Food insecurity is a public health concern in both developed and developing countries, affecting the urban and rural poor. Food security is defined by the World Food Summit as a state where ‘all people, at all times, have physical and economic access to sufficient, safe and nutritious food to meet their dietary needs and food preferences for an active and healthy life’(1). Globally it is estimated that about 854 million people are food insecure, with 820 million of these living in developing countries(2).
Poor dietary intake is described as an integral characteristic of food insecurity that may result in poor nutritional state and quality of life(Reference Campbell3). Recent methodological advances in the measurement of food insecurity have allowed exploration of the influence of food insecurity on dietary consumption of adults in both developed(Reference Frongillo, Olson and Rauschenbach4–Reference Tarasuk7) and developing countries(Reference Gulliford, Mahabir and Rocke8–Reference Shariff and Khor10). These studies have revealed that food insecurity is associated with lower intakes of meat and dairy products, fruits and vegetables, as well as lower consumption of nutrients that may result in poor nutritional status and health(Reference Frongillo, Olson and Rauschenbach4, Reference Olson5, Reference Tarasuk7, Reference Gulliford, Mahabir and Rocke8, Reference Shariff and Khor10). These findings have forged a path for dietary data to be used in validation of questionnaire-based food insecurity measures in developing countries(Reference Frongillo and Nanama11, Reference Zalilah and Ang12).
Dietary patterns from most developing countries and in particular African countries are described as monotonous, comprising foods low in energy, few animal products and fruits and vegetables(Reference Gulliford, Mahabir and Rocke8, Reference Kinabo, Mnkeni and Nyaruhucha13–Reference Tatala, Svanberg and Mduma16). These patterns are increasingly being named responsible for the high rates of malnutrition and micronutrient deficiencies observed in these countries, although other non-food related factors may also partly explain the magnitude observed(17). In light of the predicted increase in the magnitude of food insecurity in East Africa(2) and the latest crises in food prices, there is a need for simple instruments that can assess dietary patterns accurately, timely and cost-effectively. The use of questionnaire-based food insecurity instruments may fill in this crucial gap.
Using a recently adapted and validated Radimer/Cornell food insecurity measure for use in rural Tanzania(Reference Leyna, Mmbaga and Mnyika18), we hypothesized that food insecurity (assessed by a questionnaire-based instrument) may predict decreased dietary intake and result in a continuum of negative outcomes, namely micronutrient deficiencies and poor nutritional status.
Methods
Study area and subjects
The study was conducted in Oria village, Moshi Rural district. The village is located at the foot of Mount Kilimanjaro, a few kilometres from the Tanzania–Kenya border and about 30 km south of the regional administrative capital, Moshi. The population mainly consists of peasants who grow maize, paddy and vegetables; petty businessmen selling agricultural products and fish; and a few employed individuals. The village has a weekly market selling a variety of commodities from fruits and vegetables to clothes and shoes. It is used by local and neighbouring villagers and by business people from Moshi town. A detailed description of the study area is given elsewhere(Reference Klouman, Masenga and Klepp19).
Study design and survey procedure
A cross-sectional study was carried out in Oria village between March and May of 2005. Before onset of the study, house-to-house registration of all eligible participants (aged 15–44 years with a permanent address in the village) was performed by trained research assistants with support from village and hamlet leaders. A total of 2093 individuals were listed and invited to participate. Consenting individuals were interviewed by the research team in or near their homes. The ethical committees of the Tanzania Ministry of Health and the Norwegian Committee for Medical Research reviewed and approved the study protocol.
Questionnaire data
All participants responded to questions on demographic characteristics, food insecurity items and a short FFQ. Food insecurity was assessed using an adapted version of the Radimer/Cornell food insecurity scale whose validity and reliability have been established in this setting(Reference Leyna, Mmbaga and Mnyika18). We defined a participant as ‘food secure’ if they responded ‘never’ to all of the Radimer/Cornell items; as ‘individual food insecure’ if they responded ‘sometimes’ or ‘always’ to items on anxiety to food depletion, monotony in diet or decreased individual food intake; and ‘child food insecure’ if they responded ‘sometimes’ or ‘always’ to any of the child items.
Dietary assessment
Dietary intake was assessed by using a thirty-eight-item FFQ. Participants were asked on average how often in the past month they had consumed each food item (e.g. meat/eggs/oranges/amaranth leaves, etc.). There were five response categories: ‘every day’, ‘several times a week’, ‘once a week’, ‘once or twice a month’ and ‘less than once a month/rarely/never’. The FFQ was pre-tested with a convenience sample of 150 and has been previously used in this study area(Reference Ottesen20). To ease analysis, the food items were condensed into eight simpler food group categories. A summary measure of frequent consumers was determined as eaten ‘several times weekly’ for fruits and vegetables and ‘at least weekly’ for animal products. This categorization has been used by other studies(Reference Gulliford, Mahabir and Rocke8).
Anthropometric measures
Height was measured to the nearest 0·1 cm using locally made portable devices equipped with height gauges and weight was measured using calibrated portable electronic scales to the nearest 0·1 kg with participants wearing light clothing and no shoes. Waist and hip circumference was measured by trained research assistants (waist measured at the level of the umbilicus and hip measured horizontally at the level of the greatest lateral extension of the hips) with participants standing, using non-stretchable tape measures; and used to construct waist:hip ratio (WHR) (cm/cm). Measurements were conducted in duplicate and an average value was used for the analysis. BMI was calculated as kg/m2 and classified as proposed by WHO(21). Participants were classified as underweight (BMI < 18·5 kg/m2), normal weight (BMI = 18·5–24·9 kg/m2), overweight (BMI = 25·0–29·9 kg/m2) or obese (BMI ≥ 30·0 kg/m2). Central obesity was defined as a WHR of ≥0·85 for women and ≥0·95 for men.
Serum micronutrients
Blood was drawn from the antecubital vein of each participant into a 5 ml Vacutainer tube wrapped with black tape. The blood samples were stored in insulated cool boxes for transportation to the Kilimanjaro Christian Medical Hospital laboratory. The blood samples were centrifuged at 2000 rpm for 15 min at room temperature within 8–10 h of collection. The sera were then separated into Nunc tubes also wrapped with black tape and labelled with sample number. The plasma samples were subsequently stored in a freezer at −20°C and flown to Norway for analysis by VITAS AS laboratories. Serum retinol concentration was determined by HPLC and serum ferritin was measured by ELISA. Iron stores were characterized on the basis of serum ferritin concentration as depleted when <15 μg/l and vitamin A status was characterized as deficient if serum retinol concentration was <0·70 μmol/l.
Statistical analysis
Data were analysed using the SPSS for Windows statistical software package version 14·0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were used to depict overall distribution of the variables. ANOVA was used to describe means and standard deviations for continuous variables according to food insecurity status. Prevalences of categorical variables were evaluated with contingency tables, expressed as percentages, and accompanied by the χ 2 test for differences in proportion. We examined the outcome food insecurity status in relation to demographic characteristics using three categories (food secure, food insecure at individual level and food insecure at child level). In logistic regression models two dummy variables were created from food insecurity status, food insecure at individual level and food insecure at child level; the category food secure was used as the reference. We evaluated the association between food insecurity status and food consumption patterns using a summary measure where one term for frequent consumption was used. We employed analysis of covariance to examine whether anthropometric measures differed by food insecurity status controlling for potential confounders. Since sex contributes to the variation observed in both food insecurity status and anthropometric measures, we examined for possible differences among men and women separately. Finally, we examined the association between food insecurity status and serum micronutrient levels. In our multivariable logistic models we considered age, sex, marital status, education and occupation as potential confounders. Our results are given as odds ratios and their 95 % confidence intervals. Significance was set at P < 0·05.
Results
A total of 1528 (73 %) adults aged between 15 and 44 years participated in the survey. Of these, 452 (30 %) did not have children and sixty-two (4 %) had missing data for either one or more of the food insecurity items or demographic variables. In the analysis presented herein, 1014 adults were included with anthropometric measurements available for 1006 (99 %) subjects. There was no difference in the distribution of demographic characteristics and food insecurity status between participants with and without anthropometric measurements.
Food insecurity and demographic characteristics
Food insecurity was prevalent with 91 % of the subjects reporting some kind of food insecurity (food secure, 9 %; individual food insecure, 37 %; child food insecure, 54 %). Table 1 shows the prevalence and odds ratio of food insecurity status according to demographic characteristics. Food insecurity was associated with age, marital status and occupation. The adjusted odds ratio (AOR) for food insecurity at the individual level, compared with food secure, for age groups 21–30 years, 31–40 years and ≥41 years respectively, was 1·23 (95 % CI 0·67, 2·24), 2·57 (95 % CI 1·39, 4·75) and 6·10 (95 % CI 3·07, 12·11). Married participants were less likely to report individual food insecurity compared with single participants (AOR = 0·36; 95 % CI 0·21, 0·59). Further analysis revealed that this association was limited to males (AOR = 0·07; 95 % CI 0·01, 0·26). Finally, peasants were less likely to report food insecurity at the individual level compared with respondents reporting other occupations (AOR = 0·63; 95 % CI 0·42, 0·92).
Table 1 Prevalence and odds ratio of food insecurity status according to sociodemographic characteristics among adults aged 15–44 years with children in rural Kilimanjaro, Tanzania, 2005

ref, reference category.
†Odds ratio adjusted for age, sex, marital status, education level and occupation.
Statistical significance: *P < 0·05, ***P < 0·001.
Food insecurity at the child level was associated with age, marital status and occupation. In multivariable logistic regression, adults aged ≥41 years were less likely to report child food insecurity than adults aged <21 years (AOR = 0·29; 95 % CI 0·15, 0·55). There was a significant and positive association between food insecurity at child level and marital status (AOR = 2·01; 95 % CI 1·23, 3·27) and being a peasant (AOR = 1·52; 95 % CI 1·05, 2·18).
Food insecurity and food consumption patterns
The distribution and likelihood of food consumption by food insecurity status is depicted in Table 2. There was evidence of a strong and significant negative trend in the prevalence of frequent fruits and vegetables, meat, chicken and eggs consumption and food insecurity status. There was no marked difference in fish consumption across the food insecurity groups.
Table 2 Distribution and likelihood of food consumption by food insecurity status among adults aged 15–44 years with children in rural Kilimanjaro, Tanzania, 2005
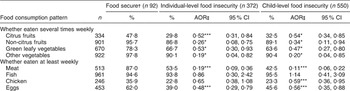
†Food secure as the reference category.
‡Odds ratio adjusted for age, sex, marital status, education level and occupation.
Statistical significance: *P < 0·05, ***P < 0·001.
Food insecurity and anthropometric measures
The mean (sd) anthropometric measurements for the study population were: height 160·0 (8·4) cm; weight 56·6 (9·7) kg; waist circumference 77·3 (11·8) cm; hip circumference 91·3 (11·7) cm; WHR 0·68 (0·18) for men and 0·61 (0·14) for women; and BMI 22·2 (3·7) kg/m2. The majority of participants were categorized as having normal weight (69 %) followed by overweight (18 %); the rest were underweight (8 %) or obese (5 %). The mean BMI and waist circumference of women varied with food insecurity status. Women categorized as having children experiencing food insecurity had lower mean (sd) BMI values than women who were food secure: 22·3 (3·9) v. 23·3 (3·9) kg/m2 (P = 0·038). Also women experiencing food insecurity at the individual level had higher mean (sd) waist circumference than women reporting food security: 80·1 (10·5) v. 78·0 (9·7) cm (P = 0·026). Other anthropometric measures did not differ according to food insecurity status (Table 3). Bivariate analysis showed borderline significance of the percentage of women with central obesity (P = 0·064), as did BMI categories (P = 0·075), with food insecurity status (Table 4). Further analysis using regression models did not reveal any significant associations.
Table 3 Anthropometric characteristicsFootnote † of study participants by food insecurity status: adults aged 15–44 years with children in rural Kilimanjaro, Tanzania, 2005

WHR, waist:hip ratio.
† Adjusted for age, marital status, education level and occupation.
Statistical significance: *P < 0.05.
Table 4 Distribution of BMI and central obesity of study participants according to food insecurity status: adults aged 15–44 years with children in rural Kilimanjaro, Tanzania, 2005

†Central obesity classified as waist:hip ratio ≥0·85 for women and ≥0·95 for men.
Food insecurity and serum micronutrient levels
The mean (sd) serum ferritin level was 58·6 (115·8) μg/l and mean (sd) serum vitamin A level was 1·26 (0·52) μmol/l. Iron depletion was prevalent in 23·5 % (230/979) of the respondents and vitamin A deficiency was observed in 10·7 % (104/971) of the respondents. There was no correlation between food insecurity status and iron depletion or vitamin A deficiency.
Discussion
The present study assessed the association between food insecurity status and food consumption patterns, anthropometric measures and serum micronutrient levels among adults in rural Kilimanjaro, Tanzania. The overall prevalence of food insecurity in this rural area was high. Food insecurity status was associated with selected demographic characteristics at both the individual and child level. These findings are similar to observations from other studies in developed countries(Reference Furness, Simon and Wold22) as well as developing countries(Reference Gulliford, Mahabir and Rocke8–Reference Shariff and Khor10, Reference Isanaka, Mora-Plazas and Lopez-Arana23, Reference Zerafati-Shoae, Omidvar and Ghazi-Tabatabaie24). Contrary to findings from studies conducted in other developing countries(Reference Gulliford, Mahabir and Rocke8–Reference Shariff and Khor10, Reference Isanaka, Mora-Plazas and Lopez-Arana23, Reference Zerafati-Shoae, Omidvar and Ghazi-Tabatabaie24), we observed no significant association between food insecurity and education. Although the majority of participants reported attending primary school, this has been described as insufficient to reduce poverty(Reference Wedgewood25). Further, a small number of participants reported having secondary education or higher which limited our statistical power to detect any differences.
We also observed a larger proportion of married men and women reporting children experiencing food insecurity compared with those who were single. In their study in the USA, Hanson et al.(Reference Hanson, Sobal and Frongillo26) observed that divorced men reported more food insecurity than never-married men. The disparity in our observation may be due to differences in the tool used to assess food insecurity and categorization of food insecurity. Additionally, differences in the study populations as well as socio-cultural factors may put more pressure on married adults in a Tanzanian context that may lead to more severe food insecurity experiences.
Data obtained on food frequency intake revealed that participants who were food insecure had low intakes of animal products, fruits and vegetables compared with food-secure participants. The low dietary intakes of animal products, fruits and vegetables in food-insecure individuals are consistent with the findings from other studies(Reference Gulliford, Mahabir and Rocke8–Reference Shariff and Khor10, Reference Zerafati-Shoae, Omidvar and Ghazi-Tabatabaie24, Reference Hadley, Mulder and Fitzherbert27). The magnitude of the difference in food consumption between food-secure and food-insecure respondents was larger for meat and eggs. This may be because foods of animal origin are generally expensive and unaffordable to the majority of the rural population, while plant sources are less costly(Reference Mazengo, Simell and Lukumanji15, Reference Codjia28). Vegetables in Africa are said to play a vital role in food security, particularly for the poor, in both rural and urban settings(Reference Kinabo, Mnkeni and Nyaruhucha13, Reference Weinberger and Msuya29). It is estimated that about 80 % of rural Tanzanians rely on home-grown fruits and vegetables particularly during the lean rainy seasons as a primary food and relish(Reference Kinabo, Mnkeni and Nyaruhucha13, Reference Mazengo, Simell and Lukumanji15, Reference Mulokozi, Lietz and Svanberg30, Reference Tanner and Lukmanji31). The magnitude of the difference in fruit and vegetable consumption between food-secure and food-insecure individuals may widen during the dry season as vegetables become scarce and households rely more on markets(Reference Frongillo and Nanama11, Reference Kinabo, Mnkeni and Nyaruhucha13).
Fruits and vegetables offer a great source of micronutrients if prepared properly and taken in adequate amounts. The prevalence of both iron depletion and vitamin A deficiency indicates that they were common in the study population. However, there was no difference in the proportion of adults who were either iron depleted or vitamin A deficient by food insecurity status. Low serum levels of micronutrients including vitamin A, carotenoids and vitamin E have been described among adults from food-insufficient families in developed countries(Reference Dixon, Winkleby and Radimer32). Our findings show that a modest proportion of the population consumed fruits and vegetables regularly. The ready availability of leafy green vegetables from home gardens during the rain season may have allowed for most of the micronutrient requirements of the population to be met(Reference Tanner and Lukmanji31). However, the lack of correlation between food insecurity status and micronutrient status may be explained by: (i) small variation in the bioavailability of iron and pro-vitamin A in the fruits and vegetables consumed as a result of nutrient loss during preparation(Reference Mulokozi, Lietz and Svanberg30); (ii) the cut-off points used were based on acute manifestations of micronutrient deficiencies; (iii) some nutrients such as vitamin A are tightly regulated by the liver and not affected by recent dietary intake; and (iv) some infections can influence serum ferritin levels in the body(Reference Hulthén, Lindstedt and Lundberg33). More studies are needed to assess food preparation habits of food-secure and food-insecure households and the influence it may have on micronutrient status.
There are plausible biological mechanisms to suggest that food insecurity has negative health outcomes on those experiencing it(Reference Campbell3). Studies assessing the association between food insecurity and nutritional status in both developed(Reference Townsend, Peerson and Love34–Reference Wilde and Peterman36) and developing countries(Reference Gulliford, Mahabir and Rocke8, Reference Shariff and Khor10) have produced conflicting results. Our findings suggest that increased adiposity among women may be associated with food insecurity at the individual level while decreased adiposity among women may be associated with food insecurity at the child level; similar to what other studies have observed(Reference Shariff and Khor10, Reference Frongillo and Nanama11, Reference Chaput, Gilbert and Tremblay37). In light of the prevalence of food insecurity, it is expected that underweight due to a lack of adequate food would be the primary manifestation of food insecurity in this setting; however, nutritional status assessed by anthropometric measures is considered a distal outcome of food insecurity and is not only determined by access to food(Reference Frongillo and Nanama11). The lack of a significant association between food insecurity and anthropometric measures in the multivariable analysis may be because of the time lag between inadequate food intake and observable physical changes. Moreover, anthropometric measures are sensitive to other variables such as infection, sanitation and hygiene(17). Our study was conducted at the onset of the pre-harvest lean (rainy) season when food shortages and increased energy expenditure from agricultural work had just begun. Thus, physical changes may not have occurred sufficiently to be detected during the survey. Further studies are needed to examine changes in nutritional status that accompany seasonal variation in food insecurity in rural Tanzania.
Limitations of the present study lie in the temporal nature of the data, specifically the fact that food insecurity data were not coincident with data on food consumption, anthropometry and micronutrient status. This raises important questions about cause and effect. Another limitation is the use of a non-validated FFQ to assess dietary patterns. The inherent recall bias associated with the FFQ would make it less likely to correctly describe usual dietary intake. However, allowing for control of factors known to influence under-reporting such as age, sex and education and pre-testing of the FFQ, we believe our results are a fair representation of actual dietary patterns(Reference Bedard, Shatenstein and Madon38). The strength of the present survey lies in the use of a validated self-report measure of food insecurity, assessing food insecurity at an individual level to allow direct comparison with nutritional outcomes, and inclusion of the whole adult population with a fairly good response proportion.
Conclusion
Our study has shown that food insecurity was highly prevalent and associated with food consumption patterns, waist circumference and BMI of women in rural Tanzania. Further studies should apply self-report measures in assessing food insecurity to larger and more diversified populations.
Acknowledgements
Sources of funding: The study was supported by a grant from the Norwegian Programme for Development, Research and Higher Education (NUFU) and the Centre for Prevention of Global Infections (GLOBINF) at the University of Oslo, Norway. The study was facilitated by the collaborating institutions: Muhimbili University of Health Sciences, Kilimanjaro Christian Medical College, the Centre for Education Development (Arusha, Tanzania), and the Universities of Oslo and Bergen, Norway. Conflict of interest declaration: None. Authors’ contributions: G.H.L., conception of study, data collection, analysis and interpretation of results, drafting of manuscript; E.J.M., conception of study, data collection, review of manuscript; K.S.M., data collection, review of manuscript; A.H., interpretation of results, review of manuscript; K.-I.K., conception of study, interpretation of results, critical review of manuscript. Acknowledgements: The authors are grateful to the participants for their time and to the hardworking spirit of the research team.






