Introduction
In the U.S., second-generation antipsychotics (SGAs) are the de-facto first line antipsychotic agents for the treatment of schizophrenia, displacing first-generation antipsychotics (FGAs) for this indication. SGAs are also widely used in the treatment of major affective disorders, with selected drugs having U.S. Food and Drug Administration (FDA) approval for the treatment of bipolar I disorder and treatment-resistant major depressive disorder (MDD).
Compared to the general population, people with serious mental illnesses (SMI; schizophrenia, bipolar I disorder, severe MDD) have a higher risk for type 2 diabetes, hereafter diabetes, cardiovascular disease, and other cardiometabolic morbidity (Lindekilde et al., Reference Lindekilde, Scheuer, Diaz, Rubin, Plana-Ripoll, Henriksen and Pouwer2022; Momen et al., Reference Momen, Plana-Ripoll, Agerbo, Benros, Børglum, Christensen and McGrath2020; Takayanagi et al., Reference Takayanagi, Ishizuka, Laursen, Yukitake, Yang, Cascella and Sawa2021; Wimberley et al., Reference Wimberley, Horsdal, Brikell, Laursen, Astrup, Fanelli and Dalsgaard2022). Evidence exists that for diabetes and other insulinopathies, this excess risk may stem at least in part from shared genetic loading as reflected in familial aggregation (Huang et al., Reference Huang, Chen, Huang, Hsu, Bai, Cheng and Chen2019; Su et al., Reference Su, Shih, Lin, Chen, Chen, Hsiao and Wang2022; van Welie et al., Reference van Welie, Derks, Verweij, de Valk, Kahn and Cahn2013), with dysregulation of insulin signaling as a potential mechanism for both sets of disorders (Fanelli et al., Reference Fanelli, Franke, De Witte, Ruisch, Haavik, van Gils and Bralten2022). Studies have found that antipsychotics, particularly SGAs, contribute to this risk relative to FGAs or to no drug. The preponderance of evidence, much of it derived from populations with schizophrenia, suggests that olanzapine and clozapine confer a high risk for cardiometabolic morbidity and its risk factors (weight gain and metabolic dysregulation), quetiapine and risperidone confer an intermediate risk, and aripiprazole confers a lower risk (Holt & Mitchell, Reference Holt and Mitchell2015; Mazereel, Detraux, Vancampfort, van Winkel, & De Hert, Reference Mazereel, Detraux, Vancampfort, van Winkel and De Hert2020). In the U.S., concern about the association between SGAs and diabetes grew through the early 2000′s, culminating in an FDA warning about the increased risk of diabetes associated with all SGAs and in professional societies raising alarms about this risk (American Diabetes Association et al., 2004). The significance of diabetes among people with SMI stems from its higher prevalence and severity relative to those without SMI (Holt & Mitchell, Reference Holt and Mitchell2015) and its contribution to their risk of cardiovascular disease (Osborn et al., Reference Osborn, Wright, Levy, King, Deo and Nazareth2008) and premature mortality (Walker, McGee, & Druss, Reference Walker, McGee and Druss2015).
Prior studies on the association between SGAs and diabetes risk among individuals with SMI have some limitations, however. Randomized trials have been mainly designed to assess antipsychotic drugs' efficacy (Holt & Mitchell, Reference Holt and Mitchell2015) and safety assessments have focused on risk factors for diabetes rather than diabetes itself (Pillinger et al., Reference Pillinger, McCutcheon, Vano, Mizuno, Arumuham, Hindley and Howes2020). Otherwise, most evidence arises from observational studies designed using a variety of comparison arms: any SGA (Smith et al., Reference Smith, Hopkins, Peveler, Holt, Woodward and Ismail2008) or specific SGAs (Citrome et al., Reference Citrome, Holt, Zachry, Clewell, Orth, Karagianis and Hoffmann2007) v. any FGA; or antipsychotic drugs v. specific FGAs, typically haloperidol (Hartling et al., Reference Hartling, Abou-Setta, Dursun, Mousavi, Pasichnyk and Newton2012), or v. no antipsychotic drug (Kessing, Thomsen, Mogensen, & Andersen, Reference Kessing, Thomsen, Mogensen and Andersen2010). Because cardiometabolic morbidity risk may vary by specific SGAs, results from pooled analyses may mask effects. Studies that compare SGA outcomes to individuals receiving no antipsychotic drug treatment inform about whether to prescribe rather than which drug to prescribe; while the former is perhaps appropriate for individuals with affective disorders, it is almost never appropriate for individuals with schizophrenia.
SGAs and haloperidol have been associated with a higher mortality risk among elderly adults with dementia. This evidence, produced both by randomized trials and observational studies, suggests that the risk is highest for haloperidol (Reus et al., Reference Reus, Fochtmann, Eyler, Hilty, Horvitz-Lennon, Jibson and Yager2017). Evidence on antipsychotic drug mortality risk specific to individuals with SMI comes largely from observational studies, most focused on schizophrenia, with few having adequate comparators. While two studies compared SGAs or FGAs to no antipsychotic (Taipale et al., Reference Taipale, Tanskanen, Mehtälä, Vattulainen, Correll and Tiihonen2020; Tiihonen & Taipale, Reference Tiihonen and Taipale2019), a third compared antipsychotic monotherapy to antipsychotic polypharmacy (Katona, Czobor, & Bitter, Reference Katona, Czobor and Bitter2014). We are aware of only one recently published study that conducted pairwise comparisons between multiple antipsychotic drugs and oral paliperidone (Tang et al., Reference Tang, Shen, Huang, Qiu, Watanabe, Goh and Liu2022), and of one study among adults with treatment-resistant MDD that compared augmentation with a SGA to a second antidepressant (Gerhard et al., Reference Gerhard, Stroup, Correll, Setoguchi, Strom, Huang and Olfson2020). Across these studies conducted in SMI cohorts, aripiprazole appeared among the safest, with haloperidol's risk comparatively higher.
In this paper, we used novel causal inference methods in a large, racially/ethnic diverse population to re-examine the effect of commonly used antipsychotic drugs on the risk of diabetes and mortality in a publicly insured U.S. adult population with SMI. Because of aripiprazole's purportedly lower risks, we compared each antipsychotic drug to aripiprazole. We estimated antipsychotic drug effect heterogeneity associated with race/ethnicity and primary diagnosis because little is known on whether these characteristics modify the drugs' safety effects.
Methods
Data sources, study cohort, and design
In the U.S., Medicaid is a joint federal and state program that provides health coverage to low-income adults and disabled individuals, while Medicare is a federal health insurance program for those 65 years or older and disabled non-elderly adults with past employment. Some individuals may be dually enrolled in Medicaid and Medicare due to a qualifying disability (hereafter dual eligibles). We used administrative health care billing data from both programs to assemble information on eligibility, demographic characteristics, diagnoses, and service and pharmacy utilization; for dual eligibles, information from both Medicare and Medicaid files was linked.
The study was reviewed and approved by the authors' IRBs. We followed the STROBE reporting guideline (see online Supplementary Table S3).
We included adults aged 18–64 of non-Latinx Black (hereafter Black), Latinx, non-Latinx White (hereafter White), or other race/ethnicity having dual Medicaid-Medicare and Medicare-only coverage, residing in any of seven states (California, Georgia, Iowa, Mississippi, Oklahoma, South Dakota, and West Virginia), who (a) between 1 July 2008 and 30 June 2013, filled at least one prescription for any of the most commonly utilized SGAs (quetiapine, risperidone, aripiprazole, olanzapine, ziprasidone) or haloperidol, and (b) were diagnosed with an SMI, i.e., schizophrenia, bipolar I disorder, or severe MDD (as a proxy for treatment-resistant MDD). These states were selected for their racial/ethnic diversity.
We identified individuals who met criteria for antipsychotic drug monotherapy with any of the six (‘index’) drugs and who were relatively new antipsychotic drug users. Monotherapy was defined as ⩾ two fills for the same index antipsychotic drug totaling a minimum supply of 31 days during a 90-day period, with the date of the first fill denoted the index date. We defined relatively new antipsychotic drug users as individuals who in the 6-month period preceding the index date (hereafter, the pre-period) had no fills for any antipsychotic drug other than their index drug.
The final cohort included (1) individuals with at least 6 months of continuous enrollment in the pre-period (used to assess for confounders) and at least 6 months of continuous enrollment in the period immediately following the index date (post-period). We included those who died during the post-period if they were continuously enrolled up to the month prior to the month of death; and (2) those not having diabetes conditions other than type 1 diabetes, other cardiometabolic morbidity (dyslipidemia, hypertension, and cardiovascular disorders) associated with diabetes risk or death, or conditions such as polycystic ovaries syndrome whose management frequently involves use of antidiabetic drugs during the pre-period. Individuals were followed from the index date up to 3 years regardless of the duration of the monotherapy, unless a censoring event was observed, which in hierarchical order, included (a) end of the study period (31 December 2013), (b) turning 65 years, or (c) loss of insurance coverage. Individuals could only contribute one episode to the cohort (see online Supplementary material, A.1 and A.2).
Measures
Outcomes
The primary outcomes were newly diagnosed type 2 diabetes or all-cause death. Type 2 diabetes was ascertained with ICD-9 diagnosis codes observed as (a) a primary diagnosis in ⩾ one inpatient discharge claims, or (b) primary or secondary diagnosis in ⩾ two outpatient claims during a 12-month period, or in one outpatient claim if a National Drug Code (NDC) for an oral antidiabetic drug. All-cause death was defined based on variables denoting the person's date of death available in the datasets.
Treatment
Our treatment variables were indicators for quetiapine, risperidone, aripiprazole, olanzapine, ziprasidone, or haloperidol monotherapy.
Subgroups
We categorized patients by their primary SMI diagnosis (schizophrenia, bipolar I disorder, severe MDD) as well as by race/ethnicity (Black, Latinx, White, or other race/ethnicity which also includes individuals with missing race/ethnicity information).
Confounders
Confounders, all measured during the pre-period, included: ageFootnote 1Footnote † and sex; health status, assesse with three variables: (a) other chronic medical conditions potentially associated with diabetes or having the potential to affect service utilization and thus likelihood of diagnosis (e.g. HIV & other chronic infections, Type 1 diabetes & other endocrine disorders, malignancies), (b) risk factors for cardiometabolic morbidity (e.g. obesity, pre-diabetes), and (c) psychiatric comorbidity (e.g. other affective disorders, post-traumatic stress disorder); service utilization, assessed with nine variables capturing counts of psychiatric, injury-related, and non-psychiatric inpatient days, emergency department visits, and outpatient visits; metabolic testing, which captured lipid or glucose laboratory tests; exposure to drugs with cardiometabolic effects, including antidiabetic drugs, anti-hypertensive drugs, and other drugs with potential weight-related and cardiometabolic effects; exposure to the index antipsychotic drug; payer, based on dual Medicaid-Medicare v. Medicare coverage of the index fill; and year of the index fill (see online Supplementary material, A.3).
Statistical analysis
We estimated the difference in the rate of the primary outcomes between each antipsychotic drug and aripiprazole using targeted minimum loss-based estimation (TMLE), a doubly-robust estimator implemented with machine learning (Schuler & Rose, Reference Schuler and Rose2017; van der Laan & Rose, Reference van der Laan and Rose2011) to reduce the risk of model misspecification (Pirracchio, Petersen, & van Der Laan, Reference Pirracchio, Petersen and van Der Laan2015). We modified the algorithm to use minimum loss-based estimation with a categorical treatment variable (Poulos et al., Reference Poulos, Horvitz-Lennon, Zelevinsky, Cristea-Platon, Huijskens, Tyagi and Normand2022). TMLE uses machine learning models trained on potentially risk-modifying patient characteristics to estimate the expected outcome as if all subjects were treated with each antipsychotic drug, by reweighting an initial outcome estimate with a function of estimated treatment probabilities. Average absolute outcome differences were obtained by differencing the TMLE estimates of the expected outcome under each drug and the expected outcome under the comparator (aripiprazole), and average relative outcome differences were obtained by scaling the absolute difference by the observed (unadjusted) outcome rate in the aripiprazole group. 95% confidence intervals (CIs) for the average absolute differences were constructed using the efficient influence curve (Poulos et al., Reference Poulos, Horvitz-Lennon, Zelevinsky, Cristea-Platon, Huijskens, Tyagi and Normand2022). Data were analyzed using the super learner (Polley, Rose, & van der Laan, Reference Polley, Rose, van der Laan, van der Laan and Rose2011; van der Laan, Polley, & Hubbard, Reference van der Laan, Polley and Hubbard2007), an ensemble method that employs cross-validation to select the optimal weighted average of estimators obtained from a pre-selected library of classification algorithms (see online Supplementary material, A.4). To assess consistency of the main findings, we averaged the estimated differences in the expected outcome rates within the subgroups defined by primary SMI diagnosis and again by race/ethnicity. We made no adjustment for multiplicity of the pairwise comparisons.
Sensitivity to unmeasured confounding
We identified a subgroup of young (less than 45 years old) individuals having no exposure to the index antipsychotic drug in the pre-period. These individuals are (a) less likely than older individuals or those with prior exposure to the index drug to have developed undetected or uncoded diabetes or its risk factors; and (b) because of no pre-period exposure to the index drug, less information is available to prescribers on early risk signals, suggesting this subgroup is less vulnerable to unmeasured confounding. We averaged the estimates obtained from the full cohort within the subgroup, computing the diabetes incidence and mortality risk differences between each antipsychotic drug and aripiprazole.
Results
Characteristics of the study cohort
Our final cohort included 38 762 individuals who were receiving one of the study drugs and were followed for up to 3 years between 2008 and 2013 (or died before the 3-year follow-up) (see online Supplementary Fig. S1). Median follow-up was identical across all drug groups (1094 days), and mean follow-up was nearly identical (998–999 days). The cohort was predominantly White (65%), male (52%), and older than 45 (53%); most were residents of California (55%) and individuals with dual Medicaid-Medicare coverage (74%). The primary SMI diagnosis for most of the cohort was schizophrenia (55%); a sizable minority had comorbidity, with both psychiatric (17.5%) and other chronic medical conditions (21.8%) common. Most individuals (81%) had some pre-period exposure to the index drug, and on average, they were on antipsychotic drugs for 109 days (s.d. = 69.7). Metabolic monitoring with lipid or glucose lab tests was infrequent (18%) (Table 1).
Table 1. Cohort characteristics
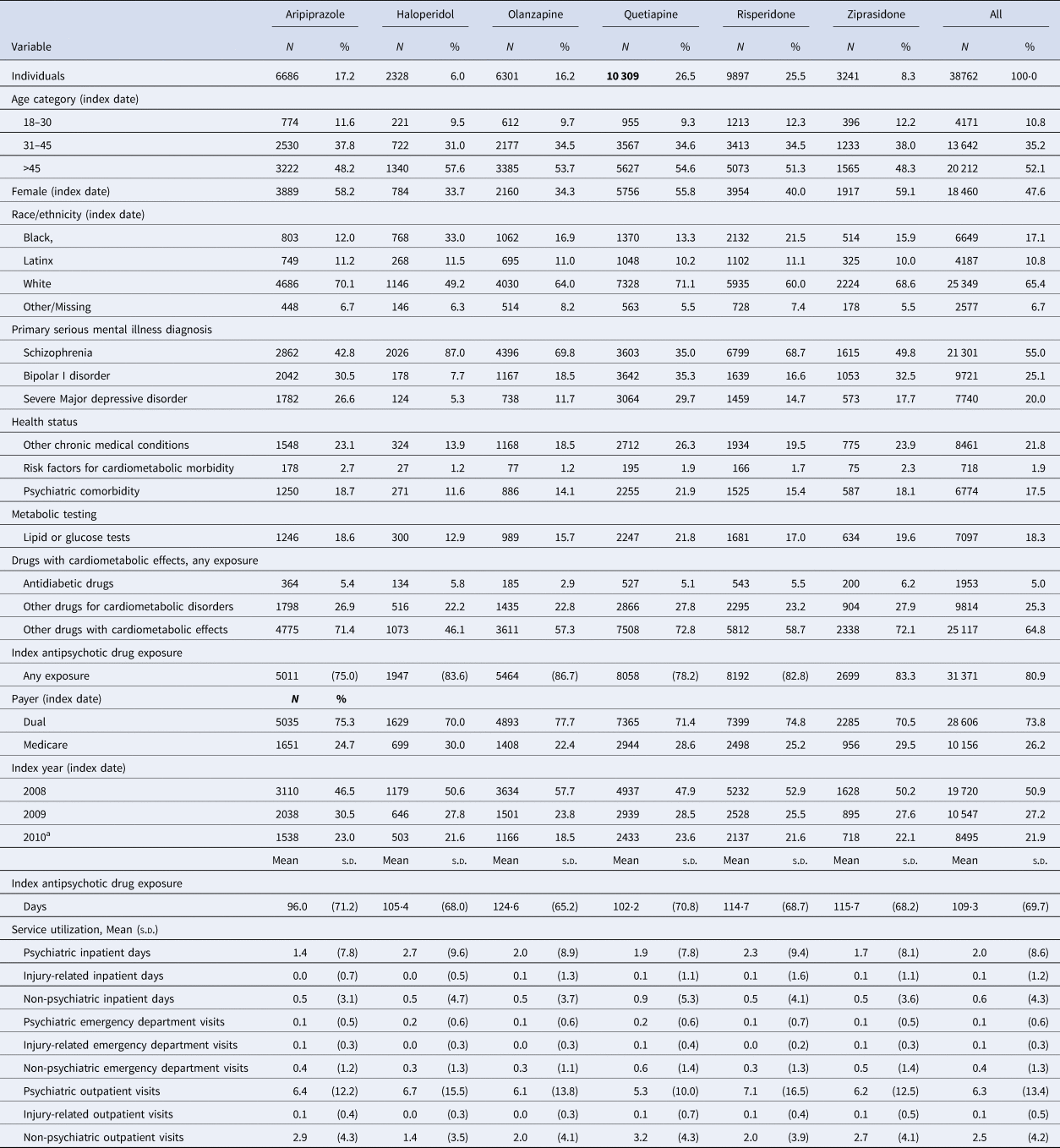
Assessed in the 6-month period prior to the index drug fill.
a Indicates summary statistics for the index years of 2010 and 2011.
Aripiprazole was initiated in less than one in five individuals; haloperidol in only 6% of the cohort; and quetiapine in 26.5% (Table 1). Differences in cohort characteristics across the antipsychotic drug groups were apparent (Table 1); for instance, 43% of aripiprazole initiators had schizophrenia compared to 87% of haloperidol initiators, and 12% of haloperidol initiators had a psychiatric comorbidity compared to 22% of olanzapine initiators.
Antipsychotic drug safety
Incident diabetes occurred in 9.3% and all-cause death in 5% of the cohort over the 3-year observation period (Table 2). While olanzapine was associated with the lowest unadjusted rate of diabetes (6.7%), aripiprazole was associated with the lowest unadjusted death rate (3.4%).
Table 2. Three-year outcomes among 38 762 publicly insured adults receiving antipsychotic drug monotherapy
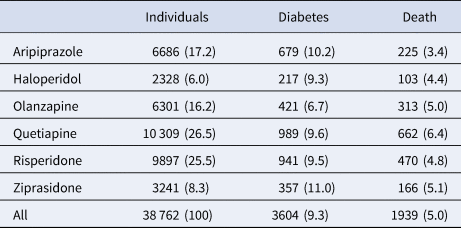
Number (percent) receiving each drug and having each outcome.
Adjusting for confounders, we estimate that if all patients were treated with haloperidol or olanzapine, their diabetes risk would be 1.9 (95% CI: 1.2–2.6) percentage points lower than if all patients were treated with aripiprazole (Table 3); the point estimate represents a 18.6 percentage point reduction compared with the unadjusted risk of diabetes among those treated with aripiprazole (10.2%, Table 2). There was a 0.5 (0.1–1.2) percentage point absolute reduction in diabetes risk favoring risperidone over aripiprazole, which represents a 4.9 percentage point relative risk reduction. We found no evidence of a relative safety benefit of aripiprazole over quetiapine or ziprasidone.
Table 3. Average absolute outcome differences in percentage points (95% confidence intervals) for five antipsychotic drugs compared with aripiprazole (comparator drug)
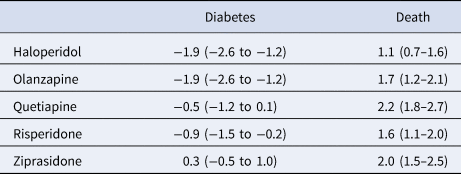
Positive values indicate an advantage for aripiprazole.
Aripiprazole appeared safer than all the other study drugs (haloperidol, olanzapine, quetiapine, risperidone, and ziprasidone) for the mortality endpoint. The estimated percentage point absolute risk increases for the five drugs ranged between 1.1 and 2.2 percentage points (Table 3), representing risk increases ranging between 32.4 and 64.7 percentage points relative to the unadjusted mortality risk among those treated with aripiprazole (3.4%, Table 2).
Treatment effect heterogeneity
Findings across the diagnostic subgroups supported the main findings. Haloperidol and olanzapine reduced the risk of diabetes relative to aripiprazole for each diagnostic subgroup, with absolute risk reductions ranging between 1.8 percentage points (0.6–2.9) and 2.0 (1.1–2.9), for patients with bipolar I disorder and schizophrenia, respectively. Mortality effects were consistent with those in the overall cohort (see online Supplementary Table S1).
Findings across the racial/ethnic subgroups were generally consistent with the main findings (see online Supplementary Table S2).
Sensitivity to unmeasured confounding
In analyses restricted to younger patients with no pre-period exposure to the index antipsychotic drug (n = 31 371), the diabetes advantages for haloperidol, olanzapine, and risperidone compared with aripiprazole found in the overall sample were not robust to the sensitivity analysis (Table 4). The main findings for mortality effects, with all study drugs having an increased risk compared with aripiprazole, were also not robust to the sensitivity analyses except for quetiapine, found to have an increased absolute mortality risk of 1.6 percentage points (0.4–2.7).
Table 4. Average absolute outcome differences in percentage points (95% confidence intervals) for five antipsychotic drugs compared with aripiprazole (comparator drug) for patients aged less than 45 years and no pre-period antipsychotic drug exposure
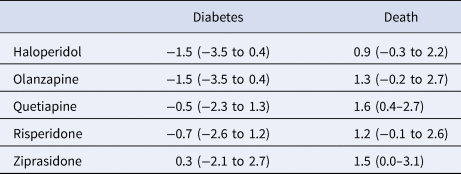
Positive values indicate an advantage for aripiprazole.
Discussion
The results of this study contribute to a large body of evidence on antipsychotic drug-related risks for non-elderly adults with SMI during real-world use. Previous research suggests that the FGA haloperidol, less frequently used than SGAs in the U.S. overall, has a favorable cardiometabolic profile relative to SGAs, while aripiprazole, a more widely used drug, has lower cardiometabolic risk relative to other SGAs (e.g. [Huhn et al., Reference Huhn, Nikolakopoulou, Schneider-Thoma, Krause, Samara, Peter and Leucht2019; Pillinger et al., Reference Pillinger, McCutcheon, Vano, Mizuno, Arumuham, Hindley and Howes2020]). However, their comparative risk has rarely been directly assessed. In this study, we observed a reduction in diabetes risk associated with initiating haloperidol, olanzapine, or risperidone treatment relative to initiating aripiprazole treatment. However, these diabetes advantages found in the overall sample, small for risperidone, did not hold in a sensitivity analysis that used a subgroup with a lower risk of confounding by indication. Mortality advantages of initiating aripiprazole relative to all study drugs were observed, but the only result that held in sensitivity analysis was the mortality advantage of initiating aripiprazole relative to quetiapine.
The similarity between our main estimates and estimates conditional on diagnosis is reassuring given that most studies have focused on schizophrenia populations; heterogeneity was plausible because each of these serious illnesses contributes to diabetes risk independently of antipsychotic drug exposures (Holt & Mitchell, Reference Holt and Mitchell2015). While there are differences across racial/ethnic groups in how drugs are metabolized or tolerated (Horvitz-Lennon, Mattke, Predmore, & Howes, Reference Horvitz-Lennon, Mattke, Predmore and Howes2017), suggesting plausible mechanisms for effect heterogeneity by race/ethnicity, our analyses did not reveal effect modification by race/ethnicity.
This study is among the first to (a) leverage a doubly-robust machine-learning based method to assess the relative safety of antipsychotic drugs using observational data; (b) directly compare diabetes and mortality risk for aripiprazole and haloperidol, a drug regarded as having low cardiometabolic risk and, among individuals with dementia, a high mortality risk but with less evidence about their comparative risk in adults with SMI, as well as aripiprazole and several widely used SGAs, including olanzapine, a drug regarded as having high cardiometabolic risk, and (c) investigate whether SMI diagnosis and race/ethnicity modify the relative safety of these drugs.
Limitations
Our study has some limitations. First, like all observational studies, unmeasured confounding may account for some of our results, including the absence of an aripiprazole advantage over the other SGAs. A potential source of unmeasured confounding is systematic drug assignment decisions based on prescribers' notions of the drugs' risks and unobserved patient risk factors. We lack the ability to identify prescribers (and leverage information on their prescribing patterns); however, we control for patient state of residence in the pre-period, which might absorb geographic variation in prescribing patterns. Moreover, we lack data on patients' medication adherence or lifestyle factors. While we control for pre-period differences in some risk factors for diabetes (obesity and other risk factors for cardiometabolic morbidity and utilization of drugs with cardiometabolic effects), we do not control for other risk factors such as patients' weight, smoking status, or family history. We note however that prior studies are similarly limited by observational designs. Additional limitations in prior studies include the specific comparisons made, e.g., SGAs v. any FGA (Citrome et al., Reference Citrome, Holt, Zachry, Clewell, Orth, Karagianis and Hoffmann2007), antipsychotic drugs v. no use of antipsychotic drugs (Taipale et al., Reference Taipale, Tanskanen, Mehtälä, Vattulainen, Correll and Tiihonen2020), or antipsychotic monotherapy to antipsychotic polypharmacy (Katona et al., Reference Katona, Czobor and Bitter2014), and their reliance on regression-based methods which are more vulnerable to confounding and model misspecification. Second, given the low risk of mortality (< 5%), we did not account for the competing risk of death in the diabetes analyses, nor did we take censoring into account. We do note that median follow-up was identical across drug groups, and mean follow-up was nearly identical. Third, because some drug subgroups defined by primary diagnosis or race/ethnicity were associated with small sample sizes, our method may have failed to detect real subgroup effects that exist within the population due to low statistical power. For example, the CIs of mortality differences with respect to race/ethnicity subgroups are wide, and in the setting of a low mortality rate and small mortality differences, they effectively rule out meaningful effects. Last, we did not adjust the CIs for multiplicity of estimation, so our findings should be replicated in similar cohorts.
Conclusions
Relative to aripiprazole, quetiapine increased the 3-year mortality risk, a result that is consistent with some (Katona et al., Reference Katona, Czobor and Bitter2014; Tang et al., Reference Tang, Shen, Huang, Qiu, Watanabe, Goh and Liu2022) but not all (Taipale et al., Reference Taipale, Tanskanen, Mehtälä, Vattulainen, Correll and Tiihonen2020; Tiihonen & Taipale, Reference Tiihonen and Taipale2019) existing evidence. Although confirmatory research is needed, our findings suggest caution in the use of quetiapine in the care of non-elderly adults with SMI, even when used in low doses, as some evidence exists of the drug's risks at low dose (Berge, Abri, Andell, Movahed, & Ragazan, Reference Berge, Abri, Andell, Movahed and Ragazan2022). Also, we did not find that aripiprazole reduced diabetes risk compared to the other study SGAs including olanzapine, a finding that may be counter-intuitive for some observers but is in keeping with evidence produced by several previous studies (e.g. [Rajkumar et al., Reference Rajkumar, Horsdal, Wimberley, Cohen, Mors, Børglum and Gasse2017; Smith et al., Reference Smith, Lindenmayer, Davis, Kelly, Viviano, Cornwell and Vaidhyanathaswamy2009; Stroup et al., Reference Stroup, Lieberman, McEvoy, Davis, Swartz, Keefe and Hsiao2009; Stroup et al., Reference Stroup, McEvoy, Ring, Hamer, LaVange, Swartz and Lieberman2011; Vancampfort et al., Reference Vancampfort, Correll, Galling, Probst, De Hert, Ward and Stubbs2016]). Ultimately, prescribing decisions should account for all potential SGA risks. Key among others, also associated with cardiovascular disease, are SGAs' risks for weight gain and dyslipidemia, for which aripiprazole and ziprasidone appear to have a clear advantage over other SGAs (Newcomer et al., Reference Newcomer, Campos, Marcus, Breder, Berman, Kerselaers and McQuade2008; Pillinger et al., Reference Pillinger, McCutcheon, Vano, Mizuno, Arumuham, Hindley and Howes2020; Stroup et al., Reference Stroup, Lieberman, McEvoy, Davis, Swartz, Keefe and Hsiao2009, Reference Stroup, McEvoy, Ring, Hamer, LaVange, Swartz and Lieberman2011; Weiden, Newcomer, Loebel, Yang, & Lebovitz, Reference Weiden, Newcomer, Loebel, Yang and Lebovitz2008; Wu et al., Reference Wu, Siafis, Hamza, Schneider-Thoma, Davis, Salanti and Leucht2022).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291723001502
Funding statement
This research was supported by the National Institute of Mental Health (R01 MH106682) and the National Institute of Minority Health and Health Disparities (R01 MDO12428).
Competing interest
The author(s) declare none except for Dr Newcomer who has received support as an unrelated consultant from Biohaven and Merck and as a member of data safety monitoring boards for Amgen and Teva.







