INTRODUCTION
In early 2009, a novel swine origin influenza A (H1N1) virus emerged in North America and Mexico [Reference Perez-Padilla1, 2]. The efficient transmission of this virus and the lack of immunity in most populations enabled it to rapidly spread across the world and necessitated the declaration of a pandemic by the World Health Organization [Reference Fraser3–5].
On 16 May 2009, the first case of pandemic H1N1 influenza was reported in Beijing, China [6]. However, it was not until August 2009, that cases were routinely detected via sentinel hospital surveillance [Reference Yang7]. As of 29 November 2009, 9207 laboratory-confirmed cases of 2009 H1N1 influenza, including 28 deaths, had been reported in Beijing [8].
There are significant differences between 2009 H1N1 virus and seasonal influenza viruses both genetically and antigenically, and 2009 H1N1 virus was never detected in humans or animals before [9]. To date the disease has been of moderate severity, with mild influenza-like illness (ILI) occurring in most patients. While there have been cases of complications leading to hospitalization and mortality, this has occurred mainly in those with underlying disease conditions [9–Reference Echevarría-Zuno12]. Many of those hospitalized with severe illness were suffering from underlying diseases/conditions such as asthma, obstructive airways disease, diabetes, immunodeficiency, pregnancy, chronic renal failure, and morbid obesity [Reference Jain11–Reference Louie17].
Due to the difficulty in distinguishing 2009 H1N1 influenza from seasonal influenza and other respiratory infections, reliance was initially placed on the use of laboratory testing to confirm cases. However, as the outbreak expanded and became widespread, laboratory testing became increasingly impractical and extremely resource-intensive. On 24 July 2009, the United States Centers for Disease Control and Prevention discontinued reporting of individual cases of 2009 H1N1 influenza, but continued to track hospitalizations and deaths [18]. Given that laboratory-confirmed data will be an underestimation of the true number of cases and that influenza and pneumonia syndromic reports are less specific to influenza, a new approach is needed to determine the real infection rate of 2009 H1N1 influenza. As estimating the numbers of infected cases and/or vaccinated persons is not as precise as the laboratory-based methods, it is essential to undertake a population-based serological survey to estimate the immunological level in the population against 2009 H1N1 influenza.
Previous studies have established that: (1) the population infection rate combined with the vaccination coverage rate is closely associated with the immunological level against a disease in a population, and that (2) the immunological level is able to strongly influence the intensity of activity and the circulating trend of pandemic influenza [Reference Ferguson19]. We undertook a cross-sectional serological survey to examine the prevalence of antibody to 2009 H1N1 influenza in residents of Beijing, China between 29 November and 5 December 2009, 1 month after the epidemic peak in Beijing [Reference Yang7].
METHODS
Subjects and survey design
Subjects were recruited from patients visiting six tertiary hospitals in Beijing, which are open to the general population of Beijing. Any patient who required a blood test from the outpatient blood collection rooms in these six hospitals was screened for eligibility by the presiding nurse. All patients, apart from those who had been attending the infectious disease/respiratory departments, were eligible to participate. Patients were excluded from the infectious disease/respiratory departments due to the possibility that the reason for their hospital visit may have been related to 2009 H1N1 influenza. Any member of the public who was donating blood at Beijing Blood Donation Center was also invited to participate in this survey. During the survey period (between 29 November and 5 December 2009), each hospital and Beijing Blood Donation Centre aimed to randomly invite 100 subjects to participate in this survey.
The following information was collected on the subjects: (1) demographics (age, sex); (2) uptake of seasonal and 2009 H1N1 influenza vaccine and (3) history of respiratory symptoms (from September 2009 to present). A blood sample was also collected from each of the subjects for testing the antibody against 2009 H1N1 influenza.
Laboratory testing
All samples were transported within 24 h of collection to the laboratory at the Beijing Center for Disease Prevention and Control (CDC). After centrifuging, sera were obtained, aliquoted, and then frozen at −80°C. The serum of each subject was treated with receptor-destroying enzyme (RDE) of 4 volumes at 37°C for 18 h, and then incubated at 56°C for 30 min. The serum was then absorbed with turkey erythrocytes of equal volume to serum. Six titrations (1:10, 1:20, 1:40, 1:80, 1:160, 1:320) were first prepared for each serum sample to test for specific antibody against 2009 H1N1 virus by haemagglutination inhibition (HI) assay. Four HA units of the antigen of 2009 H1N1 virus [A/California/07/2009 (H1N1; CA07)] were used [20]. The serum samples with 1:320 titre were rediluted into eight titrations (1:10, 1:20, 1:40, 1:80, 1:160, 1:320, 1:640, 1:1280) for the HI assay to test if there was a higher titre for each of these serum samples. Control serum samples were included in all assays, with immunized chicken serum sample against CA07 strain used as positive controls (titre 1:160), and human pooled serum samples from healthy blood donors collected in 2008 used as negative controls. In this assay, 1% turkey erythrocytes were used. The HI titre was calculated as the reciprocal of the highest dilution of serum that was able to inhibit the haemagglutination of the turkey erythrocytes induced by the influenza virus. The ⩾1:40 titre was regarded as seropositive against 2009 H1N1 virus, i.e. the participant was immune against this virus [Reference Olsen21, Reference Chen22].
Statistical analysis
The database was maintained in Microsoft Excel (version 2003, Microsoft Corporation, USA), and analysed using SPSS 11.5 statistical package (SPSS Inc., USA). Frequencies were calculated for categorical variables. The rates of seropositivity of antibody to 2009 H1N1 influenza (HI titre ⩾1:40) were compared between subgroups using χ2 test. Geometric mean titres (GMTs) were compared between subgroups using rank sum analysis. Univariate and multivariate unconditional logistic regression analyses were conducted to determine factors associated with seropositivity in subjects without 2009 H1N1 influenza vaccination. The variables with P<0·10 in univariate analysis were included in multivariate analysis. Backward logistic regression was conducted by removing variables with P>0·10. Statistical significance was defined as P<0·05.
RESULTS
Between 29 November and 5 December 2009, 710 subjects were randomly recruited. The demographic characteristics of the subjects are listed in Table 1.
Table 1. Baseline characteristics of subjects in a serological survey of 2009 H1N1 influenza in Beijing (n=710)
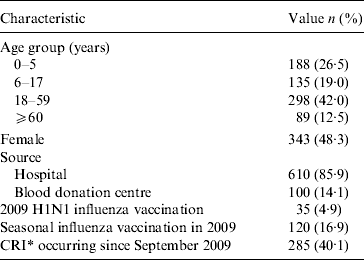
Data are no. (%) of subjects.
* CRI, Clinical respiratory illness, at least two of the following symptoms existing simultaneously: fever, cough, sore throat, nasal congestion, rhinorrhoea.
Forty percent (40·1%, 285/710) of subjects reported having a clinical respiratory illness (CRI, defined as having at least two of the following symptoms simultaneously: fever, cough, sore throat, nasal congestion, rhinorrhoea [Reference Carrat23]) at some point between September 2009 and the time of recruitment.
Of the subjects, only 98 (13·8%) were seropositive to CA07, with a titre of ⩽1:320. Subjects aged 6–17 years recorded the highest seroprevalence rate (17·8%, 24/135), while the lowest rates were recorded for subjects aged ⩾60 years (4·5%, 4/89). The age-weighted seroprevalence of 2009 H1N1 influenza was 14·0% in Beijing, according to the proportions of various age groups in the population of Beijing [Reference Guo and Yin24]. Influenza surveillance conducted in Beijing showed that the epidemic peak of the pandemic occurred in early November 2009, which preceded this serological survey; therefore it can be hypothesized that the seroprevalence at the epidemic peak would be <14%. A significant difference in seroprevalence was found between age groups (P=0·028, Fig. 1 a) but not between gender (P=0·563, Fig. 1 b). Of the 710 subjects, only 35 (4·9%) reported receiving the 2009 H1N1 influenza vaccine. Vaccinated subjects were significantly more likely to be seropositive against 2009 H1N1 influenza (51·4%, 18/35) than unvaccinated subjects (11·9%, 80/675) (P<0·001, Fig. 1 c). Similar differences were also found between: (1) age groups (P<0·001); (2) gender (P=0·278) and (3) vaccination status (P<0·001) using GMTs.

Fig. 1. Reverse cumulative distribution curves of antibody titres against 2009 H1N1 influenza by (a) age group, (b) gender and (c) 2009 H1N1 influenza vaccination. According to the criteria that the titre of ⩾1:40 was regarded as seropositive, there were statistically significant differences in seroprevalence between age groups (P=0·028) and vaccination status (P<0·001), but no difference between gender (P=0·563), by χ2 test. Similar differences were also found between: (1) age groups (P<0·001); (2) gender (P=0·278) and (3) vaccination status (P<0·001) using GMTs (geometric mean titres) by rank sum test. Titres of antibodies against haemagglutination below the lower limit (1:10) were determined at a value of 1:5 for calculating GMT.
Unvaccinated subjects aged 0–5, 6–17 and 18–59 years were significantly more likely to be seropositive than those subjects aged ⩾60 years (0–5 years: OR 5·32, 95% CI 1·22–23·20, P=0·026; 6–17 years: OR 6·26, 95% CI 1·41–27·80, P=0·016; 18–59 years: OR 5·50, 95% CI 1·29–23·53, P=0·021) on both univariate and multivariate analysis. For unvaccinated subjects, reporting a CRI was a significant independent factor associated with being seropositive (OR, 2·55, 95% CI 1·57–4·17, P<0·001) on both univariate and multivariate analysis. Receiving a seasonal influenza vaccine was also significantly associated with being seropositive to 2009 H1N1 influenza on univariate analysis (OR 2·05, 95% CI, 1·20–3·51, P<0·009). However, it did not remain significant on multivariate analysis (Table 2).
Table 2. Univariate and multivariate analysis for factors associated with seropositivity in subjects without 2009 H1N1 influenza vaccination
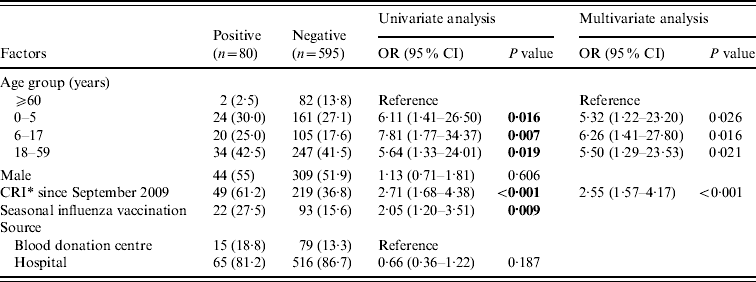
OR, Odds ratio; CI, confidence interval.
Data are no. (%) of subjects, unless otherwise indicated.
Boldface indicates P<0·1, and those variables with P<0·1 in univariate analysis were included in multivariate analysis.
* CRI, clinical respiratory illness, at least two of the following symptoms existing simultaneously: fever, cough, sore throat, nasal congestion, rhinorrhoea.
DISCUSSION
Influenza surveillance in Beijing showed that the epidemic peak of 2009 H1N1 influenza occurred in early November 2009, which preceded the time-frame of this serological survey. Our study showed that in early December 2009 following months of transmission and the availability of the pandemic vaccine, only 14% of the samples had protective antibody to 2009 H1N1 influenza in Beijing. We found a lower seropositivity rate in subjects aged ⩾60 years compared to subjects in the other age groups (0–5, 6–17, 18–59 years).
The seroprevalence obtained in our study was very low, and even for subjects aged ⩾60 years, the seroprevalence was lower than that in sera taken prior to exposure to the pandemic strain in the USA and Europe [4, Reference Miller25, Reference Hancock26]. However, the findings are not unique, as a recent study from Singapore also found that only 13% of their subjects seroconverted following the epidemic wave, and the seroconversion rate recorded in the elderly was lower than that in the young [Reference Chen27]. There are a number of possible reasons that could account for the differences in seroprevalence between these studies. First, the difference may arise because of regional variations in viral spread; second, they may also result from sensitivity issues in the assays used for the studies. Given that the HI assay used in our study had been previously adopted by other studies we feel that this is unlikely to be a contributing reason [4, Reference Miller25, Reference Chen27].
The low seroprevalence of 2009 H1N1 influenza in the Beijing community may be the result of low pre-existing immunity against the pandemic virus. The low pre-existing antibody in the Chinese population has been previously stated in other Chinese studies [Reference Chen22, Reference Zhu28, Reference Liang29] and is supported by the low seroprevalence result in the elderly (4·5%) from our study. It can be assumed that the protective effect of pre-existing antibody in the Chinese population was very limited, and both the young and the elderly in China were equally susceptible to 2009 H1N1 virus when the emerging virus began to spread. In comparison, studies conducted in many Western countries have found high rates of pre-existing antibody in the elderly [4, Reference Miller25, Reference Hancock26]. The difference in pre-existing antibody of the elderly between Western countries and China may be because the elderly population in Western countries had been previously exposed to a virus that was genetically and antigenically similar to the 2009 H1N1 virus, but by contrast, this previous exposure did not affect the Chinese population of similar age [Reference Liang29].
In our study, the seropositivity rate in subjects aged ⩾60 years was much lower than in those subjects in other age groups (0–5, 6–17, 18–59 years). In multivariate analysis (excluding vaccinated subjects), subjects aged ⩾60 years were less likely to be seropositive compared to subjects in other age groups (0–5, 6–17, 18–59 years). In contrast to older generations, the contact mixing patterns of younger age groups are very different. Young people are more likely to gather in large groups, or attend venues where there are likely to be large numbers of people and contacts, e.g. schools, universities, concerts or sports events. This key difference may be the reason why the elderly in this study had a lower infection risk from 2009 H1N1 influenza.
If the basic reproduction number (R 0) of 2009 H1N1 virus was regarded as 1·4 [Reference Fraser3], it could be assumed that the epidemic would peak at a point where 30% of the population were infected with 2009 H1N1 virus. However, as demonstrated by the surveillance data this was not the case, as the epidemic peak occurred at a time when the rate of infection and vaccination was considerably less than 30%. This finding suggests that factors, other than natural infection and vaccination, may have had an important role in containing the epidemic of 2009 H1N1 influenza in Beijing. Possible factors include: (1) the treatment and isolation of cases; (2) the medical observation and prophylactic treatment of the close contacts; (3) social distance measures; (4) good personal hygiene habits, and (5) health education programmes for the population.
In China, a vaccine against 2009 H1N1 influenza (monovalent, non-adjuvanted split-virus vaccine) was approved to be used in persons aged ⩾3 years. The H1N1 vaccination campaign in Beijing began on 20 October 2009. Prior to 16 November 2009, the vaccine was only provided to the following groups: school students, healthcare workers, public service workers, employees in critical public organizations and persons aged ⩾60 years. After that date, the vaccine was available to all citizens in Beijing.
Just over half of the subjects, who had been vaccinated against 2009 H1N1 influenza in our study, had positive antibody to this virus. This rate is lower than the proportion reported in previous studies [Reference Zhu28–Reference Greenberg30]. Given the time period that our study was conducted in and the date that the vaccine was made generally available, we assumed that the interval between the vaccination and the sampling date for most of the vaccinated subjects was too short to elicit an immune response. Another serological study we conducted in Beijing in mid-January 2010 (L. Tian et al. unpublished observations), showed that over 60% of the vaccinated subjects (who were enrolled 28 days post-vaccination) had a positive antibody. This low prevalence brings into question doubts on the efficacy of pandemic vaccine when used in large populations.
From early September 2009 onwards, the number of ILI hospital consultations and the rate of positive 2009 H1N1 influenza cases detected increased sharply in Beijing, according to ILI surveillance and virological surveillance conducted in Beijing [Reference Yang7], corresponding to the rapid spread in the community at that time. This probably explains why CRI became a significant independent factor which was associated with 2009 H1N1 influenza seropositivity in unvaccinated subjects.
In our study, being vaccinated against seasonal influenza in 2009 was not a significant independent factor for being seropositive against 2009 H1N1 influenza. This demonstrates that seasonal influenza vaccination was not able to produce a cross-reactive antibody response to 2009 H1N1 virus. This conclusion was also found in previous studies [4, 31].
Our study has a number of limitations. First, the data presented in this study is based on samples collected 1 month after the epidemic peak in Beijing, China, therefore we are unable to comment on the prevalence of cross-reactive antibody in the community prior to the onset of the pandemic. We are also unable to determine the seroconversion rate in the population. Second, given that most of our subjects were enrolled from hospitals, our study sample may not be representative of the general population of Beijing. However, we feel that this is not a major limitation given that the source of the subjects was not a significant independent factor associated with seropositivity in the subjects. Due to the urgent need for information of this kind, it was more convenient and rapid to collect samples from these sites, than from the general community. We believe that the methods employed in this study were appropriate for providing timely and precise information during a certain period, which can then be fed back to health departments to assist with their preparedness and response.
Our survey suggests that the seroprevalence against 2009 H1N1 influenza was still very low in Beijing, even after the peak of the pandemic, and it was the contribution of many factors, not just the level of immunogenicity against 2009 H1N1 influenza in the community that affected the spread of the virus within the population of Beijing. These factors may have also contributed to the differences in serostatus amongst the different age groups. The elderly had limited prior immunity to 2009 H1N1 virus and lower infection risk. Seasonal influenza vaccination is not able to produce cross-reactive antibody response to 2009 H1N1 virus.
ACKNOWLEDGEMENTS
Supported by grants from the National High Technology Research and Development Program of China (863 Program) (2008AA02Z416), Beijing Natural Science Foundation (7082047), Program of China during the 11th Five-Year Plan Period (2009ZX10004-315) and Program of Research on Strategy of Prevention and Control for 2009 H1N1 influenza in Beijing from Beijing Science and Technology Commission.
DECLARATION OF INTEREST
None.





