 mmHg at the population level might reduce mortality from stroke by about 10 %, and mortality from IHD or other vascular diseases by about 7 %(Reference Lewington, Clarke and Qizilbash2).
Polyphenols are a wide class of bioactive compounds abundant in plant foods such as fruits, cereals, legumes and vegetables. In population studies, lower cardiovascular event rates were observed in subjects with a higher consumption of flavonoids, i.e. polyphenols with a C6–C3–C6 basic structure(Reference Hertog, Feskens and Hollman3–Reference Yochum, Kushi and Meyer6). Grape products contain polyphenols, such as anthocyanins, flavanols and flavonols, as well as phenolic acids(Reference Xu, Simon and Welch7). The effects of grape products on cardiovascular risk markers have been reported in several human intervention studies and include an improvement in plasma lipid profiles(Reference Preuss, Wallerstedt and Talpur8, Reference Castilla, Echarri and Davalos9), an enhancement of endothelial function(Reference Clifton10–Reference Barona, Aristizabal and Blesso14) and an inhibition of platelet aggregation(Reference Freedman, Parker and Li15–Reference Shenoy, Keen and Kalgaonkar17). For the effects of grape products on BP, evidence was not convincing in some of the human studies(Reference Clifton10, Reference van Mierlo, Zock and van der Knaap18–Reference Sano, Uchida and Saito20), while other human studies have shown beneficial effects on BP(Reference Barona, Aristizabal and Blesso14, Reference Park, Kim and Kang21, Reference Sivaprakasapillai, Edirisinghe and Randolph22) or on its nocturnal dip(Reference Dohadwala, Hamburg and Holbrook23). Recent clinical evidence has shown that other flavanol-rich foods such as dark chocolate and black tea may also lower BP in hypertensive populations(Reference Ried, Sullivan and Fakler24, Reference Hodgson, Puddey and Woodman25).
In grapes, the highest concentrations of polyphenols are found in the skin, stems and seeds. Sivaprakasapillai et al. (Reference Sivaprakasapillai, Edirisinghe and Randolph22) recently reported that a specific grape seed extract (GSE) reduced BP by 9 mmHg v. placebo in metabolic syndrome patients. Due to a specific extraction method, this GSE consists mainly of polyphenols (approximately 80 % of the dry weight) with an enhanced content of monomeric and oligomeric polyphenols. Also, levels of gallic acid and de-gallated catechins are increased due to a tannase treatment. Thus, this GSE extract has, compared with other polyphenol extracts, relatively high concentrations of low-molecular-weight phenolic compounds, which are probably more bioavailable than large polymeric polyphenols(Reference Sivaprakasapillai, Edirisinghe and Randolph22, Reference Manach, Williamson and Morand26, 27). Therefore, this specific GSE seemed promising and possibly more effective than other GSE in lowering BP.
The metabolic mechanisms by which polyphenol-rich extracts and foods could affect BP are not well understood, although several pathways have been suggested. In the present study, we explored these pathways including the following: potent vasoconstrictor endothelin-1 (ET-1)(Reference Yanagisawa, Kurihara and Kimura28, Reference Dhaun, Goddard and Kohan29); metabolites of NO which modulate vascular tone and reactivity(Reference Verma, Buchanan and Anderson30); asymmetric dimethylarginine (ADMA) which inhibits the production of NO by competing with l-arginine as the substrate for endothelial NO synthesis(Reference Vallance, Leone and Calver31); plasma renin activity which indicates the influences on, or actions through, the renin–angiotensin system. We also explored the possible effects of GSE on platelet function. Platelet function plays a prominent role in defining CVD risk(32), and some studies have shown that grape polyphenols may reduce platelet aggregation(Reference Freedman, Parker and Li15–Reference Shenoy, Keen and Kalgaonkar17).
Thus, in the present study, we investigated the effects of an encapsulated GSE, rich in low-molecular-weight polyphenolic compounds, on daytime ambulatory systolic BP (SBP) and diastolic BP (DBP) in untreated subjects with pre- and stage I hypertension. Furthermore, we explored some mechanistic pathways that are possibly involved in the BP-lowering efficacy of GSE, as well as the effect of GSE on platelet aggregation.
Experimental methods
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Medical Ethics Committee of Wageningen University, The Netherlands. The study was conducted according to the Dutch Medical Research Involving Human Subjects Act and the International Conference on Harmonisation Guidelines for Good Clinical Practice. The study took place from April to July 2009 at Unilever R&D Vlaardingen, The Netherlands. Written informed consent was obtained from all subjects. The study was registered at ClinicalTrials.gov (identifier no. NCT00869193).
Study population
In the present study, seventy male and postmenopausal female subjects were recruited. Subjects were eligible when they met the following main selection criteria: aged 35–75 years; no reported current or previous metabolic diseases, chronic gastrointestinal disorders or cardiovascular or renal diseases; having slightly elevated BP which was defined as a mean 6 h ambulatory SBP ranging between 120 and 159 mmHg (120 mmHg is the upper threshold for a normal BP, whereas 159 mmHg is the upper threshold for hypertension stage I(33) and was taken as a cut-off point to avoid the inclusion of subjects probably requiring anti-hypertensive therapy) and DBP < 100 mmHg; having a BMI between 18·5 and 30 kg/m2; having no or willing to refrain from lifestyle habits that could have an impact on BP including extreme physical exercise and the consumption of polyphenol-rich foods and/or supplements; no use of medication that could influence the study outcomes (BP-lowering drugs, antibiotics, acetylsalicylic acid or similar-type painkillers); having ≤ 30 % error readings during the 6 h ambulatory BP (ABP) measurements at screening; having signed the informed consent.
Study design
The present study was designed as a double-blind, placebo-controlled, randomised, parallel-group study with a 1-week run-in period (days 1–7) and an 8-week intervention period (days 8–63). Subjects were randomly allocated to receive either capsules with GSE (n 35) or placebo capsules (n 35). A statistician randomised the personal codes to one of the two different treatments while stratifying the subjects according to sex. Pre- and post-intervention, subjects visited the test facility on three consecutive test days (days 5–7 and 61–63, respectively). Daytime ABP measurements were conducted on days 5, 7, 61 and 63. On days 6 and 62, 24 h urine was collected (until the mornings of days 7 and 63, respectively) and blood samples were drawn in the fasted state (before breakfast) and in the non-fasted state (2 h after breakfast (+capsule)). A standardised breakfast was served on all six test days. To check compliance and the subjects' well-being, and to encourage them to follow the protocol as requested, subjects visited the test facility 2 weeks after the start of the intervention, and they were reminded by telephone after 5 weeks.
Test products and dietary instructions
Subjects consumed either gelatin capsules (size 0) containing 300 mg GSE (MegaNatural BP®; Polyphenolics) or microcrystalline cellulose as placebo (excipients were used in equal amounts in both test products). As published by Edirisinghe et al. (Reference Edirisinghe, Burton-Freeman and Tissa34), the distribution of phenolic compounds in this GSE was expected to be on average 9 % monomers, 69 % oligomers and 22 % polymers. Capsules were produced by Metagenics. Based on the 1H NMR technique and the Folin–Ciocalteu method, the polyphenol content of the pure GSE was determined to be 77–80 % (as expressed in free gallic acid equivalents). We were able to identify only 18 % of the polyphenols; the major identified compounds were monomeric procyanidins (catechins) (4·3 %), dimeric procyanidins (6·1 %), trimeric procyanidins (2·5 %) and gallic acid (4·8%), whereas minor compounds were galloylated catechins (0·04 %), flavonols (0·02 %) and stilbenes (0·003 %). Unidentified polyphenols probably included oligomers and polymers that are more difficult to identify. Compared with the amounts of 5–15 % monomeric procyanidins reported for other batches of this GSE(Reference Edirisinghe, Burton-Freeman and Tissa34), the identified amount of monomeric procyanidins in the batch used in the present study was at the lower end of the range. The placebo capsules did not contain any detectable polyphenols. Test products were microbiologically checked (analysis of total viable count, enterobacteriaceae, yeasts and moulds) before providing them in blister packs to the subjects. Test and placebo capsules were identical with respect to size and colour. The treatment code of the two test products was blinded for subjects and all staff involved in the study.
Subjects were instructed to consume one capsule per d, in the morning directly after breakfast, receiving the final dose on the last test day (day 63). They were asked to maintain their normal diet and lifestyle during the study, but to refrain from a selection of foods that are typically rich in polyphenols, i.e. dark chocolate, red wine and grape juice, and to restrict consumption of tea and red fruit to a maximum of two servings per d each. We deliberately avoided restrictive intake of all polyphenol-containing foods, because this would have had an impact on the subjects' diet and lifestyle behaviour in such a way that it may have interfered with the treatment effect. At 36 h before each test day until the end of the test occasion, drinking alcoholic beverages was not allowed. Food intake was standardised on all the six test days. This was done by complete 3 d dietary records of individual food intakes on days 5, 6 and 7, and by the instruction to exactly repeat this food pattern on days 61, 62 and 63, respectively. Any deviations from the dietary instructions and protocol were recorded by the subjects in their personal diaries. In addition, subjects kept daily written records of their test product intake. At the end of the intervention, subjects returned all empty blister packs and any unused capsules. These were counted to estimate compliance.
Measurements
Daytime ABP and heart rate recordings were taken every 20 min between 08.30 and 20.30 hours, using an ABP monitor (Spacelabs monitor type 90 217; Spacelabs Healthcare) on the non-dominant arm. Breakfast was consumed shortly (within 30 min) before the start of the daytime ABP recordings. Subjects were asked to refrain from any strenuous exercise and to follow their habitual daily living and working pattern on the BP measurement days. Each subject used the same BP monitor throughout the study.
Blood sampling was done in the fasted state (overnight fast of at least 10 h) and in the non-fasted state (2 h after a served breakfast (+capsule)). In the fasted state, one hirudin tube was collected for determining platelet aggregation based on the single platelet count method(Reference Siotia, Buckland and Judge35) using four different agonists (HEPES, adenosine di-phosphate, collagen and thrombin receptor-activating protein). This assessment required immediate analysis after sample collection. K3-EDTA tubes were collected for measuring NO metabolites by nitrate and nitrite assays, ET-1 by RIA using commercial human ET-1 antibody (Bachem) and radiolabelled ET-1 (Perkin-Elmer), ADMA by using a competitive ADMA-ELISA (DLD Diagnostika) and plasma renin activity by a commercial RIA kit (Dia Sorin). In the non-fasted state, additional K3-EDTA tubes were collected for measuring NO metabolites, ET-1, ADMA and plasma renin activity. Urine was collected during 24 h. The subjects stored the non-acidified urine at room temperature. When the collected urine was handed in, it was immediately homogenised and weighed and samples were taken for measuring ET-1 and NO metabolites as described above. Na and K contents were measured using the Hitachi 912 auto-analyser (Rochi Diagnostics), and polyphenol metabolites were measured using a GC/MS (Agilent). More detailed information on blood analyses and 24 h urine analyses is provided in the Supplementary Material (available online). All samples, except those required for assessing platelet aggregation, were stored at − 80°C until analysis.
Adverse events were recorded on a case report form and classified according to the International Classification of Diseases, ninth revision (ICD-9).
Statistics
With seventy subjects in the study and assuming a within-subject variation of 30 mmHg for duplicate ABP measurements, the study had a statistical power of 80 % to detect a mean SBP reduction of 3·7 mmHg (α = 0·05; two-sided). Data were analysed according to both the intention-to-treat principle and the per-protocol principle excluding data from subjects for which events outside the protocol might have affected the validity of the outcomes. Here, we report the results based on the intention-to-treat analysis only; the per-protocol analysis yielded similar results.
Differences in the changes from baseline in ABP and heart rate between active treatment and placebo were statistically analysed with a repeated-measurements mixed-model analysis (the average per h was included). Treatment, sex, date of inclusion in the trial and hour of BP measurement were included as fixed factors. Age, BMI, change in body weight, change in Na, K and Na:K ratio were included as covariates. Subjects were included as a random factor. A banded Toeplitz(Reference Lewington, Clarke and Qizilbash2) covariance structure was assumed. The model was simplified by backward elimination; variables were only retained when their significance was below 0·10. Differences in changes in secondary parameters were explored by means of an ANCOVA. P values are based on two-sided testing. Statistical analyses were performed using SAS software (SAS Institute, version 9.2).
Results
Subjects and compliance
Of the 190 subjects that underwent actual screening, eighty-four subjects were eligible for the study. Of these, ten subjects were randomly excluded and four subjects were assigned to be spare subjects to allow for some dropout before the start of the intervention. During the run-in period, one subject dropped out for personal reasons and was replaced by a spare subject. Finally, seventy healthy subjects, thirty-eight males (54 %) and thirty-two (46 %) females, were randomised to active or placebo treatments. An overview of the subjects' characteristics is provided in Table 1. During the intervention, one subject dropped out due to the prescription of BP-lowering medication. Count of returned capsules indicated an almost full compliance of 99·7 %. Compliance to dietary restrictions was also high as judged by inspection of the subjects' diaries. Overall, body weights were stable during the study period.
Table 1 Characteristics of the study population at screening (Mean values with their standard errors)
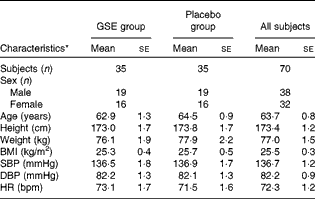
GSE, grape seed extract; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; bpm, beats per min.
* The GSE group and the placebo group were not significantly different at screening (P>0·05) for all the study population characteristics. The means of SBP, DBP and HR are the average of the 6 h ambulatory measurements.
Daytime ambulatory blood pressure and heart rate
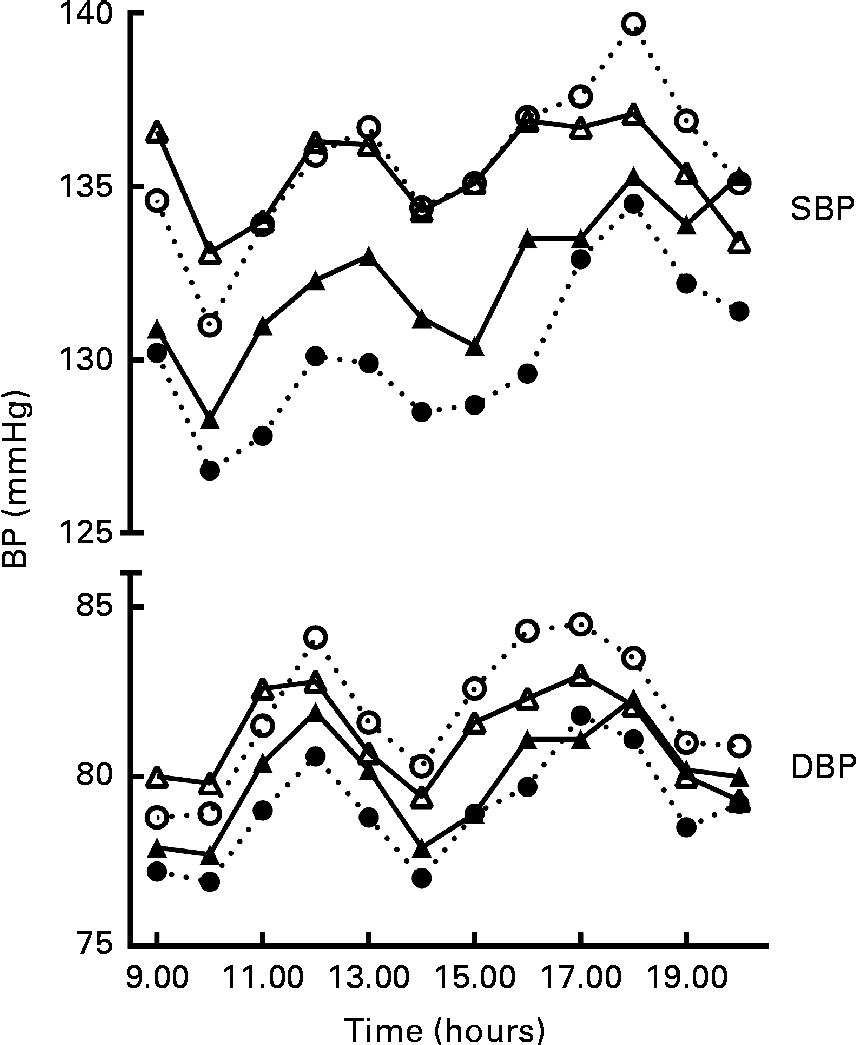
At baseline, the mean daytime ambulatory SBP was 135·8 (se 1·3) mmHg and DBP 81·5 (se 0·9) mmHg. After 8 weeks of intervention, SBP was changed by − 5·2 (95 % CI − 7·7, − 2·8) mmHg in the GSE group and by − 2·2 (95 % CI − 4·7, 0·2) mmHg in the placebo group. DBP was changed by − 2·5 (95 % CI − 4·0, − 1·0) mmHg in the GSE group and by − 1·1 (95 % CI − 2·5, 0·4) mmHg in the placebo group. Compared with placebo, BP values were not significantly lower after the grape seed treatment with a difference of − 3·0 (95 % CI − 6·5, 0·5) mmHg for SBP and − 1·4 (95 % CI − 3·5, 0·6) mmHg for DBP (Table 2 and Fig. 1). Heart rate was not significantly affected by the GSE treatment v. placebo (0·0 beats per min, 95 % CI − 2·8, 2·9).
Table 2 Ambulatory blood pressure and heart rate (HR) (Mean values with their standard errors; 95 % confidence intervals)
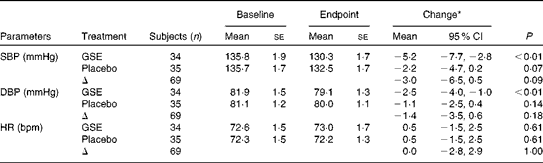
SBP, systolic blood pressure; GSE, grape seed extract; DBP, diastolic blood pressure; bpm, beats per min.
* Adjusted model; change adjusted for baseline values and covariates with P< 0·1: for SBP, change in weight and change in ratio Na:K; for DBP, sex and age; for HR, sex, age and change in ratio Na:K.

Fig. 1 Systolic blood pressure (SBP) and diastolic blood pressure (DBP) patterns between 08.30 and 20.30 hours (n 69); the first hour is the average blood pressure (BP) between 08.30 and 09.30 hours, the second hour is the average BP between 09.30 and 10.30 hours, etc. ○, ● (![]() ), BP pattern of the grape seed extract group; △, ▲ (
), BP pattern of the grape seed extract group; △, ▲ (![]() ), BP pattern of the placebo group. ○ and △, BP pattern at baseline; ● and ▲, BP pattern at the end of the intervention.
), BP pattern of the placebo group. ○ and △, BP pattern at baseline; ● and ▲, BP pattern at the end of the intervention.
Vasoactive biomarkers, plasma renin activity and platelet aggregation
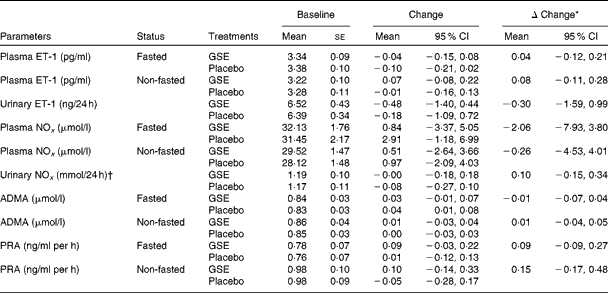
Compared with placebo, the GSE intervention did not significantly affect plasma and urine concentrations of the vasoconstrictor ET-1 and of the endogenous NO metabolites nitrite and nitrate. Also, plasma concentrations of ADMA and the activity of plasma renin were not significantly affected by the GSE treatment (Table 3). Additionally, 8 weeks of GSE intervention did not change platelet aggregation as induced by several agonists when compared with placebo, except for a modest reduction in platelet aggregation after 1 min of HEPES stimulus (change in the amount of free platelets: 5·4 % (95 % CI 0·2, 10·7)).
Table 3 Vasoactive biomarkers and plasma renin activity (Mean values with their standard errors; 95 % confidence intervals)

ET-1, endothelin-1; GSE, grape seed extract; NOx, NO metabolites; ADMA, asymmetric dimethylarginine; PRA, plasma renin activity.
* Adjusted model: change adjusted for baseline concentration and covariates with P< 0·1.
† Extreme outliers (urinary nitrite >80 μmol/l) were excluded (n 4).
Urinary phenolic metabolite excretion
Based on a principal component analysis, the urinary phenolic acids that contributed most to correctly classifying subjects in the GSE and placebo groups were 4-O-methylgallic acid, 3-hydroxymandelic acid, 3-hydroxyphenylacetic acid, pyrogallol, vanillic acid, 3,4-dimethoxyphenylacetic acid, ferulic acid and caffeic acid. Of these, only 4-O-methyl gallic acid and pyrogallol were significantly increased in the GSE group compared with the placebo group (ratio GSE:placebo: 4·7 (95 % CI 3·8, 5·8) and 2·0 (95 % CI 1·5, 2·7), respectively). Out of forty-five metabolites, six metabolites showed weak ( < 0·2[R] < 0·5) correlations with SBP changes and two with DBP changes, which we considered not biologically meaningful.
Adverse events
The reported adverse events were mild and the incidence was low (n 71), with major complaints being headache (n 16), joint pain or lumbago (n 6), allergic rhinitis (n 5) and (naso-) pharyngitis (n 4). Between the two treatment groups, there was no statistically significant difference in the number of subjects reporting adverse events and in the nature and frequency of these events.
Discussion
The findings of the present study suggest that polyphenol-rich GSE has no major effect on BP in subjects with pre- and stage I hypertension. The 3 mmHg fall in SBP that we observed was not statistically significant and much smaller than the 9 mmHg reduction in SBP that has been previously reported for this specific GSE(Reference Sivaprakasapillai, Edirisinghe and Randolph22). Because we anticipated a larger BP-lowering effect based on this previous finding(Reference Sivaprakasapillai, Edirisinghe and Randolph22), the present study was not sufficiently powered to pick up more modest effects. Nevertheless, a 3 mmHg reduction in SBP would be clinically meaningful when considered at the population level, particularly in view of the large population of people with pre- and stage I hypertension. Further studies should therefore confirm or reject more modest effects of GSE on BP.
The composition of the GSE used in the present study is unique because of its high content of low-molecular-weight phenolic compounds, which should theoretically increase bioavailability(Reference Manach, Williamson and Morand26). Unfortunately, the GSE batch used in the present study possibly contained lower levels of monomeric procyanidins (4 %) compared with what has been seen in other batches (5–15 %)(Reference Edirisinghe, Burton-Freeman and Tissa34). We cannot exclude that this may have influenced the efficacy of the GSE used in the present study. The present study was not specifically designed to investigate the bioavailability and metabolism of polyphenol compounds, as this would require multiple blood sampling over the day. Nevertheless, we estimated urinary excretion of polyphenol metabolites over a 24 h sampling period. Up to four times higher levels of urinary phenolic acids were detected in the GSE group v. the placebo group, indicating that at least part of the polyphenol compounds or their metabolites formed in the body's tissues or by the colonic microflora(Reference Scalbert and Williamson36) must have reached the circulation.
To our knowledge, five earlier human studies on GSE and BP have been published(Reference Clifton10, Reference van Mierlo, Zock and van der Knaap18–Reference Sano, Uchida and Saito20, Reference Sivaprakasapillai, Edirisinghe and Randolph22), as summarised by Feringa et al. (Reference Feringa, Laskey and Dickson37). The majority of these studies, applying GSE doses ranging from 150 to 2000 mg/d during 2–12 weeks, have failed to show a significant effect of GSE on BP in various populations (e.g. healthy adults, hypertensives, hypercholesterolaemics). Although these studies did not find significant effects, average SBP and DBP were in almost all studies slightly lower after a GSE intervention than after a placebo(Reference van Mierlo, Zock and van der Knaap18–Reference Sano, Uchida and Saito20), which is in agreement with the present results. Only Sivaprakasapillai et al. (Reference Sivaprakasapillai, Edirisinghe and Randolph22) reported a large significant reduction in daytime SBP of about 9 mmHg v. placebo in metabolic syndrome patients after 4 weeks of intervention with 150 and 300 mg/d of GSE. In this study and in the present study, the same GSE was used as the active ingredient. The discrepancy in findings between the studies may be explained by differences in study populations. First, subjects in the present study were presumably healthier than the metabolic syndrome patients included in the study by Sivaprakasapillai et al. (Reference Sivaprakasapillai, Edirisinghe and Randolph22). Second, the total polyphenol intake from the background diet was possibly higher in our Dutch population compared with their US population, which may have attenuated polyphenol-induced effects on BP in the present study.
The main regulators of BP, such as the renin–angiotensin–aldosterone system and the concentrations of cortisol and catecholamines, follow certain circadian rhythms(Reference Gordon, Wolfe and Island38, Reference Linsell, Lightman and Mullen39). Therefore, the timings of administration of a substance acting on this pathway may be important to elicit a response on BP(Reference Snoep, Hovens and Pasha40). The once-daily administration of the test products in the present study might therefore have affected the outcomes of the study. We did not measure BP during night-time and could thus potentially have missed a nocturnal effect as reported earlier with a high dose of polyphenol-rich grape juice consumed during breakfast and dinner(Reference Dohadwala, Hamburg and Holbrook23). We cannot exclude that GSE at higher intake levels or administered in multiple doses throughout the day would have resulted in more pronounced BP effects.
Several mechanisms by which GSE could affect BP have been suggested. In one study in rats, it has been demonstrated that red wine extract (Provinols) lowered BP via an NO synthesis-dependent pathway(Reference Ralay, Diebolt and Andriantsitohaina41). Also, in vitro studies have indicated the effects of GSE on NO availability(Reference Edirisinghe, Burton-Freeman and Tissa34, Reference Feng, Wei and Hong42). NO is released in response to a variety of chemical and physical stimuli and causes the smooth muscle in the vessel wall to relax. In addition, cellular studies with wine polyphenols have indicated that these compounds may inhibit the generation of ET-1(Reference Khan, Lees and Douthwaite43), which is a potent vasoconstrictor(Reference Yanagisawa, Kurihara and Kimura28, Reference Luscher and Barton44). These findings are in line with a human study showing that specific polyphenols can reduce ET-1 concentrations and increase NO status(Reference Loke, Hodgson and Proudfoot45). Additionally, reduction of plasma levels of ADMA may improve vessel wall function and lower BP by increasing NO bioavailability(Reference Vallance, Leone and Calver31). Degradation of ADMA occurs via dimethylarginine dimethylaminohydrolase, an enzyme that converts ADMA into citrulline and dimethylarginine. Polyphenol-rich products may decrease ADMA levels and increase dimethylarginine dimethylaminohydrolase activity(Reference Tang, Hu and Chen46, Reference Xiao, Jun and Lu47). In the present study, plasma NO metabolites, ET-1 and ADMA were unaffected by the GSE treatment, which would not support that these markers can explain a possible relationship between GSE and BP. However, in view of the only modest and non-significant difference in BP, the present study was probably not suited to demonstrate small changes in these markers.
At least two studies(Reference Freedman, Parker and Li15, Reference Keevil, Osman and Reed16) have shown an inhibition of platelet aggregation after consumption of grape products (5–7·5 ml purple grape juice/kg body weight per d with approximately 2·26 g/l gallic acid equivalents) in healthy subjects. In the present study, inhibition of platelet aggregation could not be demonstrated, except for a small increase in the number of free platelets after stimulation by HEPES. HEPES resembles the physiologically relevant effect of shear stress not related to the tested agonists. However, the observed difference between the GSE and placebo groups was small and therefore physiological relevance is unclear. The lack of a clear effect may be partly due to the smaller dose of polyphenols used in the present study than the dose used in the two earlier studies. Future larger-sized studies should preferably test higher doses of GSE.
In summary, the present study shows that GSE rich in polyphenols has no major BP-lowering effect in middle-aged males and females with pre- and stage I hypertension. If anything, the effect is smaller than reported earlier. However, because even modest BP reductions of the order of 2 mmHg could have a significant impact on CVD at the population level(Reference Lewington, Clarke and Qizilbash2), future larger-scale studies are warranted to further investigate the BP-lowering potential of grape seed polyphenols. These studies should preferably include subjects at an increased risk of CVD (e.g. metabolic syndrome patients) and measure BP over a 24 h period. Also, we recommend testing higher polyphenol doses with relatively more monomeric procyanidins, consumed twice per d.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S000711451300161X
Acknowledgements
We would like to acknowledge Wieneke Koppenol for her contribution to the conduct of the study, Arne Jol and Michiel Gribnau for performing the statistics, Young de Graaf for the analysis of platelet aggregation and ADMA and Ferdi van Dorsten and Sonja Peters for the analysis of urinary polyphenols.
R. T. R., Y. E. M. P. Z., D. J. W. and R. D. designed the research. R. T. R. and Y. E. M. P. Z. conducted the research. N. R. J. analysed the urine and plasma samples. All authors wrote the paper. R. T. R., P. L. Z. and R. D. had primary responsibility for the final content.
The present study was conducted and funded by Unilever R&D Vlaardingen, The Netherlands. R. T. R., P. L. Z. and R. D. are employed by Unilever R&D Vlaardingen. Y. E. M. P. Z. was employed by Unilever R&D Vlaardingen at the time of the study conduct. Unilever markets food products, some of which address cardiovascular risk factors. D. J. W. and N. R. J. had no conflicts of interest.