Barley is a high-fibre cereal grain that contains significant levels of both β-glucan and insoluble fibre, and it has been classified as having a low glycaemic index (GI). Health professionals recommend choosing foods that elicit a low postprandial glycaemic response (PPGR), without a concomitant increase in insulin, to improve glycaemic control( Reference Dworatzek, Arcudi and Gougeon 1 ). Furthermore, the potential for the utilisation of barley in functional foods is high( Reference Ames, Rhymer and Rossnagel 2 ). Barley has been evaluated for incorporation into several food products, including bread( Reference Jacobs, Izydorczyk and Preston 3 – Reference Chaudhary and Weber 5 ), pasta( Reference Kaur, Sharma and Nagi 6 ), biscuits( Reference Sudha, Vetrimani and Leelavathi 7 ), noodles( Reference Lagasse, Hatcher and Dexter 8 ), tortillas and chips( Reference Ames, Rhymer and Rossnagel 2 , Reference Prakash, Naik and Hussain 9 ). Government-approved health claims have focused on the effect of the soluble fibre (β-glucan) content of barley on PPGR( 10 ), but there are several factors that could be manipulated in barley foods to optimise PPGR, including the amount and type of fibre and the type of starch.
Barley starch, like that of other cereal grains, contains two forms of starch, amylose and amylopectin, each with specific physico-chemical properties. Amylose is a starch that has a tightly packed structure, which slows and sometimes (in the case of resistant starch formed from retrograded amylose) prevents digestive enzymes from breaking it down to glucose. Amylopectin is a starch that has a branched structure and is easier to digest( Reference Byrnes, Miller and Denyer 11 ). Numerous studies have shown that meals made with high-amylose or high-resistant starches (rice, maize and wheat) elicit a lower PPGR than meals made from high-amylopectin starches( Reference Byrnes, Miller and Denyer 11 – Reference Hospers, Van Amelsvoort and Westrate 14 ). Therefore, it is plausible that a barley variety with a significantly higher amylose:amylopectin ratio would result in a food product with a lower PPGR. However, a study on plasma glucose and insulin after the consumption of barley varying in amylose content (7–44 % amylose) showed marginal differences in glycaemic response( Reference Granfeldt, Liljeberg and Drews 15 ), which suggests that other constituents, such as fibre, might play a role. By choosing a barley food with higher fibre levels, one would anticipate a blunted PPGR( Reference Bourdon, Yokoyama and Davis 16 – Reference Liljeberg, Granfeldt and Bjorck 18 ). However, fibre can be classified as soluble or insoluble. β-Glucan is a soluble fibre which has been shown to lower PPGR. Numerous clinical trials have demonstrated that oat and barley foods containing β-glucan can lower blood glucose levels through several different mechanisms in both the upper gastrointestinal tract (GIT) and the lower intestinal tract( Reference Tosh 19 ). In the upper GIT, soluble β-glucan can develop a high viscosity, thereby slowing the mixing of the meal with digestive enzymes( Reference Marciani, Gowland and Spiller 20 ) and slowing the rate of gastric emptying( Reference Braaten, Wood and Scott 21 – Reference Panahi, Ezatagha and Temelli 23 ). Insoluble dietary fibre (IDF), which includes resistant starch, like soluble dietary fibre, cannot be digested into glucose, so it is unclear whether the total fibre or the type of fibre has a greater effect on postprandial glucose levels. Barley fibre composition is significantly influenced by variety to a greater extent than other cereal grains are( Reference Izydorczyk and Dexter 24 ). Large variation in fibre composition has been reported for barley genotypes from different origins, with the levels of total dietary fibre in wholegrain ranging from as low as 9 % to greater than 30 %( Reference Andersson, Lampi and Nystrom 25 – Reference Yalcin, Celik and Akar 30 ). Similarly, a wide range of β-glucan content has been reported for barley (3–15 %)( Reference Andersson, Lampi and Nystrom 25 – Reference Oscarsson, Andersson and Salomonsson 28 , Reference Yalcin, Celik and Akar 30 , Reference Panfili, Fratianni and Di Criscio 31 ), with Canadian hull-less varieties typically containing 4–9 % β-glucan( Reference Bhatty 32 , Reference Zheng, Rossnagel and Tyler 33 ). It is also possible to further manipulate the fibre composition of barley through milling( Reference Ames, Rhymer and Rossnagel 2 , Reference Chaudhary and Weber 5 , Reference Klamczynski and Czuchajowska 34 – Reference Izydorczyk, Dexter and Desjardins 36 ). The physiological response to food products made with specific barley cultivars that have undergone optimised milling and processing treatments is unknown; this knowledge is critical in determining whether or not these optimisation treatments will have a beneficial influence on glycaemia and whether they are superior from a nutritional perspective.
The present study investigated the use of cultivar selection and milling fractions to formulate 100 % barley flour tortillas with varying proportions of amylose/amylopectin, resistant starch, soluble fibre and insoluble fibre. Subsequently, a single-site, double-blind, randomised, controlled clinical trial was carried out with the objective of better understanding the relationship between the consumption of barley tortillas with varying starch and fibre compositions and PPGR while keeping available carbohydrate constant. Hormones that play a role in PPGR and satiety, such as the incretin glucagon-like peptide-1 (GLP-1) and the satiety hormone peptide YY (PYY), were also measured.
Experimental methods
Barley samples and compositional analysis
Grain samples of four hull-less barley genotypes were obtained from the Crop Development Centre, University of Saskatchewan, including two waxy, low-amylose varieties (CDC Candle, CDC Fibar) and two high-amylose types (SB94893, SH99250). Starch amylose content was confirmed according to a method developed by Schoch( Reference Schoch and Whistler 37 ). Grain was milled into wholegrain flour (WGF) and several fractions, including straight-grade flour (SGF), ‘dusted flour from shorts’, ‘dusted flour from bran’, ‘concentrated shorts’, ‘concentrated bran’ and ‘pearlings’, as described previously by Harding et al. ( Reference Harding, Storsley and Thandapilly 38 ). A commercial barley fraction with concentrated β-glucan content was obtained from PolyCell Technologies. The composition of the flour fractions was analysed using American Association of Cereal Chemists (AACC) International Approved Methods for insoluble and total dietary fibre (method 32-07.01( 39 )), β-glucan (method 32-23.01( 40 )), total starch (method 76-13.01( 41 )) and resistant starch (method 32-40.01( 42 )) as well as available carbohydrate content( Reference McCleary 43 ). Based on the composition results (Table 1), CDC Fibar was selected for use as the low-amylose barley (0 % amylose in its starch), because it had the highest fibre levels. Barley genotype SH99250 was selected for use as the high-amylose variety (42 % starch amylose) (Table 1). Starch was extracted from the whole grain of each cultivar, and its amylose content was measured in order to confirm the amylose content of the different genotypes. High-amylose barley cultivars have been shown to have increased levels of dietary fibre because they have higher levels of resistant starch after heat processing( Reference Björck, Eliasson and Drews 44 ), which may result in a lower glycaemic response. Therefore, it was hypothesised that SH99250 as a high-amylose cultivar would contain higher resistant starch levels after being cooked into tortillas, would result in a low glycaemic response and would consequently have the potential for industrial significance in the future.
Table 1 Content of various carbohydrates in milling fractions from four barley genotypes

db, Dry basis; WM, wholemeal; SGF, straight-grade flour; DFFB, dusted flour from bran; DFFS, dusted flour from shorts; CB, concentrated bran; CS, concentrated shorts; P, pearlings.
Preparation of barley flour blends
By varying the proportions of different milling fractions and making small adjustments to the serving sizes, several flour blend scenarios were created and examined for possible use in test meal treatments (Table 2). We selected five scenarios to supply a consistent 50 g of available carbohydrate per serving while varying the following starch/fibre levels: (1) low amylose (0 %) v. high amylose (42 %) with similar β-glucan and insoluble fibre content; (2) low β-glucan (4·5 g) v. medium β-glucan (7·8 g) v. high β-glucan (11·6 g) with similar amylose and insoluble fibre content; (3) low insoluble fibre (7·4 g) v. high insoluble fibre (19·6 g) with similar amylose and β-glucan content. All barley test meal formulations had more than 4 g of β-glucan per serving (Table 2). The high-amylose variety SH99250 was used for the high-amylose dusted flour fractions (HA-DFF) flour blend; it was prepared by combining dusted flour from bran (37·7 %) and dusted flour from shorts (62·3 %). All other flour blends (WGF, SGF, bran flour with high β-glucan (BF-BG) and bran flour with high IDF (BF-IDF)) were prepared from the low-amylose cultivar CDC Fibar, as described by Harding et al. ( Reference Harding, Storsley and Thandapilly 38 ). These flour blends were used to prepare the scenarios indicated earlier: (1) WGF (low amylose) v. HA-DFF (high amylose); (2) SGF (low β-glucan) v. WGF (medium β-glucan) v. BF-BG (high β-glucan); (3) WGF (low insoluble fibre) v. BF-IDF (high insoluble fibre).
Table 2 Barley test meal treatment scenarios calculated to deliver varying amounts of fibre components in a single serving
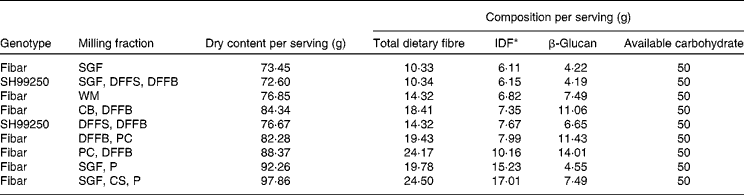
IDF, insoluble dietary fibre; SGF, straight-grade flour; DFFS, dusted flour from shorts; DFFB, dusted flour from bran; WM, wholemeal; CB, concentrated bran; P, pearlings; PC, Polycell Technologies high β-glucan fraction; CS, concentrated shorts.
* IDF was estimated for flour blend scenario calculations (IDF = total dietary fibre − β-glucan soluble fibre). All other components were calculated using analytical data obtained from the milling fractions.
Test meal processing, analysis and presentation
Barley tortillas made only from barley flour and water, as developed previously at the Cereal Research Centre, Agriculture and Agri-Food Canada( Reference Ames, Sopiwnyk and Therrien 45 ), were chosen as the test food product, because they required no additional ingredients that could interfere with the results. The five selected flour blends were processed into tortillas in the Metabolic Kitchen Facility at the Richardson Centre for Functional Foods and Nutraceuticals (Winnipeg, Manitoba, Canada) using the following basic protocol: mix 200 g of flour with water for 5 min (GRL200 Mixer, Muzeen & Blythe), rest dough at room temperature for 10 min, sheet dough (National MFG Company), die cut to 14·5 cm diameter and cook on a 250°C tortilla grill (Bakery Equipment and Service Company). The water amount, sheeting thickness and cooking times were optimised for each flour blend to manage variation in the dough-handling properties between treatments. Tortilla moisture content was measured using the two-stage AACC International Approved Method 44-15A( 46 ) and used to calculate the serving size required to deliver 50 g of available carbohydrate (therefore, serving sizes ranged from approximately seven to ten tortillas depending on the treatment; the average weight of each tortilla was 15·5 g, and the average approximate diameter was 14·5 cm). Full chemical analysis of the tortillas (using the methods described earlier for milling fraction analysis) confirmed the final composition of the prepared test meals (Table 3). A difference between the available carbohydrate and total starch content of the tortilla treatments was noted in the analysis. This difference was partly a result of the fact that glucose in starch is in an anhydrous form. When glucose is released after being exposed to amylases, it binds to another water molecule (per glucose), i.e. the molecular weight of glucose increases from 162 to 180 g/mol; therefore, the total starch expressed as available carbohydrate is 11·1 % higher. The remaining difference between available carbohydrate and starch content was likely due to free sugars in the tortilla samples, although these were not measured. Acid extraction of the tortilla samples was carried out( Reference Greenberg and Whitmore 47 ), and viscosity measurements of the acid extracts were made using a Brookfield DV-II+Pro Viscometer (LV-4 spindle, 60 rpm; Brookfield Engineering Laboratories) to determine whether the tortilla samples differed in viscosity (see online supplementary Fig. S1). As can be seen in Fig. S1, the tortillas differed in viscosity; the high-β-glucan tortillas (BF-BG) were more viscous than the other treatments were.
Table 3 Barley tortilla test meal treatment composition confirmed by analytical chemistry
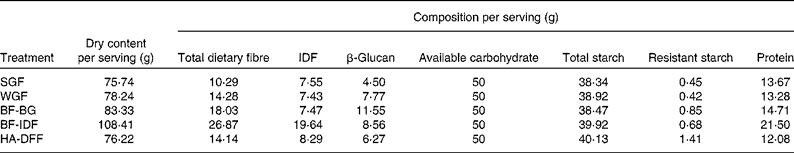
IDF, insoluble dietary fibre; SGF, straight-grade flour (low β-glucan/low IDF); WGF, wholegrain flour (medium β-glucan/low IDF); BF-BG, bran flour with high β-glucan/low IDF; BF-IDF, bran flour with high IDF/medium β-glucan; HA-DFF, high-amylose dusted flour fractions (medium β-glucan/low IDF).
Tortillas were stored frozen until use in the clinical trial. Before presenting test meals to the participants, tortillas were reheated in a Black & Decker three-tier food steamer for 10 min wrapped in wax paper. Warmed tortillas were then wrapped in aluminium foil and placed in a Bravetti buffet server until served.
Clinical trial
A single-site, double-blind, randomised, controlled, cross-over study was conducted at the I. H. Asper Clinical Research Institute at St Boniface Hospital in Winnipeg, Manitoba, Canada.
There were a total of twelve participants, aged 19–35 years with a glycated Hb of < 6 % and BMI between 20 and 31 kg/m2. They did not have any allergies to barley flour or any chronic diseases, such as CVD, hypertension, disorders affecting the GIT tract or thyroid disease, or require medications for these conditions. They did not require medication for glycaemic control or consume supplements which had an effect on blood glucose response. See Table 4 for details on the demographics of the participants.
Table 4 Demographics
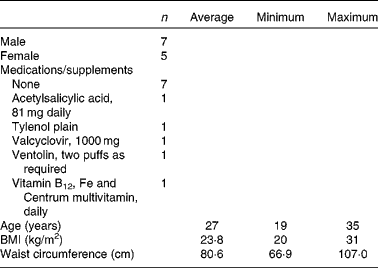
The present study was performed according to the guidelines in the Declaration of Helsinki. Ethics approval for the clinical trial was obtained from the University of Manitoba Biomedical Research Ethics Board and the St Boniface Hospital Research Review Committee. The present study was registered on http://www.clinicaltrials.gov (NCT00831285). The first participant's initial visit was on 6 May 2009, and the final visit of the last participant was completed on 18 February 2010. Participants provided written informed consent before undergoing any study-related procedures. A total of thirteen participants provided written informed consent, but one participant dropped out of the study after the first visit due to a physiological aversion to blood draws. The remaining twelve participants came in for six visits that were separated by at least 1 week. The test products consumed included one of five different barley tortillas (Table 3) or an orange-flavoured Trutol Glucose Tolerance Beverage containing 50 g dextrose (from Fisher Scientific) assigned in random order. Participants arrived at each clinic visit in the morning after an overnight fast. Blood was collected at fasting and at 15, 30, 45, 60, 120 and 180 min after the first bite/sip of the test product. Blood was collected into lithium heparin tubes for glucose analysis and EDTA tubes for hormone analysis. Dipetidyl peptidase IV inhibitor (Millipore) and Halt protease inhibitor (Pierce) were added immediately to the EDTA tubes and centrifuged at 1800 g for 10 min at 4°C to separate plasma. Plasma was stored at − 80°C until analysis.
Plasma glucose concentrations at each time point were determined using an enzymatic colorimetric kit (Genzyme Diagnostics). Plasma insulin concentrations at each time point were measured using an ultrasensitive ELISA kit from Alpco Diagnostics. Active GLP-1 and PYY concentrations at the 0, 30, 60, 120 and 180 time points were determined using an electrochemiluminescent immunoassay from Meso Scale Discovery. All samples and respective standards were analysed in triplicate for glucose and in duplicate for the hormones with a percentage CV of less than 10 %. Incremental AUC (iAUC) for glucose, insulin, GLP-1 and PYY was calculated as described by Brouns et al. ( Reference Brouns, Bjorck and Frayn 48 ). GI was calculated by dividing the glucose iAUC for each tortilla by the glucose drink iAUC and multiplying by 100.
Randomisation procedures
A list of unique product code numbers was prepared to ensure that volunteers received the treatments in random order. Randomisation was performed by the research team. After randomisation occurred, the study nurse was responsible for ensuring that the assigned randomisation number was applied to all study documentation for that particular volunteer and for dispensing the foods with the matching randomisation number.
Blinding
Although participants knew whether they were consuming a food product or a liquid, both the research team and the volunteer were blinded from the time of randomisation and for the duration of the study in regards to which tortilla they ate during any given visit.
Statistical analysis
Mäkeläinen et al. ( Reference Mäkeläinen, Anttila and Sihvonen 49 ) incorporated similar methodology to explore of the effect of β-glucan on the glycaemic and insulin index with oat products. Their findings showed that n 10 participants were sufficient for attaining evaluable results. We recruited n 12 to allow us to reach our target sample while accounting for a small percentage of withdrawals by subjects who might be unable to complete the study.
The present study was designed to examine three effects: (1) the effect of low v. high amylose; (2) the effect of low, medium and high β-glucan; and (3) the effect of low v. high IDF on glycaemic response. For effects (1) and (3), paired t tests were used to determine differences in GI, iAUC and percentage change from baseline at 30 min between the groups. For effect (3), data were analysed using PROC MIXED with tortilla as a fixed effect and subject as a random variable using the SAS system version 9.2 (SAS Institute Inc.). PROC MIXED repeated measures analysis was used to determine differences between time points. Differences among means were determined by Lsmeans.
The primary outcome was iAUC for glucose. The secondary outcome was iAUC for insulin. Additional outcomes were GI and percentage change from baseline at 30 min.
Results
Glycaemic index
Foods can be classified as low GI ( < 55), medium GI (56–69) or high GI (>70). The plasma glucose curve after consumption of the glucose reference drink is shown in Fig. 1. The WGF tortillas (low amylose, medium β-glucan and low IDF) were classified as medium GI, whereas all of the other tortilla treatments were low GI (Table 5). The high-β-glucan (BF-BG) tortilla treatment had a 56–60 % lower GI as compared to both the low-β-glucan (SGF) and medium-β-glucan (WGF) tortilla treatments (Table 5). There was no significant difference in GI between the low-amylose (WGF) and high-amylose (HA-DFF) treatments (Table 5) or between the low-IDF (WGF) and high-IDF (BF-IDF) treatments (Table 5).

Fig. 1 Plasma glucose concentrations after consuming glucose reference drink. Values are means (n 12), with their standard errors represented by vertical bars. a,b,c,dMean values with unlike letters were significantly different (P< 0·05).
Table 5 Glycaemic index (GI) of barley tortillas (Mean values with their standard errors, n 12)
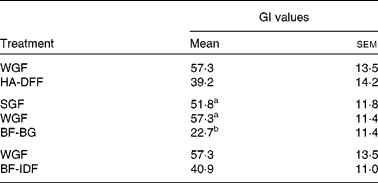
WGF, wholegrain flour (low amylose/medium β-glucan/low insoluble dietary fibre); HA-DFF, high-amylose dusted flour fractions (high amylose); SGF, straight-grade flour (low β-glucan); BF-BG, bran flour with high β-glucan (high β-glucan); BF-IDF, bran flour with high insoluble dietary fibre (high insoluble dietary fibre).
a,bMean values with unlike superscript letters were significantly different (P< 0·05).
Plasma glucose
There was no difference in the fasting plasma glucose concentrations between the participants at each visit (Fig. 2(A), (D) and (G)). There was no significant increase in plasma glucose from baseline at any time point after eating the high-β-glucan tortillas (Fig. 2(D)). Plasma glucose concentrations were 9–16 % higher at 30 min and 7–15 % higher at 45 min as compared to baseline levels for all of the other treatments, but plasma glucose concentrations returned to baseline levels by 45 min for the high-IDF tortillas (Fig. 2(A), (D) and (G)).

Fig. 2 Effect of barley tortillas on plasma glucose concentrations. Low v. high amylose (A–C); low v. medium v. high β-glucan (D–F); low v. high insoluble dietary fibre (IDF) (G–I). Plasma glucose over time (A, D, G); glucose incremental AUC (iAUC) (B, E, H); percentage change in plasma glucose from baseline at 30 min (C, F, I). WGF (![]() ), wholegrain flour (low amylose/medium β-glucan/low IDF); HA-DFF (
), wholegrain flour (low amylose/medium β-glucan/low IDF); HA-DFF (![]() ), high-amylose dusted flour fractions (high amylose); SGF (
), high-amylose dusted flour fractions (high amylose); SGF (![]() ), straight-grade flour (low β-glucan); BF-BG (
), straight-grade flour (low β-glucan); BF-BG (![]() ), bran flour with high β-glucan (high β-glucan); BF-IDF (
), bran flour with high β-glucan (high β-glucan); BF-IDF (![]() ), bran flour with high IDF. Values are means (n 12), with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05). * Mean value was significantly different from baseline for WGF treatment (P< 0·05). † Mean value was significantly different from baseline for BF-IDF treatment (P< 0·05). ‡ Mean value was significantly different from baseline for SGF treatment (P< 0·05). § Mean value was significantly different from baseline for HA-DFF treatment (P< 0·05).
), bran flour with high IDF. Values are means (n 12), with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05). * Mean value was significantly different from baseline for WGF treatment (P< 0·05). † Mean value was significantly different from baseline for BF-IDF treatment (P< 0·05). ‡ Mean value was significantly different from baseline for SGF treatment (P< 0·05). § Mean value was significantly different from baseline for HA-DFF treatment (P< 0·05).
The high-β-glucan treatment had a 61 % lower glucose iAUC as compared to the low-β-glucan treatment (Fig. 2(E)). The high-β-glucan treatment had a percentage change from baseline at 30 min that was 3·9–5·1 times lower than both the low- and medium-β-glucan treatments (Fig. 2(F)). There was no difference in the glucose iAUC or percentage change from baseline at 30 min between the low- and high-amylose treatments (Fig. 2(B) and (C)) or between the low- and high-IDF treatments (Fig. 2(H) and (I)).
Plasma insulin
Fasting plasma insulin concentrations were not significantly different between visits (Fig. 3(A), (D) and (G)). All treatments had significantly higher insulin concentrations at 15–60 min as compared to baseline levels (Fig. 3(A), (D) and (G)). The high-amylose and high-β-glucan treatments returned to baseline levels by 120 min, whereas the low-β-glucan treatment returned to baseline by 180 min. Plasma insulin concentrations were still higher than baseline levels at 180 min after eating the low-amylose/medium-β-glucan treatments with low IDF (WGF) and high IDF (BF-IDF).

Fig. 3 Effect of barley tortillas on plasma insulin concentrations. Low v. high amylose (A–C); low v. medium v. high β-glucan (D–F); low v. high insoluble dietary fibre (IDF) (G–I). Plasma insulin overtime (A, D, G); insulin incremental (iAUC) (B, E, H); percentage change in plasma insulin from baseline at 30 min (C, F, I). WGF (![]() ), wholegrain flour (low amylose/medium β-glucan/low IDF); HA-DFF (
), wholegrain flour (low amylose/medium β-glucan/low IDF); HA-DFF (![]() ), high-amylose dusted flour fractions (high amylose); SGF (
), high-amylose dusted flour fractions (high amylose); SGF (![]() ), straight-grade flour (low β-glucan); BF-BG (
), straight-grade flour (low β-glucan); BF-BG (![]() ), bran flour with high β-glucan (high β-glucan); BF-IDF (
), bran flour with high β-glucan (high β-glucan); BF-IDF (![]() ), bran flour with high IDF. Values are means (n 12), with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05). * Mean value was significantly different from baseline for WGF treatment (P< 0·05). † Mean value was significantly different from baseline for BF-IDF treatment (P< 0·05). ‡ Mean value was significantly different from baseline for SGF treatment (P< 0·05). § Mean value was significantly different from baseline for HA-DFF treatment (P< 0·05). ∥ Mean value was significantly different from baseline for BF-BG treatment (P< 0·05).
), bran flour with high IDF. Values are means (n 12), with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05). * Mean value was significantly different from baseline for WGF treatment (P< 0·05). † Mean value was significantly different from baseline for BF-IDF treatment (P< 0·05). ‡ Mean value was significantly different from baseline for SGF treatment (P< 0·05). § Mean value was significantly different from baseline for HA-DFF treatment (P< 0·05). ∥ Mean value was significantly different from baseline for BF-BG treatment (P< 0·05).
The high-β-glucan tortilla treatment had a 39 % lower iAUC for insulin as compared to the medium-β-glucan tortillas, which in turn had a 33 % lower insulin iAUC as compared to the low-β-glucan treatment (Fig. 3(E)). The low-β-glucan tortillas had a 64–176 % higher percentage change from baseline at 30 min for plasma insulin as compared to both the medium- and high-β-glucan tortillas (Fig. 3(F)). There was no significant difference between the high- and low-IDF tortillas in terms of insulin iAUC (Fig. 3(H)), but the high-IDF tortillas had a higher percentage change of plasma insulin from baseline at 30 min as compared to the low-IDF tortillas (Fig. 3(I)). There was no significant difference in insulin iAUC or percentage change from baseline at 30 min between the low- and high-amylose treatments (Fig. 3(B) and (C)).
Plasma glucagon-like peptide-1
There was no significant difference in fasting GLP-1 concentrations between the treatments (Fig. 4(A), (D) and (G)). All of the treatments had significantly higher plasma GLP-1 concentrations at 30 min. The GLP-1 concentrations after eating the high-amylose (HA-DFF) and high-β-glucan (BF-BG) tortillas returned to baseline by 60 min. The low-amylose/medium-β-glucan/low-IDF (WGF) and low-β-glucan (SGF) tortillas had plasma GLP-1 concentrations that were not significantly different from baseline by 120 min, but the GLP-1 concentrations after eating the low-β-glucan tortillas were higher than baseline at 180 min. The high-IDF tortillas had higher GLP-1 concentrations than baseline at every time point (Fig. 4(G)).

Fig. 4 Effect of barley tortillas on plasma glucagon-like peptide-1 (GLP-1) concentrations. Low v. high amylose (A–C); low v. medium v. high β-glucan (D–F); low v. high insoluble dietary fibre (G–I). Plasma GLP-1 over time (A, D, G); GLP-1 incremental AUC (iAUC) (B, E, H); percentage change in plasma GLP-1 from baseline at 30 min (C, F, I). WGF (![]() ), wholegrain flour (low amylose/medium β-glucan/low IDF); HA-DFF (
), wholegrain flour (low amylose/medium β-glucan/low IDF); HA-DFF (![]() ), high-amylose dusted flour fractions (high amylose); SGF (
), high-amylose dusted flour fractions (high amylose); SGF (![]() ), straight-grade flour (low β-glucan); BF-BG (
), straight-grade flour (low β-glucan); BF-BG (![]() ), bran flour with high β-glucan (high β-glucan); BF-IDF (
), bran flour with high β-glucan (high β-glucan); BF-IDF (![]() ), bran flour with high IDF. Values are means (n 12), with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05). * Mean value was significantly different from baseline for WGF treatment (P< 0·05). † Mean value was significantly different from baseline for BF-IDF treatment (P< 0·05). ‡ Mean value was significantly different from baseline for SGF treatment (P< 0·05). § Mean value was significantly different from baseline for HA-DFF treatment (P< 0·05). ∥ Mean value was significantly different from baseline for BF-BG treatment (P< 0·05).
), bran flour with high IDF. Values are means (n 12), with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05). * Mean value was significantly different from baseline for WGF treatment (P< 0·05). † Mean value was significantly different from baseline for BF-IDF treatment (P< 0·05). ‡ Mean value was significantly different from baseline for SGF treatment (P< 0·05). § Mean value was significantly different from baseline for HA-DFF treatment (P< 0·05). ∥ Mean value was significantly different from baseline for BF-BG treatment (P< 0·05).
There was no significant difference in GLP-1 iAUC or percentage change from baseline at 30 min between the low- and high-amylose treatments (Fig. 4(B) and (C)) or between the low-, medium- and high-β-glucan treatments (Fig. 4(E) and (F)). The high-IDF treatment had a 54 % higher GLP-1 iAUC as compared to the low-IDF treatment (Fig. 4(H)). There was no difference between the low- and high-IDF treatments in the percentage change from baseline at 30 min (Fig. 4(I)).
Plasma peptide YY
There was no significant difference in plasma PYY concentrations between time points or between treatments. There was also no significant difference in PYY iAUC or percentage change from baseline at 30 min between treatments (data not shown).
Discussion
Overall, the results of the present research provide knowledge about the effects of high-fibre barley consumption on one of the physiological factors that is associated with heart disease and diabetes: postprandial glucose and insulin response. The Canadian Diabetes Association recommends that people with diabetes replace high-GI foods with low-GI foods to improve glycaemic control( Reference Dworatzek, Arcudi and Gougeon 1 ), and the present study showed that barley tortillas made with high β-glucan or high IDF are low-GI foods. All of the barley tortillas elicited lower PPGR as compared to the glucose drink; however, increasing the amount of barley β-glucan in the tortillas improved PPGR further, whereas increasing the amylose or insoluble fibre content had no effect on PPGR.
Using barley flour from a genotype that is rich in amylose (SH99250, which has 42 % amylose content in its extracted starch) did not improve PPGR, which is in line with the report by Granfeldt et al. ( Reference Granfeldt, Liljeberg and Drews 15 ). High-amylose rice (23 % amylose content)( Reference Trinidad, Mallillin and Encabo 50 ), high-amylose maize starch (70–75 % amylose content)( Reference Granfeldt, Drews and Bjorck 51 ) and high-amylose wheat (38 % amylose content)( Reference Hallström, Sestili and Lafiandra 52 ) have been shown to reduce PPGR, but these foods contain negligible amounts of β-glucan. Any benefit of increasing amylose content may be masked by the effects of β-glucan in foods that contain barley. Moreover, the tortillas made with high-amylose barley flour contained 1·41 g resistant starch per serving as compared to 0·42 g in the low-amylose tortillas. Therefore, the dosage of resistant starch may not have been sufficient to reduce the glycaemic response in general and/or to reduce the glycaemic response further than what was achieved by the β-glucan content. The present results do not support the use of barley varieties based on the amylose:amylopectin ratio to improve the PPGR of food products made from barley flour.
The European Food Safety Authority has approved a health claim for β-glucans from oats or barley for their beneficial effect of improving PPGR( 10 ). According to this claim, a beneficial physiological effect requires the consumption of 4 g of β-glucan from oat or barley for each 30 g of available carbohydrate consumed per meal. A recent review( Reference Tosh 53 ) demonstrated that in order to achieve a significant lowering of PPGR, 3 g of β-glucan per meal is sufficient if the β-glucan is consumed as intact cooked or fermented grains, and 4 g of β-glucan per meal is sufficient if the β-glucan is processed. The present tortilla study used β-glucan doses of 4·5, 7·8 and 11·6 g per 50 g of available carbohydrate. Although all of the barley tortilla treatments showed a 20 % reduction in glucose response as compared to consuming glucose alone, the maximum reduction was observed with the treatments that contained the highest amount of β-glucan (which also corresponded with the highest viscosity). These results suggested that β-glucan doses of more than 4 g could be more effective at blunting glycaemic response, and this warrants future clinical study to examine the dose-dependent effect of β-glucan on PPGR. Considering the acute nature of the present study, the role of β-glucan fermentation products, such as acetate, propionate and butyrate, on glucose metabolism could not be determined( Reference Wood, Arrigoni and Miller 54 , Reference Cummings and Englyst 55 ). Accordingly, slowed glucose absorption as a result of increased gut viscosity might have been responsible for the acute effects that were observed( Reference Battilana, Ornstein and Minehira 56 ); this idea was supported by the supplementary viscosity data. Future long-term studies in people with type 2 diabetes are needed to validate these effects and to determine whether there is an added benefit of increased-fermentation products in the lower GIT for maintaining lower blood glucose in the longer term.
Although previous studies have shown that the consumption of IDF is associated with a reduced risk of type 2 diabetes( Reference de Munter, Hu and Spiegelman 57 ) and lowered PPGR 75 min after consuming a meal( Reference Samra and Anderson 58 ), in the present study, the differential intake of low v. high IDF (7·4 v. 19·6 % flour basis, respectively) did not alter glycaemic or insulinemic responses. As in a previous report( Reference Bourdon, Yokoyama and Davis 59 ), the present study showed that insulin concentrations remained elevated during the study period, whereas glucose concentrations returned to baseline. We speculate that this discrepancy might have resulted from the fact that low levels of insulin may have been required to control the slow release of glucose. In addition, in the present study, the high-IDF tortillas elicited the highest GLP-1 response, which suggested increased satiety. Future studies should include visual analogue scales and measure ad libitum energy intake at the next meal(s) to determine if these high-IDF tortillas result in increased satiety and/or reduced energy intake.
Overall, the present results indicated that the type of dietary fibre present in barley flour and its milling fractions could alter postprandial glucose and insulin responses. The selection of high-β-glucan barley genotypes and specialised milling fractions could be used to optimise barley-based functional foods to reduce PPGR. This opens new opportunities and novel ways to optimise the health benefits of barley tortillas as low-GI foods. The results of the present acute study indicated that barley food products enriched with β-glucan could be useful as part of a dietary approach for controlling and improving PPGR in humans. The results also indicated that barley tortillas with high IDF could possibly improve satiety, which has implications for weight control and obesity management. The data also supported the hypothesis that increasing β-glucan intake specifically is beneficial for helping control PPGR. More studies using a wholefoods approach for glycaemic control are needed for barley foods and wholegrain foods in general.
Supplementary material
To view supplementary material for the present article, please visit http://dx.doi.org/10.1017/S0007114515000367
Acknowledgements
The authors would like to thank and acknowledge Dr Brian Rossnagel, University of Saskatchewan, for providing the barley samples for the study and the AAFC staff, particularly Tracy Exley and Camille Rhymer, for their technical support.
The authors would also like to acknowledge Angela Wilson (clinical trial coordinator/nurse), Brenda Wright and Danielle Stringer for their technical support and Alanna Baldwin for preparing the initial regulatory documents for approval by the University of Manitoba Research Ethics Board and St Boniface General Hospital Research Review Committee.
Funding for the present project was provided by Agriculture and Agri-Food Canada as well as the Alberta Barley Commission (MII 53755). Additionally, H. B. received a postdoctoral fellowship from the Manitoba Health Research Council.
The authors' contributions are as follows: N. A. was the principal investigator of the study, conceived and designed the study, conducted the food development and analysis, created the treatments, designed the clinical trial and prepared the manuscript; H. B. prepared the amendments, sought the necessary regulatory approvals for study-related documents, recruited participants, screened participants, administered test products to participants, performed pre-analytical blood preparation, analysed blood samples, performed the statistical analysis of the clinical trial data and drafted the sections of the manuscript that describe the clinical trial; J. S. contributed to the data analysis and the manuscript preparation; S. J. T. contributed to the manuscript preparation and editing; P. Z. provided oversight for the clinical trial and sample analyses and edited the manuscript; and C. T. contributed to the design of the clinical trial, provided oversight for the clinical trial and edited the manuscript.
There are no conflicts of interest.












