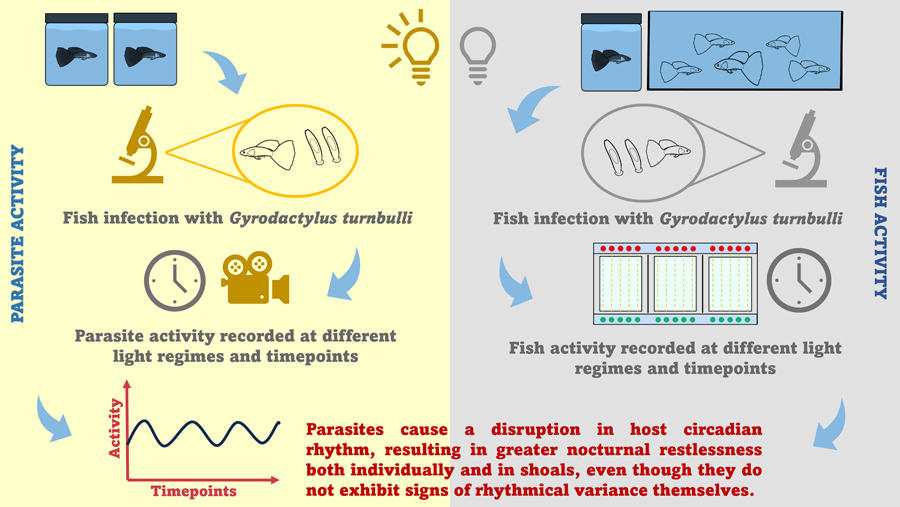
Introduction
Circadian rhythms are intrinsic timekeeping mechanisms responsible for the cyclic repetition of metabolic, behavioural and psychological processes in all living organisms, typically over a 24-h period (Liang et al., Reference Liang, Bushman and FitzGerald2015; Sollars and Pickard, Reference Sollars and Pickard2015). They are endogenously generated by self-sustaining biological clocks, encoded by ‘clock genes’, and entrained by environmental cues such as light and temperature (Piggins, Reference Piggins2002). Their disruption can affect an array of biological activities such as rest–activity cycles, immunity and disease susceptibility (Bass and Lazar, Reference Bass and Lazar2016), as shown in humans if natural circadian cues are ignored due to shift work, jet-lag and general sleep deprivation (Takahashi et al., Reference Takahashi, Lin and Sakai2008).
Sleep is a complex enigma that serves multiple functions (Krueger et al., Reference Krueger, Frank, Wisor and Roy2016), most notably provisioning critical restorative and repair processes (Adam, Reference Adam1980; Benington and Craig Heller, Reference Benington and Craig Heller1995; Cirelli and Tononi, Reference Cirelli and Tononi2008; Helvig et al., Reference Helvig, Wade and Hunter-Eades2016). The general assumption that all species ‘sleep’ is controversial, with some animals entering a restful state that does not necessarily fulfil descriptors depicting true sleep (Siegel, Reference Siegel2008). Recent evidence of true sleep (including Rapid Eye Movement sleep phase; REM), however, has now been reported in zebrafish (Leung et al., Reference Leung, Wang, Madelaine, Skariah, Kawakami, Deisseroth, Urban and Mourrain2019). Furthermore, a consistent observation across taxa is that disturbances to ‘rest–activity’ cycles, and thus disruption of circadian rhythms, can have detrimental consequences for health with respect to disease, even reducing life expectancy (Kripke et al., Reference Kripke, Garfinkel, Wingard, Klauber and Marler2002; Davidson, Reference Davidson2006).
In fish, circadian rhythms govern biological activities ranging from reproduction, spawning, smoltification and maturation to immune responses. Circadian rhythms have been observed in activity patterns of various fish of economic importance including the golden shiner (Notemigonus crysoleucas), goldfish (Carassius auratus), lake chub (Couesius plumbeus), Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss) (see Reebs, Reference Reebs2002). In aquaculture, manipulating photoperiods, such as extending the light period in diurnal species, can improve rearing quality and promote increased growth rates (Boeuf and Le Bail, Reference Boeuf and Le Bail1999). In the extreme, constant light is used to improve feed utilization (Boeuf and Le Bail, Reference Boeuf and Le Bail1999) or control maturation and puberty (Taranger et al., Reference Taranger, Carrillo, Schulz, Fontaine, Zanuy, Felip, Weltzien, Dufour, Karlsen, Norberg and Andersson2010). However, this may have negative implications for health and disease resistance, as immune functions are often highly rhythmic, enabling organisms to mount their most efficient response at times when risk of infection or injury is highest (Ellison et al., Reference Ellison, Wilcockson and Cable2021). Conversely, immune factors and infections can affect expression of molecular clocks (Castanon-Cervantes et al., Reference Castanon-Cervantes, Wu, Ehlen, Paul, Gamble, Johnson, Besing, Menaker, Gewirtz and Davidson2010; Adams et al., Reference Adams, Castanon-Cervantes, Evans and Davidson2013). So, disruption of normal circadian cycles can impact immune responses and may increase disease risks (Ellison et al., Reference Ellison, Wilcockson and Cable2021). Given the increased understanding of the intricate link between fish body clocks and their immune systems, harnessing knowledge of circadian rhythms may be hugely beneficial against infectious diseases. However, for chronobiological approaches to tackle infectious diseases, rhythms of both the fish and their associated parasites must be considered.
Parasites can directly impact host rest–activity cycles (Ibarra-Coronado et al., Reference Ibarra-Coronado, Pantaleón-Martínez, Velazquéz-Moctezuma, Prospéro-García, Méndez-Díaz, Pérez-Tapia, Pavón and Morales-Montor2015), which are associated with activation of immune defences (Preston et al., Reference Preston, Capellini, McNamara, Barton and Nunn2009). Moreover, individuals are most at risk of acquiring parasitic infections when sleep deprived (Bryant et al., Reference Bryant, Trinder and Curtis2004; Majde and Krueger, Reference Majde and Krueger2005). Thus, the reciprocal interaction between rest–activity cycles and immune functioning is complex (Opp, Reference Opp2009). The underlying mechanism appears to be stress-related, which in turn affects the immune system, causing increased susceptibility to infection and subsequently higher mortality rates (Penev et al., Reference Penev, Kolker, Zee and Turek1998; Davidson, Reference Davidson2006). In fish, immune responses to infectious diseases have been extensively studied in the past. Now, increasingly more studies investigate the disruption of fish circadian rhythms by parasites, as in the case of zebrafish (Danio rerio), where established Pseudoloma neurophilia infections induced major transcriptional changes in the host brain (Midttun et al., Reference Midttun, Vindas, Whatmore, Øverli and Johansen2020). However, little is known about how parasites might alter fish resting periods and the long-term implications of disrupted circadian rhythms.
Parasite circadian rhythms are critical in the co-evolution of host–parasite systems, as synchronization of their rhythms can influence infection dynamics and transmission potential (O'Donnell et al., Reference O'Donnell, Schneider, McWatters and Reece2011). Parasite circadian rhythms are apparent in oviposition (Schistosoma haematobium see Theron and Combes, Reference Theron and Combes1995; Passalarus ambiguous see Rinaldi et al., Reference Rinaldi, Russo, Schioppi, Pennacchio and Cringoli2007), timing of asexual reproduction (Plasmodium chabaudi see Mideo et al., Reference Mideo, Reece, Smith and Metcalf2013) as well as expression of certain metabolism genes (Trypanosoma brucei see Rijo-Ferreira et al., Reference Rijo-Ferreira, Pinto-Neves, Barbosa-Morais, Takahashi and Figueiredo2017). Circadian rhythms have also been implicated in detachment of parasites from their host (Doube, Reference Doube1975), as well as host immune evasion by secretion of chemical signals (DuRant et al., Reference DuRant, Hopkins, Davis and Romero2015). For monogenean ectoparasites, rhythmical variance has been observed in egg laying and hatching (Discocotyle sagittata see Gannicott and Tinsley, Reference Gannicott and Tinsley1997; Entobdella soleae see Kearn, Reference Kearn1967; Benedenia ludjani see Ernst and Whittington, Reference Ernst and Whittington1996). With circadian rhythms seemingly affecting various aspects of a parasite's life cycle, the impact of circadian rhythms on infection potential and dynamics needs to be further explored.
One of the most ubiquitous groups of monogenean parasites infecting teleost fish are the gyrodactylids. These parasites are known to infect multiple fish of aquacultural importance, including cyprinids (Zietara and Lumme, Reference Zietara and Lumme2002) and salmonids (Harris et al., Reference Harris, Shinn, Cable and Bakke2004), and can have a major economic impact on the industry. Little is known regarding daily activity rhythms of gyrodactylids, such as movement on the host and host-seeking behaviour, with the exception of 1 study which reported greater variation of in vitro parasite activity in dark compared to light conditions (Brooker et al., Reference Brooker, Grano Maldonado, Irving, Bron, Longshaw and Shinn2011). From a host perspective, sticklebacks (Gasterosteus aculeatus) were more susceptible to Gyrodactylus gasterostei when exposed to prolonged photoperiods; due to changes in host physiology, condition and immune responses (Whiting et al., Reference Whiting, Mahmud, Bradley and MacColl2020). However, whether gyrodactylids exhibit a light-dependant behaviour or parasite activity has true circadian rhythmicity has yet to be studied.
The current study investigates (a) the impact of an ectoparasitic infection on host rest–activity cycles, and (b) the rhythmical variance in parasite activity and behaviour. For this study, we used the tropical Trinidadian guppy (Poecilia reticulata)–Gyrodactylus turnbulli model; a system that has been subject to extensive epidemiological and behavioural investigations (Bakke et al., Reference Bakke, Cable and Harris2007). Although this parasite has been known to cause behavioural modifications in its typically diurnal host (e.g. foraging and swimming performance; Cable et al., Reference Cable, Scott, Tinsley and Harris2002; Kolluru et al., Reference Kolluru, Grether, Dunlop and South2009), the daily dynamics of guppy-gyrodactylid interactions have, until now, been overlooked. Therefore, we are exploring the behaviour of infected hosts compared to their uninfected conspecifics both when isolated and in shoals and we discuss the implications that this may have for host health and aquaculture in general.
Materials and methods
Host and parasite origins and maintenance
Trinidadian guppies (P. reticulata) originating from the Lower Aripo River, Trinidad (wild-type strain), or from a commercial wholesaler (ornamental strain) were transported to Cardiff University Aquarium. Fish stocks were housed separate in 70 L tanks of dechlorinated water (approx. 1 fish/1 L water, as recommended by OATA; Ornamental Aquatic Trade Association), in 24 ± 1°C in a 12:12 h light: dark regime and fed daily with Aquarian® tropical fish flakes supplemented with live Daphnia magna and freshly hatched Artemia nauplii. For all experiments, female or juvenile guppies were used and size-matched to avoid size variability, which is known to affect parasite load (Cable and van Oosterhout, Reference Cable and van Oosterhout2007). For each experiment, only 1 fish stock and single sex fish were used, to avoid confounding variables.
For experimental infections, the Gt3 strain of G. turnbulli was used; isolated in 1997 from, and subsequently maintained on ornamental guppies (as in Stewart et al., Reference Stewart, Jackson, Barber, Eizaguirre, Paterson, van West, Williams and Cable2017). For all experimental infections, a sacrificed donor was placed close to a recipient fish anaesthetized with 0.2% tricaine methanesulfonate (MS222). Direct contact between hosts facilitated transfer of gyrodactylids, as observed under a dissecting microscope with fibre optic illumination. Fish were infected with 30 parasites each, representative of burdens reached after 5 days for an individually isolated fish experimentally infected with 2 worms on Day 0 (e.g. Van Oosterhout et al., Reference Van Oosterhout, Harris and Cable2003).
Experimental design
Overall, 4 experiments were performed: 2 compared the rhythmical activity of the guppy host when uninfected and infected with G. turnbulli and 2 explored the rhythmical variance in activity of the actual parasite. For all experiments, we report the Zeitgeber Time (ZT) system, where ZT is a unit of time based on light Zeitgeber. The ZT denotes when the lights go on and off, in this case, ZT0 denotes lights on and ZT12 lights off (Karatsoreos and Silver, Reference Karatsoreos and Silver2017). There was no light fade to simulate sunrise/sunset conditions. For nocturnal observations, infrared lights (light intensity 1.2–1.3 Lux; Precision Gold Digital Light Meter) were used compared to the white light (500 Lux intensity) used during the day, as infrared illumination is invisible to the animals being observed but visible to infrared cameras (Widder et al., Reference Widder, Robison, Reisenbichler and Haddock2005).
For all experiments, uninfected control fish were sham infected to account for handling time and then returned to 1 L dechlorinated water pots to recover. No anaesthetic associated mortalities occurred during this study and the anaesthesia process, with a 0.02% MS222 dose, seemed to have no effect on host and parasite behaviour and survival (Chambel et al., Reference Chambel, Pinho, Sousa, Ferreira, Baptista, Severiano, Mendes and Pedrosa2015). Following infection (and sham infection), fish were transferred to the experimental tank in a small glass dish containing dechlorinated water, ensuring the fish was never out of water nor was there any risk of nets dislodging the ectoparasites. Once all experimental trials concluded, infected fish were treated with an anti-helminthic drug, 0.1% Levamisole, to eliminate any parasites and then screened clear under the microscope 3 consecutive times to ensure that they were parasite-free (Schelkle et al., Reference Schelkle, Shinn, Peeler and Cable2009).
Automated monitoring of host behaviour
Behavioural arrays used in experiment 1 for monitoring infected and uninfected individual fish consisted of 3 acrylic tanks (22 cm length × 10 cm width × 20 cm depth; Fig. 1), positioned within 2 rows of TriKinetics behavioural monitors. Each behavioural array tank was filled with 1.25 L of dechlorinated water and white card paper on each side of the tanks avoided any external disturbances to the fish. Ten infrared beams passed through each tank, 5 from the top monitor and 5 from the bottom monitor, from the infrared emitters to the receivers. The monitors were connected to the TriKinetics software, which automatically recorded how many times a fish passed through a beam within a certain time period.

Figure 1. 2D schematic showing the set-up of the behavioural arrays for experiment 1. (a). Birds eye view of the behavioural arrays with 2 rows of 5 infrared beams (yellow dotted line) going through each fish tank from the light emitters (green) to the light receivers (red). (b). Side view of the behavioural arrays with 2 rows of monitors outside of each tank with the light emitters going through the tank to the receivers on the other side, with light emitters and receivers alternating between rows. The water level is indicated (blue dotted line) along with the paper dividers between the tanks (black line).
Experiment 1: impact of infection on daily activity of isolated guppies
To observe whether there is a difference in activity between uninfected and infected isolated wild-type guppies under a 12:12 h light: dark regime, female adult guppies were size-matched (15.68 ± 0.95 mm) and 2 experimental groups were created: uninfected controls (n = 11) and infected experimental fish (n = 10). Fish remained in individual 1 L containers for 7 days prior to start of the experiment. On Day 1, experimental guppies were infected with exactly 30 gyrodactylids, whilst control fish were sham infected to control for handling time. Each fish was then placed into a 1 L dechlorinated pot to recover, before being transferred to a behavioural array tank for acclimation. At 07:00, the following day (Day 2), the arrays started monitoring guppy activity every minute for 48 h. On Day 4, fish were removed from the tanks, anaesthetized and screen under the microscope. The experimental fish were screened to record their final parasite load (mean intensity 73, range 49–93) and the control fish were screened in order to ensure that no contamination had occurred, with control fish indeed remaining parasite free. Fish activity was recorded as the counts of infrared beam breaks per tank, as retrieved from the TriKinetics software and investigated hourly from Day 1 (08:00). As the arrays monitored guppy activity every minute, recordings were then averaged per hour, to follow the ZT system.
Experiment 2: impact of infection on daily activity of guppy shoals
To observe whether there is a difference in activity between uninfected and infected wild-type guppy shoals under a 12:12 h light: dark regime, female adult guppies were size matched (13.21 ± 0.67 mm) into shoals of 5 individuals (n = 16 groups). Each shoal was housed in 6 L familiarization tanks for a minimum of 12 days (Griffiths and Magurran, Reference Griffiths and Magurran1997) prior to trials. On Day 1 of the experiment, each familiarized shoal was transferred to a test arena (150 cm length × 20 cm width × 16 cm depth) for a 24 h acclimation period. At 08:00 the following day (Day 2), fish were removed from the arena, and 1 guppy was anaesthetized and infected with 30 gyrodactylids, whilst the remaining 4 fish in each shoal were sham infected to account for handling time. Fish were placed in individual 1 L pots for 30 min recovery time, whilst remaining in visual contact to one another. On Day 3, an observer (partially hidden by a screen) recorded the proportion of time (sec) an infected and a randomly selected uninfected fish spent actively swimming during a 5-min focal follow over 5 time points; 3 diurnal (ZT1: 08:00, ZT6: 13:00 and ZT11: 18:00 h) and 2 nocturnal (ZT15: 22:00 and ZT18: 01:00 h). Fish were deemed actively swimming when propelling themselves forward. After the 5-min focal follow, both fish were screened to account for any parasites transfer. Data collected from the uninfected individuals were used as a control.
Experiment 3: impact of photoperiod on parasite daily activity
To identify whether there is rhythmical variance in parasite activity under the 2 light regimes (12:12 h light: dark and 24 h constant darkness; constant darkness often used a ‘free-running’ condition – a test of endogenous circadian rhythms; Brown et al., Reference Brown, Quan and Eichling2011), we monitored the host-seeking motion of the parasite (number of probes), which is part of their exploratory behaviour (Bakke et al., Reference Bakke, Cable and Harris2007). For both light conditions (12:12 h light: dark and 24 h darkness), wild-type juvenile guppies (n = 60 for each experiment) were size-matched (10.75 ± 0.40/11.10 ± 0.9 mm) and each fish infected with 2 gyrodactylids, before being placed individually in 1 L dechlorinated water pots. After an acclimation period of 7 days, during which parasite number on each host increased naturally, in a 12:12 h light: dark regime, fish were anaesthetized and parasite activity recorded for a 2-min period under a dissecting microscope, using a Longse standard box camera. The activity of 3 randomly selected parasites on the fins of each fish was analysed. For the first condition, parasite activity was recorded both in light and dark depending on the ZT point, whereas for the second condition at ZT0 the light remained off, so all recordings took place in constant darkness with infrared light. Once recordings concluded, the host parasite load was also recorded. For these observations, timepoints monitored were ZT0 (07:00 h), ZT4 (11:00 h), ZT8 (15:00 h), ZT12 (19:00 h), ZT16 (23:00 h) and ZT20 (03:00 h).
Experiment 4: impact of photoperiod on parasite transmissibility
To examine whether daily variation in parasite activity affected their transmissibility to a new host, ornamental female adult guppies (n = 120) were size-matched (12.94 ± 1.3 mm) into dyads. One guppy from each dyad (n = 60) was infected with 2 gyrodactylids and all guppies were placed individually in 1 L pots. After an acclimation period of 7 days in a 12:12 h light: dark regime, infected individuals were screened to determine their parasite load. Then, both infected and uninfected guppies from each dyad were placed together in 25 mL of dechlorinated water for 1 h, resulting in 10 dyads at each of the following time points: ZT0 (07:00 h), ZT4 (11:00 h), ZT8 (15:00 h), ZT12 (19:00 h), ZT16 (23:00 h) and ZT20 (03:00 h). After 1 h, fish were separated, anaesthetized and screened to record how many parasites had transferred from the donor to the recipient or how many parasites had been dislodged.
Statistical analysis
All statistical analyses were conducted using the R statistical software (version 4.1.1, R Core Team, 2019). To analyse the data, the following packages were used: ‘lme4’ to run Generalized Linear Mixed Models (GLMMs) (Bates et al., Reference Bates, Mächler, Bolker and Walker2015), ‘emmeans’ for post hoc analyses (Searle et al., Reference Searle, Speed and Milliken1980), ‘ggplot2’ to visualize data (Wickham, Reference Wickham2009) and ‘circacompare’ to compare rhythms (Parsons et al., Reference Parsons, Parsons, Garner, Oster and Rawashdeh2020). The ‘circacompare’ package was used to compare rhythms between different conditions by assessing MESOR, amplitude and acrophase across rhythms. MESOR (Midline Estimating Statistic of Rhythm) refers to the rhythm-adjusted mean level of a response variable around which a wave function oscillates, so the mean activity level over a certain period of time. Amplitude is a measure of half the extent of predictable variation within a cycle, so the activity variation from the MESOR, which is the mean, to the peak of activity. Acrophase refers to the time at which the response variable peaks; the time that it takes to go from MESOR to Amplitude (Otsuka et al., Reference Otsuka, Cornelissen, Furukawa, Kubo, Hayashi, Shibata, Mizuno, Aiba, Ohshima and Mukai2016; Parsons et al., Reference Parsons, Parsons, Garner, Oster and Rawashdeh2020; Fig. 2).

Figure 2. Variables assessed by the ‘circacompare’ package in each rhythm and then compared between rhythms (include Mesor, the rhythm-adjusted mean level; amplitude, half the extend of predictable variation; and acrophase the time the response variable peaks).
For experiment 1, a GLMM fitted with ‘binomial error’ family and ‘logit’ link function assessed activity (count of infrared beam breaks) of infected and uninfected isolated guppies in light and dark conditions. Guppy activity was the dependent term in the model, and fixed effects were infection status (infected or uninfected) and light condition (light or dark). Fish number was included as a random term to account for repeated measures. Additionally, the ‘circacompare’ package was used to investigate and compare the rhythms of infected and uninfected individuals in 12:12 h light: dark regime over a 48 h period. For experiment 2, 1 GLMM, fitted with ‘binomial error’ family and ‘logit’ link function, was used to assess diel activity patterns of infected and uninfected guppies. In the GLMM, the proportion of time fish remained actively swimming during a 5-min period was the dependent term in the model, and fixed effects included infection status (infected or uninfected) and ZT as well as an interaction term between infection status and ZT, and the shoal number was included as a random term to account for repeated measures. For experiment 3, a Generalized Linear Model (GLM) was used to compare parasite activity (number of probes) with respect to different ZT and light conditions. An interaction term between ZT and light conditions was incorporated into the model. Moreover, the ‘circacompare’ package was used to investigate and compare rhythms of parasite activity in different light conditions. For experiment 4, 2 GLMs, fitted with ‘binomial error’ family and ‘logit’ link function assessed the proportion of parasites transmitting from an infected host to its uninfected conspecific and proportion of parasites that had been dislodged with respect to ZT, light conditions and parasite density on the host. In all tests, the level of significance was taken as P < 0.05. GLMM models were refined through stepwise deletion of non-significant terms and AIC comparisons and their robustness was assessed using residual plots, indicating that assumptions of models were met (Pinheiro and Bates, Reference Pinheiro and Bates2000). Mean standard length was not included within models, as fish were size-matched at the start of each experiment to eliminate size variability.
Results
Overall, the circadian rhythm detected in guppy activity was disturbed by infection, resulting in increased activity at night, thus nocturnal restlessness both in isolated guppies and in shoals. Even though gyrodactylid behaviour and activity did not exhibit diurnal variance, parasite activity peaked at night, coinciding with the increase in host activity.
Experiment 1: impact of infection on daily activity of isolated guppies
For both uninfected (control) and infected guppies there was a significant difference in activity between light and dark conditions (emmeans; df = 1; P < 0.0001 and P < 0.0001 respectively; Fig. 3a), with both uninfected and infected fish having significantly higher activity in the light comparing to the dark conditions. In light conditions, uninfected guppies were significantly more active than infected guppies (emmeans; df = 1; P = 0.0005), whilst the opposite was observed in dark conditions, whereby uninfected guppies were less active than their infected conspecifics (emmeans; df = 1; P = 0.036; Fig. 3a). The ‘circacompare’ package confirmed the presence of circadian rhythmicity in activity of both the uninfected (P = 0.006) and infected group (P = 0.0008; Fig. 3b). The 2 rhythms had a significant difference in MESOR (P = 0.0008), with the uninfected group having a greater rhythm-adjusted mean than infected group, in acrophase (P = 0.0004) with the uninfected group having an earlier peak and a significantly higher amplitude, which is the half of the predictable variation in activity throughout the 48 h period (P���= 0.0002; Fig. 3b).

Figure 3. (a). Activity of isolated guppies from uninfected and Gyrodactylus turnbulli infected fish in light and dark conditions. In the light, the uninfected guppies were significantly more active than the infected fish and in the dark uninfected guppies were significantly less active than their infected conspecifics. Fish activity is defined as the number of times fish went through the infrared beams per tank, as retrieved from the TriKinetics software. Dots represent outliers; the box the first and third quartile with median and the line 50% of fish activity. (b). The activity of uninfected and infected guppies monitored hourly for a 47 h period. Grey areas indicate dark periods. Error bars represent standard error.
Experiment 2: impact of infection on daily activity in guppy shoals
Swimming activity of uninfected guppies was elevated during the day and dropped drastically at night. When guppies were infected, however, they exhibited nocturnal restlessness with increased swimming activity, indicating that infection status had a significant effect on swimming activity of guppies when in shoals, which also depended on ZT (ZT × Infection status interaction; GLMM; P < 0.001). When studying shoal swimming activity at specific ZT timepoints, uninfected guppies were significantly less active than infected conspecifics at each timepoint (GLMM; df = 4; P < 0.001), evidently more so during nocturnal hours where there is a great difference in activity of uninfected and infected shoaling guppies (ZT15, ZT18; Fig. 4).

Figure 4. The proportion of time Gyrodactylus turnbulli infected and uninfected guppies remained active during 5-min focal follows at 5 ZT timepoints. Grey areas indicate dark periods. Black dots represent outliers; bars the upper and lower limits; the box the first and third quartile with median, and the dashed line 50% of the time in which guppies remained active during a focal follow.
Experiment 3: impact of photoperiod on parasite daily activity
Light conditions and ZT timepoint both had a significant effect on parasite activity (GLM; P = 0.007 and P < 0.001 respectively) as well as their interaction (Light conditions × ZT timepoints; GLM; P < 0.001). Overall, parasites were more active in the dark compared to light conditions under the 12:12 h light: dark regime (GLM; df = 1; P = 0.0004; Fig. 5a). When comparing parasite activity between the 12:12 h light: dark regime and constant darkness (Fig. 5b), there was a significant difference in ZT0 (emmeans; df = 1; P < 0.0001), ZT4 (emmeans; df = 1; P < 0.0001), ZT8 (emmeans; df = 1; P = 0.015), ZT12 (emmeans; df = 1; P = 0.004), ZT16 (emmeans; df = 1; P = 0.009) but not ZT20 (emmeans; df = 1; P = 0.342). The ‘circacompare’ package, however, did not detect a circadian rhythm in parasite activity either in 12:12 h light: dark or 48 h of darkness regime, suggesting that it is not endogenously driven, but affected by other cues (Fig. 5b).

Figure 5. (a). Activity (number of probes) of Gyrodactylus turnbulli parasites present on their guppy host in light and dark conditions. The box represents the first and third quartile with median. (b). Parasite activity recorded both in 12:12 h light: dark regime (LD) and 48 h constant darkness (DD). There was significant difference in activity at ZT0, ZT4, ZT8, ZT12 and ZT16. However, there was no rhythmicity detected in either case. Grey areas indicate dark periods. Error bars represent standard error.

Figure 6. Proportion of parasites that transferred from the host to the recipient conspecific at different ZT points in a 12:12 h light: dark regime with no significant difference recorded. Grey areas indicate dark periods. Error bars represent standard error.
Experiment 4: impact of photoperiod on parasite daily transmissibility
The proportion of parasites that transferred from an infected host to an uninfected conspecific (GLMM; df = 5; P > 0.05) or the proportion of parasites that dislodged from their host (GLMM; df = 5; P > 0.05) were not significantly different between ZT timepoints, in light vs dark conditions or dependent on parasite density of the host. Also, the ‘circacompare’ package did not detect a rhythm in parasite transmissibility in the 12:12 h light: dark regime (P > 0.05) with no significant difference detected in MESOR, amplitude and phase.
Discussion
Here, we provide the first empirical evidence of aquatic ectoparasites directly altering ‘rest–activity’ cycles of diurnal fish hosts. Using the guppy-gyrodactylid system, we showed infection changes the daily rhythms of guppy activity; infected individuals were more active at night than their uninfected conspecifics, with nocturnal restlessness exhibited both in isolated and guppy shoals. Although gyrodactylid behaviour (host-searching activity and transmissibility) did not exhibit diurnal cycles, parasites did display elevated activity at night (supporting Brooker et al., Reference Brooker, Grano Maldonado, Irving, Bron, Longshaw and Shinn2011). Our results are important because regulated rest–activity cycles are essential for physical and mental wellbeing (Besedovsky et al., Reference Besedovsky, Lange and Born2012) and most notably optimizing efficient immune functioning (Imeri and Opp, Reference Imeri and Opp2009). Sleep deprivation can result in cognitive impairment (Alhola and Polo-Kantola, Reference Alhola and Polo-Kantola2007) and increased disease susceptibility (Cohen et al., Reference Cohen, Doyle, Alper, Janicki-Deverts and Turner2009). Moreover, disease itself induces dramatic sleep alterations, although previously only reported for endoparasite infections (Norman et al., Reference Norman, Chediak, Kiel and Cohn1990; Buguet et al., Reference Buguet, Bert, Tapie, Tabaraud, Doua, Lonsdorfer, Bogui and Dumas1993; Toth, Reference Toth1995).
Ectoparasites likely inflict some degree of physical discomfort to their hosts during establishment on the host skin and throughout infection. In the case of gyrodactylids, they attach to their host primarily using hooks, and following establishment extrude digestive enzymes onto the hosts' skin from which host epidermal cells and mucus are subsequently ingested (Bakke et al., Reference Bakke, Cable and Harris2007). The frequent movement of gyrodactylids across the host's skin, potentially associated with their avoidance of localized host immune responses (Richards and Chubb, Reference Richards and Chubb1996), may irritate the guppy hosts and result in increased host nocturnal activity. In the case of host activity experiments, both when isolated and in shoals, observations started within 24 h of parasite infection, so shortly after host infection. As also shown in other parasite species, the brain-infecting Euhaplorchis californiensis cercariae had an impact on their killifish host (Fundulus parvipinnis) during parasite exposure. Host activity and metabolic rate increased, with metabolic rate remaining elevated while activity returned to normal, suggesting ongoing physiological changes are separate from behavioural effects (Nadler et al., Reference Nadler, Bengston, Eliason, Hassibi, Helland-Riise, Johansen, Kwan, Tresguerres, Turner, Weinersmith and Øverli2021). So, migration of gyrodactylids across the host's skin and subsequent irritation may have been a driver of host nocturnal restlessness.
Whilst not measured here, complex interactions between immune and hormonal modulation activated by infection may have also contributed to nocturnal restlessness. Inflammatory responses to infection, for example, significantly contribute to sleep disturbances (Ali et al., Reference Ali, Choe, Awab, Wagener and Orr2013). In fish, a typical response to ectoparasite infection is epidermal thickening (Esteban et al., Reference Esteban, Castaño, Schindler, Koch, Angerer, Casteleyn, Joas, Joas, Biot, Aerts and Kolossa-Gehring2012; Smallbone et al., Reference Smallbone, van Oosterhout and Cable2016), whereby inflammation at the site of parasite establishment occurs after tegument damage (Lindenstrøm et al., Reference Lindenstrøm, Secombes and Buchmann2004). Inflammatory responses are regulated by pro- and anti-inflammatory cytokines, which promote and inhibit rest, respectively. The production and release of pro-inflammatory cytokines has been associated with the secretion of melatonin (Srinivasan et al., Reference Srinivasan, Maestroni, Cardinali, Esquifino, Perumal and Miller2005): a regulatory hormone essential for enhancing propensity to sleep (Zhdanova et al., Reference Zhdanova, Wang, Leclair and Danilova2001; Cajochen et al., Reference Cajochen, Kräuchi and Wirz-Justice2003). Perturbances in natural oscillations of a protein like melatonin can promote restlessness (Budh et al., Reference Budh, Hultling and Lundeberg2005). So, disease can induce dramatic rest alterations, so far only emphasized in endoparasite infections (Norman et al., Reference Norman, Chediak, Kiel and Cohn1990; Buguet et al., Reference Buguet, Bert, Tapie, Tabaraud, Doua, Lonsdorfer, Bogui and Dumas1993; Toth, Reference Toth1995), leading to a constant state of restlessness. However, previous studies have shown that shortly after infection, effective localized immune responses towards gyrodactylids and infection-related changes in gene expression are exhibited (Lindenstrøm et al., Reference Lindenstrøm, Buchmann and Secombes2003; Bakke et al., Reference Bakke, Cable and Harris2007; Konczal et al., Reference Konczal, Ellison, Phillips, Radwan, Mohammed, Cable and Chadzinska2020). Therefore, these disruptions in immune responses could further promote host restlessness. Overall, there is increasing evidence of complex interactions between molecular clocks and immunity (Ellison et al., Reference Ellison, Wilcockson and Cable2021), as dysregulation of certain host clock proteins linked with cell function, defence and inflammation may lead, among others, to inflammatory diseases and immunodeficiency (Curtis et al., Reference Curtis, Bellet, Sassone-Corsi and O'Neill2014). While clock gene expression drives daily cycles in immunity, immune activation caused by infection can itself alter clock gene expression. Therefore, we suggest the observed changes in daily behaviour patterns could a result of altered clocks.
Regarding parasite activity, even though no ‘true’ circadian rhythmicity in phenotypes was detected, host seeking behaviour and activity were greater in the dark and more specifically at the end of the light period (ZT12), where there was a peak in parasite activity. Interestingly, this elevated host seeking behaviour coincides with natural guppy shoaling behaviour peaking around dusk (Croft et al., Reference Croft, Arrowsmith, Bielby, Skinner, White, Couzin, Magurran, Ramnarine and Krause2003; O'Connor and Krause, Reference O'Connor and Krause2003). Thus, an increase in parasite activity could potentially facilitate transmission between hosts, as fish are closely aggregated during shoaling (Pitcher, Reference Pitcher1983). Elevated host activity may be beneficial to individuals as infected fish move between and directly contact resting conspecifics (Reynolds et al., Reference Reynolds, Hockley, Wilson and Cable2019), potentially diluting their parasite burdens (Mooring and Hart, Reference Mooring and Hart1992). Previous studies demonstrate the benefits of successful parasite transmission in terms of ‘vaccinating’ hosts against subsequent infections (Faria et al., Reference Faria, van Oosterhout and Cable2010), but also reducing resource competition between parasites and allowing short-term evasion of a hosts' immune response (Richards and Chubb, Reference Richards and Chubb1996), concluding that a driver of parasite activity could be host behaviour.
Better understanding of both guppy and gyrodactylid behavioural and activity patterns, provides a greater insight in host–parasite dynamics. Using this knowledge of circadian rhythms may be helpful in tackling infectious diseases, as chronotherapeutic approaches could be used to yield maximum therapeutant efficiency based on host metabolism, when treating for parasites. In aquaculture, parasite infections do not always lead to fish mortality, yet still negatively impact productivity, health and welfare of fish (Shinn et al., Reference Shinn, Pratoomyot, Bron, Paladini, Brooker and Brooker2015), thus extensive use of therapeutics is used to maximize efficiency (Burka et al., Reference Burka, Hammell, Horsberg, Johnson, Rainnie and Speare1997; Grant, Reference Grant2002). However, drug efficacy and toxicity vary with time of day (Bruguerolle, Reference Bruguerolle1998), as daily rhythms in drug absorption, metabolism, detoxification and excretion have been reported in mammalian species (Smolensky and Peppas, Reference Smolensky and Peppas2007). As shown by Vera and Migaud (Reference Vera and Migaud2016).
Atlantic salmon (S. salar) treated with hydrogen peroxide (H2O2) revealed increased sublethal toxic effect during the first half of the day, providing the first evidence of chronotoxicity in Atlantic salmon. Moreover, the impact of photoperiod and infection status on immune gene activation as well as immune expression and rhythmicity was investigated by Ellison et al. (Reference Ellison, Wilcockson and Cable2021), where it was shown that circadian perturbation, that shifts the magnitude and timing of immune activity, is detrimental to fish health. These studies provide evidence for potential optimization of treatment timing in aquaculture, opening the door to treating fish diseases chronotherapeutically. In addition, non-detected infections, which alter fish behaviour such as increased restlessness, could be used as a diagnostic tool for emerging infectious diseases.
In conclusion, we demonstrate that ectoparasites alter daily rhythmic activity of their hosts, resulting in greater nocturnal restlessness both individually and in shoals. Circadian rhythmicity in activity was present and distinctly different between uninfected and infected fish. Peaks in activity may be driven parasite skin irritation as well as immune responses to infection, such as infection resolution and repair, which are elevated at night (Ellison et al., Reference Ellison, Wilcockson and Cable2021) and may have direct implications for other animal behaviour studies that overlook nocturnal activity of diurnal species. We also provide a better understanding of parasite behaviour, that does not exhibit a daily rhythmical variance, but peaks in the dark, coinciding with infected fish behaviour. As gyrodactylids pose a significant threat to many economically important fish in aquaculture (Lafferty et al., Reference Lafferty, Harvell, Conrad, Friedman, Kent, Kuris, Powell, Rondeau and Saksida2015; Shinn et al., Reference Shinn, Pratoomyot, Bron, Paladini, Brooker and Brooker2015), the use and application of chronotherapy to maximize treatment efficacy could be a potential solution to this problem.
Acknowledgements
We thank Emily Shaw, Benjamin Goodman and Sarah Hendry for technical assistance.
Author's contributions
JC conceived the project, designed by all authors. E. A. A. and M. R. conducted all experiments and analysed the data, with significant input from A. R. E. E. A. A. and M. R. drafted the article and all authors reviewed and provided feedback on the manuscript.
Data availability statement
The data that support the findings of this study are openly available in Mendeley at http://doi.org/10.17632/p23ghwjhxj.1 Arapi, Elissavet (2024), “Restless nights when sick: ectoparasite infections alter rest-activity cycles of diurnal fish hosts”, Mendeley Data, V2, doi: 10.17632/p23ghwjhxj.1
Financial support
This research was funded by a Knowledge Economy Skills Scholarship II (KESSII) to E. A. A., supported by European Social Funds (ESF) through the Welsh Government. KESS is a pan-Wales higher level skills initiative led by Bangor University on behalf of the HE sector in Wales.
Competing interests
None.
Ethical standards
All applicable institutional guidelines for the care and use of animals were followed (Kilkenny et al., Reference Kilkenny, Browne, Cuthill, Emerson and Altman2014). Procedures and protocols were conducted under UK Home Office licence (PPL 303424) with approval by the Cardiff University Animal Ethics Committee.