Introduction
Life-threatening illnesses affect physical, emotional, social, and spiritual well-being (LeMay and Wilson Reference LeMay and Wilson2008). Psychiatric disorders are common, with the prevalence of anxiety and depressive disorders estimated at around 10% and 15%, respectively, in patients with cancer (Mitchell et al. Reference Mitchell, Chan and Bhatti2011). Additionally, this confronting experience can induce existential distress by challenging one’s fundamental expectations about various aspects of life (Vehling and Kissane Reference Vehling and Kissane2018). Individuals with progressive disorders may experience a demoralization syndrome, a well-defined state of existential distress characterized by feelings of hopelessness, meaninglessness, and a sense of failure. Demoralization syndrome is clinically significant in 13%–18% of this population (Robinson et al. Reference Robinson, Kissane and Brooker2015), associated with impaired quality of life, functional compromise, and suicidal ideation (Simard et al. Reference Simard, Thewes and Humphris2013; Vehling et al. Reference Vehling, Kissane and Lo2017).
The mitigation of clinically significant psycho-existential distress remains a challenging issue in palliative care (Blinderman Reference Blinderman2016). The use of pharmacotherapy in cancer-related psychiatric conditions is common, yet meta-analysis fails to show the superiority of antidepressants over placebo (Ostuzzi et al. Reference Ostuzzi, Matcham and Dauchy2015), and there is insufficient evidence to substantiate the effectiveness of anxiolytics (Salt et al. Reference Salt, Mulvaney and Preston2017). Psycho-oncologic interventions yield significant albeit time-limited effects on existential well‐being, quality of life, and self-efficacy (Bauereiß et al. Reference Bauereiß, Obermaier and Özünal2018). Nonetheless, the results on depression and anxiety are ambiguous (Bauereiß et al. Reference Bauereiß, Obermaier and Özünal2018; Wang et al. Reference Wang, Chow and Chan2017). The need for additional effective treatments stimulates interest in exploring the therapeutic potential of psychedelic medicines. Before global prohibition in 1971, there were promising findings from early-phase clinical trials in terminal illness of classic psychedelics, such as psilocybin and lysergic acid diethylamide (LSD), characterized by agonist activity at serotonin 5-HT2A receptors and perception-altering properties (Petranker et al. Reference Petranker, Anderson and Farb2020). Entering the 21st century, the revival of psychedelic research includes re-examination of the use of these drugs in terminal illness (Fig. 1) and extends to include other hallucinogens, namely ketamine and 3,4-methylenedioxymethamphetamine (MDMA) (Reiff et al. Reference Reiff, Richman and Nemeroff2020). The entactogen MDMA (mixed serotonin and dopamine reuptake inhibitor and releaser) and dissociative anesthetic ketamine (N-methyl-D-aspartate receptor antagonist) share important similarities with serotonergic psychedelics in their ability to induce temporary but profound alterations of consciousness (Garcia-Romeu et al. Reference Garcia-Romeu, Kersgaard and Addy2016). Therefore, for the present article, the term “psychedelic” will be used to denote classic psychedelics and related compounds (i.e., ketamine and MDMA). Recent systematic reviews indicate the potential utility of these agents in terminal illness (Maia et al. Reference Maia, Beaussant and Garcia2022; Schimmel et al. Reference Schimmel, Breeksema and Smith-Apeldoorn2022).

Figure 1. Trend of end-of-life-related psychedelic publications. Number of Web of Science publications where the title or abstract contains a psychedelic drug (classic psychedelics and related compounds) and the following terms: “dying”; “palliative”; “hospice”; “life-threatening”; “terminal”; “cancer”; “tumour,” by year, from 1960 to 2021.
The accumulating evidence for psychedelic therapies at the end of life has been accompanied by growing commercial and public interest. The evolving social and legal landscape demands continuing critical appraisal of scientific evidence, particularly in light of methodological challenges and sources of bias that beset psychedelic trials (Muthukumaraswamy et al. Reference Muthukumaraswamy, Forsyth and Sumner2022). This scoping review aims to shed light on the near future of this field by summarizing proposed, registered, and ongoing clinical trials of psychedelic treatments for end-of-life anxiety, depression, and existential distress. Scoping reviews are distinguished from systematic reviews by their broader inclusion of topics and the lack of need to address specific research questions or appraise study quality (Arksey and O’Malley Reference Arksey and O’Malley2005). They serve to map available evidence, clarify concepts, examine research conduct, highlight knowledge gaps, or inform systematic reviews (Munn et al. Reference Munn, Peters and Stern2018). They are thus helpful for investigating emerging evidence and synthesizing an overview, appropriate for the objective of this article.
Method
We performed a scoping review of ongoing and upcoming trials of psychedelic medicines for end-of-life care. Registered clinical trials were identified on 20 October 2022 in 2 searchable databases: ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform. Search terms included (psilocybin OR ketamine OR esketamine OR LSD OR MDMA OR mescaline OR dipropyltryptamine OR dimethyltryptamine OR psychedelic) AND (cancer OR life OR hospice OR palliative OR terminal OR tumour OR dying OR end-of-life). Similar to Maia et al. (Reference Maia, Beaussant and Garcia2022) and Schimmel et al. (Reference Schimmel, Breeksema and Smith-Apeldoorn2022), we chose to include ketamine in the review because its psychotomimetic effects have been implicated in mediating depression and anxiety (Dore et al. Reference Dore, Turnipseed and Dwyer2019; Luckenbaugh et al. Reference Luckenbaugh, Niciu and Ionescu2014; Sos et al. Reference Sos, Klirova and Novak2013). We acknowledge that ketamine may not necessarily be used for its psychedelic properties and the distinction will be highlighted later in the review. Synonym search was enabled. The final search results were exported to Microsoft Excel, and duplicates were removed. Our search also included unregistered projects identified in recent pertinent reviews, and the websites of relevant commercial and non-profit organizations, specifically atai Life Sciences, COMPASS Pathways, Multidisciplinary Association for Psychedelic Studies (MAPS), Beckley Foundation, Heffter Research Institute, and the Ketamine Research Foundation. Collateral information on studies was gathered from press releases and published protocols via PubMed.
Randomized and open clinical trials were included if they (1) test classic psychedelics and related substances (i.e., MDMA and ketamine), (2) include participants with life-threatening illnesses or in palliative/hospice care, and (3) include psychological outcomes. We excluded trials of patients with early-stage cancer and those conducted in a postoperative setting. We also excluded trials that had published results or had been withdrawn. Ketamine trials with analgesia as the primary outcome were excluded. Only trials with registry details in English were considered; no limit was placed on the year of registration. Data were extracted and screened by 1 author (XJ). Uncertainties were resolved through discussion and consensus. Trial investigators were contacted to request information for unregistered studies.
Results
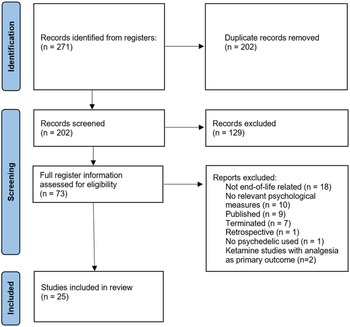
Overview
The initial search identified 271 trials, with 2 added from relevant reviews. After the removal of duplicates, 202 trial titles were screened, and 129 excluded. Seventy-three records were assessed for eligibility, and a total of 25 planned and progressing clinical trials were included for analysis (Fig. 2). Of those, 6 (24%) are Phase 1, 2 (8%) Phase 1 and 2, 13 (52%) Phase 2, and 4 (16%) unspecified. Four psychedelics feature in the trials, ketamine (n = 11), psilocybin (n = 10), LSD (n = 2), and MDMA (n = 2). There is a mix of open-label trials (n = 12) and randomized controlled trials (RCTs, n = 13). Just over half (15/25) of studies incorporate psychotherapeutic interventions, which are an uncommon feature of ketamine trials compared to trials of the other 3 drugs (2/11 vs. 13/14, chi-square = 11.4, p = 0.0007, Yates’ correction applied). Three trials employ microdosing (1 as an active placebo). Most trials specifically recruited cancer patients (n = 16). A variety of psychological outcome measures are included (Table 1).

Figure 2. Flowchart of search process.
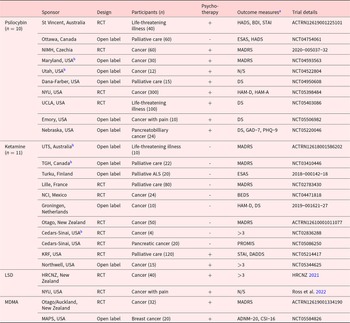
Table 1. Pipeline trial summary

N/S = Not specified, HADS = Hospital Anxiety and Depression Scale, BDI = Beck Depression Inventory, STAI = State-Trait/State Anxiety Inventory, ESAS = Edmonton Symptom Assessment System, PGIC = Patients’ Global Impression of Change scale, MADRS = Montgomery–Åsberg Depression Rating Scale, DS = Demoralization Scale, HAM-D = Hamilton Depression Rating Scale, HAM-A = Hamilton Anxiety Rating Scale, PHQ-9 = Patient Health Questionnaire-9, GAD-7 = General anxiety scale, BEDS = Brief Edinburgh Depression Scale, PROMIS = Patient-Reported Outcomes Measurement Information System, DADDS = Death and Dying Distress Scale, MEQ30 = Mystical Experience Questionnaire, ADNM-20 = Adjustment Disorder New Module, CSI-16 = Couples Satisfaction Index.
St Vincent =St Vincent’s Hospital, Ottawa = Ottawa Hospital, NIMH = National Institute of Mental Health, Maryland = Maryland Oncology Hematology, Utah = University of Utah, Dana-Farber = Dana-Farber Cancer Institute, NYU = New York University, UCLA = University of California, Los Angeles, Emory = Emory University, Nebraska = University of Nebraska, UTS = University of Technology Sydney, TGH = Toronto General Hospital, Turku = Turku University Hospital, Lille = Lille’s University Hospital, NCI = National Cancer Institute, Groningen = University Medical Center Groningen, Otago = University of Otago, Cedars-Sinai = Cedars-Sinai Medical Center, KRF = Ketamine Research Foundation, Northwell = Northwell Health, HRCNZ = Health Research Council of New Zealand, Otago/Auckland = University of Otago and University of Auckland, MAPS = Multidisciplinary Association for Psychedelic Studies.
>3 – more than 3 psychological outcome measures.
a Measures are for primary (if applicable) or secondary psychological outcomes.
b Recruitment completed.
Psilocybin
In the largest trial identified (target n = 300), a team at New York University is planning a Phase 2b, double-blind, placebo-controlled multicenter RCT in advanced-stage cancer (NYU NCT05398484). The intervention consists of single doses of either 25 mg or 1 mg (active control) of psilocybin, both delivered in conjunction with psychotherapy. This study extends a previous, smaller crossover RCT undertaken by the same principal investigator (Ross et al. Reference Ross, Bossis and Guss2016) showing enduring antidepressant and anxiolytic effects in a similar population.
In addition to low-dose psilocybin, studies are using other active placebos. For example, another RCT (St. Vincent’s Hospital [St. Vincent] ACTRN12619001225101) uses 100 mg niacin, which can induce a mild acute physiological reaction (e.g., flushing, lower blood pressure) (Bays and Rader Reference Bays and Rader2009; Mills et al. Reference Mills, Prousky and Raskin2003). Niacin has been used in doses up to 250 mg as placebo in previous psilocybin studies but, unfortunately, without assessment of the integrity of the blind (Grob et al. Reference Grob, Danforth and Chopra2011; Ross et al. Reference Ross, Bossis and Guss2016). Owing to its psychoactive properties, the short-acting benzodiazepine midazolam has previously been used as an active comparator in psychedelic trials with partial success (Muthukumaraswamy et al. Reference Muthukumaraswamy, Forsyth and Lumley2021). A Czech government-sponsored, double-blind, parallel-group study (The National Institute of Mental Health [NIMH] 2020-005037-32) is comparing 20 mg psilocybin, 200 mg ketamine, and 5 mg midazolam (active placebo), each in combination with psychotherapy, in treating cancer patients with depression. A University of California-based RCT (UCLA NCT05403086) will provide another cross-comparison, in this case single doses of psilocybin or ketamine, importantly including blinding assessment using the Treatment Allocation Questionnaire. Additionally, an open-label study at the Ottawa Hospital (Ottawa NCT04754061) will evaluate the feasibility of psilocybin microdosing (1–3 mg/day, Monday–Friday for 4 weeks) for existential distress in palliative care patients.
A Phase 2, open-label trial at Maryland Oncology Hematology (Maryland NCT04593563) explores the therapeutic potential of psilocybin-assisted group treatment in cancer patients with major depression. This study has completed recruitment, with 30 participants divided into cohorts to receive simultaneous administration of 25 mg psilocybin in a cancer center setting, complemented by group preparatory and integration sessions. The pilot project developed by the University of Utah (Utah NCT04522804) echoes this concept, where 12 recruited participants will undergo 7 group therapy sessions (1 with psilocybin).
Ketamine
Ketamine is a noncompetitive N-methyl-D-aspartate receptor antagonist with anesthetic, analgesic, dissociative, and hallucinogenic properties (Wolff and Winstock Reference Wolff and Winstock2006). However, its dose-dependent psychotomimetic effects are generally considered undesirable in antidepressant research (Garcia-Romeu et al. Reference Garcia-Romeu, Kersgaard and Addy2016). The development of ketamine-assisted psychotherapy (KAP) in recent times has aimed to harness the psychedelic properties of ketamine at relatively higher doses to treat psychiatric illnesses and existential issues (Dore et al. Reference Dore, Turnipseed and Dwyer2019). Similarly, 2 pipeline trials in this review combine ketamine with psychotherapy. The same group of Dore et al. (Reference Dore, Turnipseed and Dwyer2019) at Ketamine Research Foundation plans a Phase 2 pilot study (KRF NCT05214417) of intramuscular KAP in terminally ill patients. Two separate sessions with multiple doses of up to 100 mg per session will be administered to 18 subjects across 5 sites. The naturalistic control group will continue to receive pre-existing conventional treatment and complete the same assessments and will then be offered an optional crossover intervention. Another trial in the USA (Northwell Health [Northwell] NCT05344625) is an open-label study of repeated intramuscular KAP with individualized doses up to 60 mg or 1 mg/kg in a session. Both KAP studies will explore the impact of the intervention on existential distress, as assessed by the Death and Dying Distress Scale (DADDS).
Numerous routes of administration are examined by the other ketamine pipeline trials in the end-of-life setting, including intravenous (n = 2), intramuscular (n = 1), oral (n = 3), subcutaneous (n = 2), and intranasal (n = 1). The feasibility and efficacy of (S)-ketamine use (more potent than the racemic drug) in this population will also be investigated (White et al. Reference White, Schüttler and Shafer1985). One trial measures expectancy via the Credibility/Expectancy Questionnaire (Cedars-Sinai Medical Center [Cedars-Sinai] NCT02836288). However, it is worth noting that these trials are likely designed to minimize the psychotomimetic symptoms of ketamine due to the lower dose range employed (e.g., <0.5 mg/kg IV, <1 mg/kg oral) compared to the 2 KAP trials.
Lysergic acid diethylamide
Another RCT in development at New York University (Ross et al. Reference Ross, Agrawal and Griffiths2022) focuses on LSD-assisted psychotherapy in patients with advanced cancer pain syndromes; existential and psychiatric distresses are important secondary outcomes. A similar trial in development at the University of Auckland (Health Research Council of New Zealand [HRCNZ] 2021) evaluates the feasibility of combining microdosed LSD with meaning-centered psychotherapy. This study will provide 40 advanced cancer patients with either a titrated dose of LSD (4–12 µg) or an inert placebo twice a week for 6 weeks, both in the clinic and at home (Lisa Reynolds 2022, personal communication). Blinding maintenance and treatment expectancy will be measured as secondary outcomes.
3,4-Methylenedioxymethamphetamine
A 2-center New Zealand Phase 2 trial (Universities of Otago and Auckland [Otago/Auckland] ACTRN12619001334190) aims to recruit 32 participants with advanced-stage cancer. It will assess the effect of psychotherapy with either 120 mg MDMA or 20 mg methylphenidate. Methylphenidate shares MDMA’s stimulant properties and appears effective in blinding a prior psilocybin study (Griffiths et al. Reference Griffiths, Richards and McCann2006). Finally, an open-label trial with a novel protocol in the USA, sponsored by MAPS (MAPS NCT05584826), will recruit 10 dyads of a patient with cancer and a significant other (n = 20). Each dyad will undergo 2 MDMA-assisted therapy sessions with 100 mg MDMA and 6 additional psychotherapy sessions over 8 weeks. The primary outcomes are the Adjustment Disorder New Module (ADNM-20) and Couples Satisfaction Index (CSI-16).
Discussion
Our review of pipeline trials of psychedelic medicines for end-of-life depression, anxiety, and existential distress demonstrates the continued momentum of research in this area. The 25 included studies were heterogenous and focused chiefly on psilocybin and ketamine. A striking observation is that drug treatment combined with psychotherapy is unusual (2/11) in ketamine trials and almost universal in current trials of the other 3 drugs (13/14). This may be a consequence of most ketamine trials using the drug for other than its psychedelic properties.
The findings expected from these trials will augment existing knowledge in several ways. So far, there is limited evidence on psychedelic assisted-group therapy in patients with a life-threatening illness. A pilot study by Anderson et al. (Reference Anderson, Danforth and Daroff2020) demonstrated the efficacy of group psychotherapy with individual psilocybin administration in patients with chronic AIDS. More extensive evidence documents the feasibility of psychedelic-assisted group therapy for treating mood, personality, and alcohol use disorders (Trope et al. Reference Trope, Anderson and Hooker2019). Hence, the 2 psilocybin group intervention trials (Utah NCT04522804; Maryland NCT04593563) will set the stage for the next phase of group-focused research. Group interventions have the potential to increase the scalability and cost-effectiveness of psychedelic-assisted therapies.
Pipeline trials also explore the potential benefits of microdosing psychedelics in the end-of-life context. Although low-dose LSD and psilocybin had previously been used as short-term active placebos (Gasser et al. Reference Gasser, Holstein and Michel2014; Griffiths et al. Reference Griffiths, Johnson and Carducci2016), clinical trials are now set to evaluate the effects of repeated microdosing in patients with life-threatening illness (HRCNZ 2021; UCLA NCT05403086). Unlike full-dose regimens, microdosing in a research context refers to the regular ingestion of sub-hallucinogenic amounts of psychedelics (10%–12% of a full dose by convention) (Polito and Liknaitzky Reference Polito and Liknaitzky2022). Home dosing is possible with the less intense experience involved in this practice (Murphy et al. Reference Murphy, Sumner and Evans2021). Additionally, results from the Griffiths et al.’s (Reference Griffiths, Johnson and Carducci2016) study highlighted that more of the participants receiving high-dose psilocybin reported psychological discomfort than those in the low-dose arm. Thus, microdosing may offer an alternative approach that is more acceptable to patients and (similar to group therapy) has the potential to reduce demand on clinician time.
The diversity of ketamine trials in progress will add to emerging evidence of its use in the end-of-life setting. Although most of the pipeline trials attempt to avoid the psychotomimetic effects of ketamine with lower doses, they can still provide valuable data on the feasibility and tolerability of ketamine use in the palliative care population, which is currently lacking in the literature (Lee et al. Reference Lee, Sheehan and Chye2021). Concerns regarding durability of mood improvement appear more of an issue with ketamine than with classic psychedelics (Bahji et al. Reference Bahji, Vazquez and Zarate2021). KAP trials (KRF NCT05214417; Northwell NCT05344625) are hence particularly awaited and expected to show the extent to which ketamine benefits can be augmented or extended by the presence of psychedelic experiences.
Despite the anticipated advances, as outlined above, expected from current pipeline trials, important methodological issues and research gaps will remain. Fundamentally, the psychoactive properties of psychedelics pose significant difficulties in blinding (Nichols and Barker Reference Nichols and Barker2016), while expectancy effects, both positive and negative (Aday et al. Reference Aday, Heifets and Pratscher2022) require rigorous control. Concerns have thus been raised about the possible overestimation of treatment effects in psychedelic studies (Butler et al. Reference Butler, Jelen and Rucker2022; Muthukumaraswamy et al. Reference Muthukumaraswamy, Forsyth and Lumley2021). The substantial proportion (13/27) of RCTs among identified trials is encouraging, as is the range of active placebos employed to enhance blinding.
Unfortunately, only 3 trials plan to assess the integrity of masking and expectancy (HRCNZ 2021; Cedars-Sinai, NCT02836288; UCLA, NCT05403086). Moreover, most ketamine trials lack active placebo control. Few published psychedelic studies assess expectancy and blinding (Muthukumaraswamy et al. Reference Muthukumaraswamy, Forsyth and Lumley2021), emphasizing the importance of including such measures in future RCTs. Additionally, following the largest (n = 300) pipeline Phase 2 trial (NYU, NCT05398484), more extensive Phase 3 studies will be needed to confirm therapeutic findings and establish a safety profile in the long term. Lastly, further head-to-head comparisons between psychedelics as the 2 pipeline trials aimed at (NIMH, 2020-005037-32; UCLA, NCT05403086) are needed to identify optimal agents for the specific indications and clinical context. This would also require additional MDMA and LSD trials as they currently constitute a minority of the available evidence (Schimmel et al. Reference Schimmel, Breeksema and Smith-Apeldoorn2022).
Our review highlights both the extent and challenges of research into the use of psychedelics in palliative care. The very nature of the target population poses barriers to conducting clinical trials. Recruitment can be hindered by various factors, including engagement-limiting anhedonia (Lee et al. Reference Lee, Chang and DiGiacomo2022) and the often competing priorities of patients and families. There are also concerns about possible interactions of psychedelics when co-administered with medications commonly used at the end of life (Sarparast et al. Reference Sarparast, Thomas and Malcolm2022). Despite their promising potential, the prescription of psychedelics at the end of life also raises bioethical considerations, particularly regarding the possibility of interfering with one’s pre-existing belief system and sense of identity (Smith and Sisti Reference Smith and Sisti2021). Modern palliative care calls for a multi-dimensional approach to improve quality of life, addressing physical, psychological, social, and spiritual or existential needs (Davies and Higginson Reference Davies and Higginson2004). Available evidence indicates that psychedelic-assisted therapies can decrease physical distress related to pain and also promote improved sleep in this patient population (Maia et al. Reference Maia, Beaussant and Garcia2022). More importantly, the import of mystical-type experience, defined by feelings of unity, interconnectedness, peace, and joy, as well as senses of sacredness, ineffability, and transcendence, may address elements at the core of psycho-existential suffering (MacLean et al. Reference MacLean, Johnson and Griffiths2011). Preliminary evidence has demonstrated positive psychological effects and reduced psychiatric and existential distress following psychedelic treatments (Maia et al. Reference Maia, Beaussant and Garcia2022; Schimmel et al. Reference Schimmel, Breeksema and Smith-Apeldoorn2022). Some also propose that psychedelic experiences extend beyond the alleviation of suffering and enhance social relationships that could be deeply meaningful for people approaching end of life (Earp Reference Earp2018).
This review presents several limitations. The literature search was mainly based on data gleaned from clinical registries, which may not accurately or entirely reflect trial details in real time. One author (XJ) reviewed individual studies with active supervision, analysis, and critical input from the other authors.
Conclusion
Addressing the psychological and physical needs of patients approaching end of life is an enduring clinical priority. Existing studies support the potential role of psychedelic medicines in this area, but much uncertainty remains. Our scoping review highlights ongoing scientific interest internationally and identifies pipeline trials set to provide important additions to the evidence base. More extensive, methodologically stronger trials will be needed to address blinding and expectancy problems. There will also be a need for head-to-head comparisons of different psychedelics for particular indications.
Acknowledgments
The authors thank Dr Lisa Reynolds, University of Auckland, for providing additional information on her trial supported by the Health Research Council of New Zealand.
Funding
NRH has received research funding from MindBio Therapeutics Ltd to conduct further work in psychedelic microdosing. NRH and DBM have also contracted with atai Life Sciences for unrelated research work.
Competing interests
The authors declare none.