Introduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis complex, is a major public health concern worldwide [1]. Despite an observed marginal decline in TB incidence over the past few decades, the World Health Organisation (WHO) has estimated that over two billion people have latent TB infection (LTBI), preventing realisation of the ambitious goal of ending TB globally by 2035 [Reference Lienhardt2]. Without an effective intervention, approximately 5–10% of people infected with TB may develop active TB disease due to reactivation of latent infection [Reference Parekh and Schluger3]. In view of the huge burden of latent TB infection globally, LTBI control is an essential step towards TB elimination when used in combination with effective TB case detection and treatment [Reference Stuurman4]. Previous studies have demonstrated that identification of people who are highly susceptible to latent TB reactivation, in combination with therapy to prevent active disease and transmission, can all contribute to reduction of TB burden [Reference Sterling5]. While multiple studies have reported that isoniazid (INH) therapy is highly effective in preventing TB reactivation, the role of INH for managing disease in individuals with LTBI caused by multidrug-resistant tuberculosis (MDR-TB) remains uncertain, due to the absence of randomised clinical trial reports [Reference Churchyard, Fielding and Grant6, Reference Ayele7].
China has the third largest TB burden in the world, with an estimated 0.92 million incident cases and 38 000 TB deaths in 2015 [1]. In addition, a recent population-based, multicentre, prospective cohort study has demonstrated that one-quarter of people living in China are infected with M. tuberculosis [Reference Gao8]. The high prevalence of LTBI in China highlights the urgent need to take measures to prevent TB transmission from LTBI populations. Unfortunately, to date there is still no national guidance on LTBI management in China, with few recommendations included within an overall strategy for TB prevention in high-risk populations. One such special population consists of students who study and/or live in crowded quarters (schools) in close contact for long periods of time. Detection of people with active TB within these special populations is achieved through routine screening using tuberculin skin tests (TSTs) and chest X-rays [Reference Wang9, Reference Ma10]. LTBI individuals are required to receive 6 months of INH therapy to prevent reactivation of latent infection. Although WHO opposes the clinical application of INH therapy for prevention of drug-resistant TB, this therapy is still widely used in China for TB prevention in close contacts of MDR-TB patients, regardless of the index case drug susceptibility profile. Hence, retrospective observations should provide important information regarding the effectiveness of INH therapy for MDR-TB prevention. In this study, we identified a single MDR-TB outbreak in a high school in northern China with a TB incidence rate of 49.2 cases per 100 000 people in the general population. Our aim was to describe our retrospective findings regarding protective efficacy of INH therapy in prevention of MDR-TB activation in this cluster.
Methods
Study setting
The research site was a well-managed boarding high school in a county in North China. In December 2016, this local high school first reported a case of TB. All close contacts of the index case, defined as the students from the same class, were screened by TST and chest X-ray. From students with imaging abnormalities suspicious for TB, samples were further collected and subjected to laboratory testing that included smear microscopy, solid culture and immunological assays. Active cases were defined as individuals with positive smear results or clinical findings consistent with active TB, as defined by national guidelines. In addition, students with indurations of ⩾15 mm and normal radiological features were classified as having LTBI and were advised to receive isoniazid preventive therapy (IPT) against TB on a voluntary basis [Reference Wang11].
Treatment
As recommended by the World Health Organisation, the 9-month INH regimen was used for the preventive treatment of students with LTBI [Reference Wang11]. If students with LTBI progressed to active TB cases, they received the standardised first-line treatment regimen of 2 months of IREZ (Isoniazid, Rifampicin, Ethambutol, Pyrazinamide) plus 4 months of IR in the local TB dispensary. Upon patient arrival at the Beijing Chest Hospital, treatment regimens were formulated based on GeneXpert and phenotypical drug susceptibility testing (DST) results. Primary clinical outcomes of patients were monitored monthly for sputum culture status using the solid Löwenstein–Jensen (L–J) culture method.
Laboratory examination
Ziehl–Neelsen staining for detection of acid-fast bacilli was performed directly on sputum samples as described previously [Reference Pang12]. In addition, clinical samples were digested with N-acetyl-L-cysteine-NaOH in sodium citrate (1.5% final NaOH concentration), vortexed for 30 s and incubated for 15 min at room temperature. The processed samples were then inoculated onto L–J medium. MTB isolates identified using the Tibilia TB Rapid Test (Chuangxin, Hangzhou, China) were further subjected to phenotypic DST using the absolute concentration method on L–J media using a panel of anti-TB drugs specified by the National Clinical Laboratory on Tuberculosis, Beijing Chest Hospital, Capital Medical University [Reference Zhang13]. Meanwhile, 1 mL of sputum sample was mixed with 2 ml Xpert MTB/RIF sample reagent and incubated at room temperature for 15 min. Next, 2 ml of mixture was added to a test cartridge and loaded onto the Xpert instrument following the manufacturer's instructions. MTB and RIF resistance results were automatically scored by the instrument.
Genotyping
Crude DNA was extracted from freshly cultured bacteria by heating cell suspensions at 95 °C in a water bath for 30 min [Reference Pang14]. After centrifugation to remove cellular debris, DNA in the supernatant was used as the template for PCR amplification. Classical 24-locus Mycobacterial Interspersed Repetitive Units-Variable Numbers of Tandem Repeat (MIRU-VNTR) and spoligotyping analyses were performed to genotype isolates collected from bacteria-positive cases as previously reported [Reference Pang14, Reference Supply15]. Briefly, each PCR mixture was prepared in a volume of 20 µl containing 10 µl 2 × PCR Mix (Genestar, Beijing, China), 2 µl of crude DNA template and 0.2 µM of each primer pair. After 35 cycles of amplification, amplicon sizes were analysed by 1.5% agarose electrophoresis. After comparisons of genotypic profiles, clustered cases were defined as cases harbouring strains that shared the same MIRU-VNTR profile.
Statistical analysis
Fisher's exact test was used to evaluate the efficacy of preventive treatment for latent TB cases. All calculations in this study were carried out using the SPSS program (SPSS version 17.0, SPSS Inc., Chicago, IL, USA). Differences were declared significant for P values <0.05.
Results
In January 2017, pulmonary TB was identified in a 17-year-old boy in the third year of high school in a city in northern China. The student experienced fever, cough and chest pain in December 2016. After treatment with cephalosporin for 1 month, he was hospitalised due to the increase in the severity of symptoms. Based on observation of positive indicators using smear microscopy and radiographic examination, he was diagnosed as an active TB patient in the prefectural hospital.
As a result of this case, local health authorities conducted a close contact investigation. The TST and chest X-ray were used to screen students and faculty from the same class. Subsequently, a total of 53 close contacts of the primary case were screened and 23 students had positive TST results, yielding a TB infection rate of 43.4%. Two students were confirmed as active TB cases due to abnormal X-ray results. Of the 21 LTBI students, only five (5/21, 23.8%) received voluntary preventive treatment, whereas the other 16 students refused preventive treatment (Fig. 1).

Fig. 1. Flow chart for the close contact investigation.
Subsequently, a total of 11 cases of TB were identified during the 6-month follow-up, including eight pulmonary bacteria-positive forms and three pulmonary bacteria-negative forms of disease. Of the five LTBI students receiving IPT, two pulmonary TB cases (40.0%) emerged in March and April of 2017, for an active TB rate not significantly different from the corresponding rate for the non-IPT group (4/16, 25.0%, P = 0.598). In addition, three students (3/30, 10.0%) with negative TST results developed active TB in the follow-up period. Notably, of the 11 subsequently identified cases, five (45.5%) lived in the same dormitory with the index case at the boarding school (Figs 1 and 2).
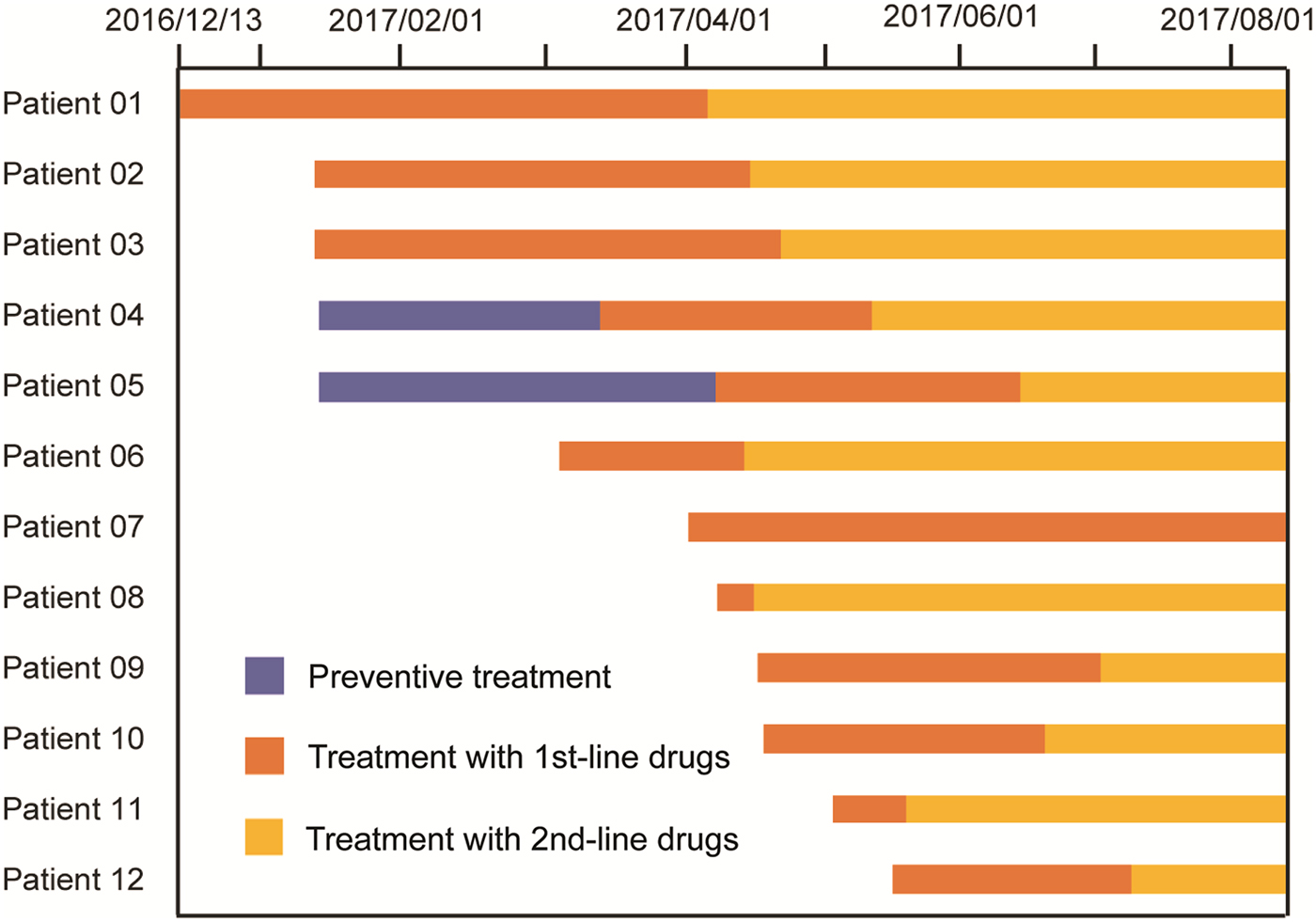
Fig. 2. Therapeutic process of 12 TB cases from this outbreak.
All TB cases were first treated with the standardised first-line regimen (2IREZ/4IR) in the local TB hospital. However, 11 out of 12 active TB cases exhibiting poor treatment outcomes were admitted to the Beijing Chest Hospital after several months of anti-TB treatment. At the Beijing Chest Hospital, GeneXpert and phenotypic DST were first conducted to determine the drug susceptibility profiles for MDR-TB infecting these patients. In April, the index case was first diagnosed with RIF-resistant MTB by GeneXpert, with additional INH resistance further confirmed by in vitro DST. Subsequent laboratory testing revealed that MTB of the other eight patients were also resistant to RIF and INH, but susceptible to all second-line drugs tested. Thereafter, treatment regimens were changed to moxifloxacin, amikacin, pyrazinamide, cycloserine, prothionamide and ethambutol. For the two patients without available DST results, the same second-line regime was used, as informed by their prior contact with the index MDR-TB case and their unfavourable clinical response to the first-line treatment regimen. In contrast, one bacteria-negative patient receiving the first-line treatment regimen exhibited a favourable outcome using criteria of clinical evaluation and chest radiography. Further genotypic data demonstrated that all nine Beijing genotype isolates from this TB outbreak harboured the same MIRU-VNTR profile, confirming the outbreak was caused by transmission of the same MDR-TB strain (Table 1).
Table 1. Demographic characteristics, genotype and drug susceptibility of 12 TB patients identified in this study

RIF, rifampicin; MTB, Mycobacterium tuberculosis; SM, streptomycin; EMB, ethambutol; KAN, kanamycin; AMK, amikacin; CAP, capreomycin; OFLX, ofloxacin; LFX, levofloxacin; MOX, moxifloxacin; PTO, protionamide; CYC, cycloserine; PAS, para-aminosalicylic acid.
a SIT from SpolDB4.0.
b The order of MIRU-VNTR loci: ETRA, ETRB, ETRC Mtub04, Mtub21, Mtub29, Mtub30, Mtub34, Mtub39, MIRU02, MIRU04, MIRU10, MIRU16, MIRU26, MIRU20, MIRU23, MIRU24, MIRU26, MIRU27, MIRU31, MIRU39, MIRU40, Qub26, Qub11b, Qub4156.
c Patients without positive culture results.
Discussion
Our study reported a longitudinal MDR-TB outbreak in a high school in northern China after epidemiological investigation, with verification using a molecular genotyping method. Numerous previous studies incorporating epidemiologic and laboratory testing have strongly suggested that community transmission of MDR-TB plays a major role in the increasing prevalence of MDR-TB in China [Reference Yang16, Reference Zhao17]. Thus, early intervention to control MDR-TB would be expected to prevent transmission of MDR-TB inside institutions where people live and work in close contact, such as schools. Because this study focused on an outbreak in a school setting, the results provide several insights. First, the results of this investigation indicate that a diagnostic delay in identification of an index case can play a primary role in transmission of TB infection among students. Specifically, after initial appearance of clinical symptoms of pulmonary TB, it took more than 1 month to reach a final diagnosis of TB for the index case in this study. This delay was due in part to the fact that clinical manifestations of TB are easily confused with symptoms of respiratory tract infections that often resolve after antibiotic treatment [Reference Li18]. However, a recent meta-analysis has estimated that a high proportion of TB suspects in China do not seek care even after 3 weeks of persistent cough, especially in rural settings [Reference Li18]. Moreover, the students in this study were all in grade 12 and thus were due to take the nationwide college entrance examinations by the end of this school year, with associated high stress during preparation for examinations [Reference Davey, De Lian and Higgins19]. Consequently, such students tend to avoid long duration anti-TB treatment that would interrupt their studies, delaying treatment until after their examinations. This common scenario highlights the urgent need to conduct routine monitoring via physical examinations of high-risk populations.
Second, an important finding of this study was that INH preventive treatment did not prevent progression of MDR-TB patient status from LTBI to active disease. Progression is mainly due to MTB drug resistance to RIF and INH and underscores the lack of effective treatment options for prevention of latent MDR-TB reactivation [Reference Fox, Oxlade and Menzies20]. In view of its potent efficacy against MTB and a low rate of severe adverse events, a report from Fox et al. suggests that fluoroquinolone (FQ) preventive therapy is likely to reduce transmission of MDR-TB among infected contacts of individuals with MDR-TB [Reference Fox, Oxlade and Menzies20]. However, this raises some concerns regarding FQ preventive therapy use in the student population in this study. FQ antibiotics are considered to be contraindicated in paediatric patients because of their potential for cartilage damage [Reference Schaaf21]. As a consequence, regimens consisting of second-line drugs to prevent MDR-TB are urgently needed for treatment of this high-risk population. In addition, the timeliness of initiation of second-line preventive therapy depends on early diagnosis of the index patient infected with MDR- or RIF-resistant (RR-) TB. Unfortunately, our data reveal that a diagnosis confirming the presence of RR-TB was issued 3 months after initial diagnosis by GeneXpert in the Beijing Chest Hospital. Consequently, this diagnostic delay hampered rational treatment using second-line antibiotic regimens for the index patient. More importantly, two LTBI students received ineffective IPT due to delayed DST results, with the potential risk of unnecessary adverse drug-associated complications. Therefore, this study emphasises the application of point-of-care nucleic acid testing rather than time-consuming phenotypic DST to achieve the best possible treatment outcomes, especially in resource-limited settings in China.
Third, although most subsequent cases in this outbreak exhibited poor responses to standard first-line therapy, this basic regimen was effective in the treatment of patient 07. Given that MDR-TB is inherently resistant to RIF and INH, one possible explanation for this unexpected result may be that this patient was an endogenous relapse case rather than exogenously re-infected by MDR-TB during this outbreak. This hypothesis should be confirmed using molecular genotyping evidence [Reference Lai22], as no positive culture was obtained from this patient. In addition, immune status differences among individuals may also explain the variable clinical outcomes observed for patients receiving only the first-line anti-TB treatment regimen. Notably, we also found that five of eight roommates of the index case developed active TB in the follow-up period, a significantly higher proportion of TB incidence than observed for other close contacts (P < 0.01). Because TB infection occurs by inhalation of airborne TB organisms emitted by individuals with active disease [Reference Miller-Leiden23], the high concentration of airborne bacteria within the dormitory may greatly contribute to the high TB incidence observed in this setting. Therefore, our observations suggest that students living in the same dormitory as the index case should receive greater monitoring during the follow-up period given the extremely high transmission of TB to close contacts.
We also acknowledge several obvious limitations of this study. First, the incidence of TB cases from this outbreak may be underestimated due to the short follow-up period. Considering that the index case was infected with MDR-TB, the duration of follow-up should be extended to 2 years to align with the WHO guidelines [24]. Second, although the diagnostic delay of the index MDR-TB case was associated with poor clinical outcomes of anti-TB preventive treatment in this report, a more rapid molecular diagnostic screening test for TB could not be evaluated due to the lack of a cost-effective screening method.
In conclusion, our data demonstrates that a diagnostic delay in identification of the index MDR-TB patient during an outbreak plays a primary role in the transmission of MDR-TB infection among students. Moreover, IPT appeared to have no effect on preventing the progression of MDR-TB cases from LTBI to active cases in this outbreak. Nevertheless, this study emphasises the application of point-of-care nucleic acid testing rather than time-consuming phenotypic DST to achieve the best possible cure rate. In view of the high prevalence of MDR-TB in China, an improved treatment regimen for latent MDR-TB infection is urgently needed for students and other high-risk populations. In addition, the high incidence of TB among roommates of the index case indicates that close contacts are highly susceptible to TB infection, and during outbreaks, they should be monitored for TB by both initial and follow-up screening.
Acknowledgements
This study was partially supported by the Tongzhou District Science and Technology Committee (KJ2017CX076) and the National Major Project (2015ZX10003001-001).
Declaration of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.





