Age-related macular degeneration (AMD) is a progressive, chronic disease of the central retina and is the leading cause of vision loss in older people worldwide( Reference Foran, Wang and Mitchell 1 ). Major risk factors include cigarette smoking, nutritional factors, CVD and genetic markers, including genes that regulate complement, lipid, angiogenic and extracellular matrix pathways( Reference Lim, Mitchell and Seddon 2 ). To date, very few epidemiological data on modifiable risk factors are consistent and well validated, so that the only well-established modifiable risk factor is smoking( Reference Lim, Mitchell and Seddon 2 , Reference Guymer and Chong 3 ). Better understanding of modifiable risk factors will facilitate to identify and change at-risk behaviour, and help in implementing preventive strategies early in the disease process( Reference Guymer and Chong 3 ).
There is accumulating evidence from published studies demonstrating that regular consumption of dairy foods could counteract the risk of obesity, the metabolic syndrome, type 2 diabetes, hypertension and CVD( Reference Louie, Flood and Rangan 4 – Reference van Meijl and Mensink 7 ). Given that systemic risk factors such as obesity, cardiovascular risk factors (e.g. hypertension) and CVD have also been linked with AMD( Reference Lim, Mitchell and Seddon 2 ), there is a potential for regular consumption of dairy products to modify the risk of AMD in older adults. Moreover, regular consumption of dairy products has been demonstrated to confer protection against inflammation, oxidative stress and endothelial dysfunction( Reference Lamarche 6 , Reference Gibson, Makrides and Smithers 8 ). Given that inflammation and endothelial dysfunction have been implicated in the pathogenesis of AMD( Reference Hollyfield, Bonilha and Rayborn 9 – Reference Verhaar, Wever and Kastelein 11 ), these could be potential pathways by which dairy product consumption modifies the risk of developing AMD lesions.
To our best knowledge, there are no epidemiological data on the relationship between habitual consumption of dairy products and the risk of incident AMD. In the present cohort study of adults aged 49 years and over, we aimed to answer the following key questions: (1) is regular consumption of dairy products (comprising three primary dairy products consumed in the present study population – milk, cheese and yogurt) and total Ca intake at baseline prospectively associated with the 15-year incidence of AMD, independent of potential confounders such as smoking, white cell count and fish consumption? and (2) do any associations found differ by the type of dairy foods consumed, i.e. regular/high-fat compared with low/reduced-fat dairy products?
Methods
Study population
The Blue Mountains Eye Study (BMES) is a population-based cohort study of common eye diseases and other health outcomes in a suburban Australian population located west of Sydney. Study methods and procedures have been described elsewhere( Reference Attebo, Mitchell and Smith 12 ). Participants were non-institutionalised residents aged 49 years or older invited to attend a detailed baseline eye examination after a door-to-door census of the study area. Selection bias at baseline was minimised after multiple callback visits, including door-knocking, telephone reminders and letters at recruitment. Baseline examinations of 3654 residents aged >49 years were conducted during 1992–4 (BMES-1, 82·4 % participation rate). Surviving baseline participants were invited to attend examinations after 5 (1997–9, BMES-2), 10 (2002–4, BMES-3) and 15 years (2007–9, BMES-4) at which 2334 (75·1 % of survivors), 1952 (75·6 % of survivors) and 1149 (55·4 % of survivors) participants were re-examined, respectively, with complete data. Participants who did not return to the 5-year visit were also invited to the 10- or 15-year visits. The University of Sydney and the Western Sydney Area Human Ethics Committees approved the study, and written informed consent was obtained from all participants at each examination.
Assessment of age-related macular degeneration
Incidence of AMD was the main outcome, 5, 10 or 15 years later. We took two 30° stereoscopic colour retinal photographs of the macula of both eyes, which were graded for the presence of early and late AMD using the Wisconsin AMD Grading System( Reference Wang, Rochtchina and Lee 13 , Reference Klein, Moss and Magli 14 ). Inter-grader and intra-grader reliability showed good agreement in identifying individual lesions( Reference Mitchell, Smith and Attebo 15 ). The detailed methodology of AMD ascertainment in this population has been reported extensively elsewhere( Reference Wang, Rochtchina and Lee 13 , Reference Klein, Moss and Magli 14 ). Incident early AMD was defined as the absence of late AMD and the presence of either (1) large (>125 μm diameter) indistinct soft or reticular drusen or (2) both large distinct soft drusen and retinal pigmentary abnormalities (hyperpigmentation or hypopigmentation) at BMES-2, -3 or -4 in either eye of persons free of early AMD in both eyes at BMES-1( Reference Klein, Moss and Magli 14 ). Similarly, incident late AMD was defined as the appearance of neovascular AMD or geographic atrophy at BMES-2, -3 or -4 in either eye of persons without AMD lesions in both eyes at BMES-1( Reference Klein, Moss and Magli 14 ). Incident any AMD was defined as having early or late AMD at BMES-2, -3 or -4. A retinal specialist (P. M.) adjudicated all uncertain retinal pathology and confirmed all late-AMD cases.
Assessment of dairy product consumption
Dietary data were collected using a 145-item self-administered FFQ, modified for Australian diet and vernacular from an early Willett FFQ( Reference Willett, Sampson and Browne 16 ) and including reference portion sizes. Participants used a nine-category frequency scale to indicate the usual frequency of consuming individual food items during the past year. The FFQ was validated by comparing nutrients from the FFQ with 3 × 4 d weighed food records collected over 1 year (n 79) in order to assess seasonal variation. Most nutrient correlations were between 0·50 and 0·60 (e.g. 0·61 for Ca) for energy-adjusted intakes, similar to other validated FFQ studies( Reference Barclay, Flood and Brand-Miller 17 , Reference Smith, Mitchell and Reay 18 ). A dietitian coded data from the FFQ into a customised database that incorporated the Australian Tables of Food Composition 1990 (NUTTAB90), and follow-up data used NUTTAB95( 19 , 20 ).
Foods listed in the FFQ were categorised into major food categories and subcategories similar to those used for the 1995 Australian National Nutrition Survey( Reference McLennan 21 ). Dairy product subcategorisation includes regular milk, reduced fat/skimmed milk, low-fat cheese, regular cheese, reduced-fat dairy dessert (e.g. low-fat yogurt) and medium-fat dairy dessert (e.g. custard and regular yogurt). For the purpose of the present analysis, total dairy products included all of the aforementioned dairy foods; low/reduced-fat dairy products included ‘reduced fat/skimmed milk’, ‘reduced-fat dairy dessert’ and ‘low-fat cheese’; while regular-fat dairy products included ‘regular milk’, ‘regular cheese’ and ‘medium-fat dairy dessert’. Quintiles of dairy product consumption were based on servings of dairy product consumed per d. The serving sizes used were 250 g for milk, 200 g for yogurt, 250 ml for custards and 40 g for cheese( Reference Louie, Flood and Rangan 4 ). Among the participants in the lowest quintile of low-fat dairy product consumption ( ≤ 0·00 servings/d), approximately 33 % consumed ≥ 1·53 servings/d of regular-fat dairy products (i.e. in the highest quintile of regular-fat dairy food intake). Similarly, among the participants in the lowest quintile of regular-fat dairy product consumption ( ≤ 0·17 servings/d), approximately 30 % consumed ≥ 1·18 servings/d of low-fat dairy product (i.e. in the highest quintile of low-fat dairy product consumption). Total Ca intake was assessed from foods consumed only and did not include Ca intake from dietary supplements.
Assessment of covariates
Covariates were selected based on previously published data showing significant associations with AMD incidence: smoking( Reference Tan, Mitchell and Kifley 22 ), white cell count( Reference Shankar, Mitchell and Rochtchina 23 ), fish consumption( Reference Tan, Wang and Flood 24 ) and AMD risk alleles( Reference Wang, Rochtchina and Smith 25 , Reference Smailhodzic, Klaver and Klevering 26 ). Information on all covariates was available at baseline (BMES-1) and then at the three subsequent follow-up examinations (BMES-2, -3 and -4). Smoking status was determined from history as never smoker, past smoker and current smoker (which included those who had ceased smoking within the last 12 months). Fasting blood samples were also processed for white cell count. We extracted separate data on the frequency of consuming fish, including salmon, tuna and sardines from the FFQ. Fruit and vegetable consumption was also considered as a potential covariate; however, it was not significant in the multivariable model and thus not included in the final parsimonious model. The complement factor H (CFH) SNP rs1061170 and the age-related maculopathy susceptibility gene 2 (ARMS2) SNP rs10490924 were genotyped or imputed using genome-wide scan data.
Statistical analyses
SAS statistical software version 9·2 (SAS Institute, Inc.) was used for analyses, including t tests, χ2 tests and logistic regression. Associations between dairy product consumption (including regular-fat and low/reduced-fat dairy products) and the risk of incident AMD (study outcome) were examined in discrete linear logistic regression models, adjusted for age, sex, current smoking at baseline, white cell count and fish consumption. Given the overlap in regular-fat and low-fat dairy product consumption, all analyses were adjusted for high-fat dairy food intake when the study factor was low-fat dairy food intake, and if the study factor was high-fat dairy food intake, then low-fat dairy food intake was included in the multivariable model. The correlation coefficient between the change in regular-fat dairy intake and low-fat dairy product intake over 15 years was moderate i.e. − 0·34 (P< 0·0001); hence, it was appropriate to adjust for both regular-fat and low-fat dairy product consumption in the final multivariable model. Supplementary analyses involved further adjustment for the presence of CFH and ARMS2 SNP, rs1061170 and rs10490924, respectively. We analysed dairy product consumption and dietary Ca intake as quintiles and used the highest quintile as the reference group( Reference Ulm 27 ). We used discrete time logistic regression modelling to investigate the associations between quintiles of total, regular-fat and low/reduced-fat dairy product consumption and the risk of incident AMD, where the change in dairy product intake in the 15-year study period was taken into account. Findings from all analyses are expressed as adjusted odds ratios and 95 % confidence intervals.
Results
Of the 3654 residents examined at baseline BMES, 2900 had baseline dietary data. Of these, 2037 had incident AMD data at BMES-2, -3 or -4 and were included for incidence analyses. The study characteristics of the participants included are shown in Table 1. The participants in the highest v. lowest quintile of total dairy product intake were more likely to consume all types of dairy foods, ≥ 1 serve/week of fish, fruits and vegetables, and to also have higher dietary intakes of saturated fat and vitamin B12 (Table 1). The participants in the highest v. lowest quintile of regular-fat dairy product consumption were more likely to be male and to have higher white cell counts, and were more likely to consume saturated fat, vitamin B12, vegetables and regular-fat milk, yogurt, cheese and custard (Table S1, available online). However, they were less likely to consume all types of low-fat dairy foods (i.e. skimmed milk, low-fat milk and yogurt). The participants in the highest v. lowest quintile of low-fat dairy product intake were less likely to be male, older, current smokers and consume all regular-fat dairy foods (regular-fat milk and yogurt). However, these participants had a higher consumption of vitamin B12, saturated fat, low-fat milk, yogurt and cheese, and fruits, vegetables and fish, and higher white cell counts (Table S2, available online).
Table 1 Baseline characteristics of the study participants stratified by quintiles of total dairy product consumption (Mean values and standard deviations; number of participants and percentages; n 2037)
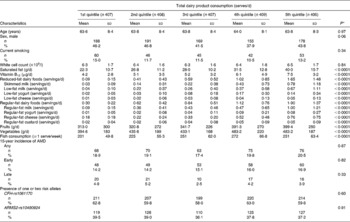
AMD, age-related macular degeneration; CFH, complement factor H; ARMS2, age-related maculopathy susceptibility gene 2.
* Unadjusted P values from test of heterogeneity across quintiles.
Table 2 presents the results from discrete time logistic regression analyses, i.e. associations between the change in dairy food intake and the 15-year incidence of AMD. After multivariable adjustment, decreasing consumption of total dairy foods over the 15 years was associated with an increased risk of developing incident late AMD (P for trend = 0·003), comparing lowest with highest quintiles of intake (OR 2·80, 95 % CI 1·21, 3·04). Over the 15 years, reduced consumption of reduced/low-fat dairy foods was associated with an increased risk of incident late AMD, comparing participants in the lowest v. highest quintile (OR 3·10, 95 % CI 1·18, 8·14, P for trend = 0·04). The change in dietary Ca intake was inversely associated with the 15-year incidence of late AMD (Table 3). After multivariable adjustment, decreasing intake of total dietary Ca over the 15 years was associated with an increased risk of developing incident late AMD (P for trend = 0·03). Supplementary analyses involved further adjustment for the presence of risk alleles (CFH-rs1061170 and ARMS2-rs10490924). Of the 2037 participants with complete dietary data and information on incident AMD, 1733 and 1601 had CFH and ARMS2 genotype data, respectively. Additional adjustment for AMD risk alleles did not appreciably change observed estimates, and significant associations still persisted when comparing participants in the lowest v. highest quintile of low-fat, regular-fat and total dairy food intakes (OR 3·31, 95 % CI 1·16, 9·44, P for trend = 0·06; OR 3·02, 95 % CI 1·26, 7·27, P for trend = 0·02; OR 3·16, 95 % CI 1·28, 7·79, P for trend = 0·01, respectively).
Table 2 Associations between the change in dairy product consumption and the 15-year incidence of age-related macular degeneration (AMD) in the Blue Mountains Eye Study during 1992–4 to 2007–9 (Adjusted odds ratios and 95 % confidence intervals; n 2037)
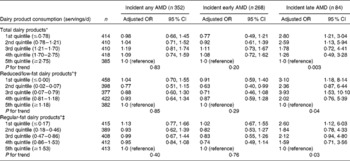
* Adjusted for age, sex, current smoking, white cell count and fish consumption.
† Further adjusted for change in regular-fat dairy product consumption over 15 years.
‡ Further adjusted for change in low-fat dairy product consumption over 15 years.
Table 3 Associations between the change in total Ca intake and the 15-year incidence of age-related macular degeneration (AMD) in the Blue Mountains Eye Study during 1992–4 to 2007–9 (Adjusted odds ratios and 95 % confidence intervals; n 2037)

* Adjusted for age, sex, current smoking, white cell count and fish consumption.
Discussion
The present novel epidemiological study shows that decreased consumption of total, reduced-fat and regular-fat dairy products, and dietary Ca over 15 years was associated with a modest increase in the risk of developing incident late AMD, irrespective of potential confounders such as age, smoking, white cell count, fish consumption and AMD risk alleles. Significant associations were not observed between intakes of dairy food and total dietary Ca and the risk of incident any or early AMD over the 15 years.
Comparisons with the present findings are difficult, given the lack of other epidemiological studies that have assessed this relationship. Previous studies have, however, shown a protective effect of habitual dairy product consumption on various health outcomes including the metabolic syndrome, hypertension and CVD( Reference Louie, Flood and Rangan 4 – Reference van Meijl and Mensink 7 ). Moreover, there is evidence to show that higher dairy product intake substantially suppresses oxidative stress and inflammation among overweight and obese adults( Reference Zemel, Sun and Sobhani 28 ). Also, prior clinical trials have shown that an increase in dairy food intake results in the suppression of circulating C-reactive protein during weight loss( Reference Zemel 29 ). There is evidence for an inflammatory process in AMD( Reference Hollyfield, Bonilha and Rayborn 9 , Reference Klein, Knudtson and Klein 10 ); hence the protection conferred by dairy foods against the development of late AMD could be at least partly explained by their anti-inflammatory properties. Despite the plausible explanations for the beneficial influence of dairy product consumption on macula health, we advise caution because of the large number of comparisons made in the present study, and hence cannot rule out the possibility that the observed protective effect is due to chance.
The primary components of most dairy products could underlie the observed beneficial effect on chronic conditions such as AMD, including Ca, vitamin D, Mg, dairy protein and a broad range of fatty acids( Reference Lamarche 6 ). In the present study, total Ca intake over the 15 years was inversely associated with the risk of developing incident late AMD. This is biologically plausible as the published literature shows that high-Ca diets can suppress systemic oxidative and inflammatory stress( Reference Zemel and Sun 30 ). Alternatively, other components of dairy products could confer protection. Vitamin D is one such nutrient; a recent US twin study has shown that a higher intake of vitamin D was associated with a reduced prevalence of AMD( Reference Seddon, Reynolds and Shah 31 ). This inverse association could be due to the anti-inflammatory and/or anti-angiogenic properties of vitamin D( Reference Schleithoff, Zittermann and Tenderich 32 ). In the BMES, we did not assess vitamin D intake and so cannot confirm this hypothesis. Alternatively, in our cohort, dairy products contribute to 34 and 29 % of vitamin B12 intake in women and men, respectively. Vitamin B12 could confer protection against AMD( Reference Rochtchina, Wang and Flood 33 ), due to its ability to lower serum homocysteine concentrations( Reference Rochtchina, Wang and Flood 33 ) and reverse endothelial dysfunction( Reference Verhaar, Wever and Kastelein 11 ), both of which have been implicated in AMD pathogenesis. Indeed, participants in the lowest quintile of total dairy product consumption had the lowest mean dietary intake of vitamin B12 (Table 1), which could have contributed to their increased risk of developing AMD. Further studies are warranted to establish the underlying causal pathways that mediate the observed associations between habitual consumption of dairy products and the risk of late AMD.
The results from the present study show that intakes of dairy food and dietary Ca do not confer any protection against the development of early AMD. The reason for the inconsistent associations observed between dairy food consumption and the different stages of AMD remains unclear. However, the present findings concur with a recent systematic review and meta-analysis which demonstrated that dietary lutein and zeaxanthin intake is not significantly associated with a reduced risk of early AMD, whereas an increase in the intake of these carotenoids may be protective against late AMD( Reference Ma, Dou and Wu 34 ). Also, prior data from the BMES have shown that frequent fish consumption reduced the risk of incident late but not early AMD( Reference Wang, Rochtchina and Smith 25 ). It is possible that the mechanisms for the development of early AMD (primary prevention) may not be the same as those involved in the progression from early to late AMD (secondary prevention)( Reference Ma, Dou and Wu 34 ), which could lead to the differential associations being observed with various dietary parameters, including dairy foods. Further longitudinal studies are needed to assess the underlying mechanisms responsible for the differential associations observed with early v. late AMD.
The present finding that habitual consumption of regular-fat dairy products is protective against the risk of late AMD could have potential public health implications. This is because new treatments are restricted to the neovascular form and mainly stabilise vision( Reference Lim, Mitchell and Seddon 2 ). Therefore, it has become imperative to identify and develop potential protective approaches to AMD. The present data suggest a possible strategy to diminish the health and economic burden of AMD by encouraging simple changes in the nutritional status of people at risk of AMD, specifically, by incorporating and maintaining adequate intakes of dairy foods in their diets( Reference Mozaffarian, Cao and King 35 ). Nevertheless, because of the small number of incident late-AMD cases, these data are suitable for hypothesis generation. We caution that any dietary recommendations from these data such as an increase in the consumption of dairy products to prevent AMD require further validation before they are incorporated into public health advice( Reference Guymer and Chong 3 ). Specifically, randomised clinical trials that determine more accurately the potential value of dairy food intake as a means of reducing the risk of developing AMD could be valuable.
The strengths of the present study include its prospective design, long-term follow-up of a stable population-based sample and robust data on major confounders and dietary parameters. Furthermore, the present study uses high-quality stereoscopic retinal photography with validated grading to assess macular conditions, and a detailed side-by-side comparison of the baseline and follow-up photographs to ensure negligible misclassification of incident AMD( Reference Tan, Wang and Flood 24 , Reference Kaushik, Wang and Flood 36 ). The present study was limited by the relatively small number of incident late-AMD cases; hence, we might not have had sufficient statistical power to detect an association between dairy product consumption and late-AMD incidence. Residual confounding by other lifestyle parameters is also plausible but is speculative based on our known knowledge of risk factors for AMD incidence in the BMES.
In summary, lower consumption of dairy products in the long term could contribute to a modest increase in the risk of developing incident late AMD. The BMES is an observational study, and we cannot prove a cause–effect link; however, it does highlight a necessity to maintain adequate consumption of dairy foods with age. Further prospective studies of the relationship between dairy product consumption and the risk of AMD lesions are nevertheless required. Additional research is also required to establish the pathway(s) that underlie the ability of dairy products to protect against AMD in older adults.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S000711451300408X
Acknowledgements
The BMES was funded by the Australian National Health and Medical Research Council (grant no. 974159, 991407, 211069 and 262120), and Westmead Millennium Institute. B. G. was supported by a Macular Degeneration Foundation and Blackmores Dr Paul Beaumont Fellowship. P. M., J. C. Y. L., V. M. F. and G. B. previously received funding from Dairy Australia on other projects related to the BMES dataset. None of the funders had any role in the design, analysis or writing of this article.
The authors' responsibilities were as follows: B. G. and P. M. contributed to the study concept and design; P. M. contributed to the acquisition of the data; G. B. and E. R. carried out the analysis of the data; B. G., V. M. F., J. C. Y. L., J. J. W., E. R. and P. M. were involved in the interpretation of the data; B. G. contributed to the drafting of the manuscript; B. G., V. M. F., J. C. Y. L., E. R., J. J. W. and PM contributed to the critical revision of the manuscript. All authors read and approved the final manuscript.
B. G., J. C. Y. L., V. M. F., E. R., J. J. W., G. B. and P. M. have no conflicts of interest.





