The cholesterol-lowering effects of oats have been studied in both human subjects and animals since the beginning of the 1960 s. This effect has mainly been ascribed to its content of the soluble fibre β-glucans, as 80 % purified oat β-glucan has been shown to reduce cholesterol levels in hypercholesterolaemic human subjects(Reference Braaten, Wood and Scott1). In 1997, the Food and Drug Administration approved a health claim for oat products based on soluble fibres from whole oats (i.e. oat bran (OB), oatmeal or rolled oats, and also whole-oat starch) after a review of thirty-seven clinical studies of the effect of oats on blood lipids(2). Daily intake of a minimum of 3 g oat β-glucans was deemed necessary to cause a relevant reduction in cholesterol levels. Health claims for β-glucans from barley have subsequently been approved(3). However, it is not completely understood what molecular structure the β-glucans should exhibit to be physiologically active or to what extent other cereal components, e.g. lipids, antioxidants and other types of dietary fibres, contribute to the effect.
Cereal β-glucans are linear polysaccharides that are present in the cell walls, and they are found in oats, barley, wheat and rye. They are composed of a chain of glucose units connected by β-(1–4) and β-(1–3) linkages. Apart from the β-glucan content, the repeating pattern of these linkages varies between cereals; it has been shown to affect the solubility and gelation properties(Reference Cui, Wood and Nishinari4). Different processing treatments of oats, e.g. bread baking(Reference Åman, Rimsten and Andersson5) or repetitive freeze–thaw treatments(Reference Lan-Pidhainy, Brummer and Tosh6), have been shown to change the molecular weight and/or the solubility of β-glucans. Such changes may possibly affect the cholesterol-lowering effects, although our knowledge about the relevant parameters is incomplete. Both the viscosity and the concentration of β-glucans after in vitro digestion have been reported to have a significant influence on the glucose response after a meal(Reference Lan-Pidhainy, Brummer and Tosh6). Another clinical study of various oat β-glucans found that there was a linear correlation between the change in plasma glucose after a meal and the viscosity of the drink consumed(Reference Wood, Braaten and Scott7). In contrast to the above-mentioned reports, studies of β-glucans of different molecular weights have shown that there is no difference in cholesterol-lowering effects either in animal models(Reference Yokoyama, Knuckles, Wood, Lee and Ho8, Reference Wilson, Nicolsi and Delaney9) or in human subjects(Reference Keenan, Goulson and Shamliyan10). In order to optimise the cholesterol-lowering effects of oat products, a more detailed understanding of the importance of different physico-chemical properties of β-glucans (including molecular weight) is needed.
Gallaher et al. (Reference Gallaher, Hassel and Lee11) investigated the cholesterol-reducing effects of hydroxypropyl methylcellulose in hamsters, and found a correlation between plasma cholesterol levels and the viscosity of the intestinal contents. Isolated β-glucan (80 % pure) and rolled oats have been shown to increase the viscosity of the small intestinal contents in rats in comparison to rats fed a diet containing cellulose(Reference Lund, Gee and Brown12). One might therefore expect that the viscous properties of oat products would play a fundamental role in their biological activity.
It has been suggested that propionate, one of the SCFA produced when β-glucans are fermented in the large intestine, can reduce hepatic cholesterol synthesis(Reference Levrat, Favier and Moundras13). Such effects of intestinal fermentation of β-glucans may thus contribute to their cholesterol-lowering effects. However, to our knowledge, the effect of the molecular weight of β-glucan on the formation of SCFA has not been investigated.
Human trials of oat products are time-consuming and costly to perform. Thus, an animal model (e.g. mice) can often be a suitable tool for screening of new potential food ingredients or to investigate the mechanisms of action. We have recently demonstrated that cholesterol-lowering effects of oats can be evaluated in C57BL/6NCrl mice that are fed an atherogenic diet(Reference Andersson, Immerstrand and Swärd14). This mouse model was used in the present study, where the main objective was to investigate the roles that molecular weight and viscous properties of oat β-glucan play in the cholesterol-lowering effects of oat products. We also wanted to evaluate their effect on the lipoprotein pattern and on TAG, and their effect on the formation of SCFA in the caecum.
Materials and methods
Experimental protocol
A control experiment was performed to investigate whether processing of OB, wet milled and amylase treated but without β-glucanase treatment, affects its cholesterol-lowering properties. After confirming that there was no difference in cholesterol-reducing effects between processed OB (POB; 1311 kDa) and OB, or in gain in body weight, feed intake and dry faeces output (data not shown), two separate experiments were performed to evaluate how different molecular weights of β-glucans in the POB preparations affect plasma cholesterol and production of SFCA in the caecum. In the first experiment, we compared the effect of POB (1311 kDa) used in the control experiment with the effect of three other β-glucanase-treated POB (241, 56 and 21 kDa). In the second experiment, the effect of an additional POB with β-glucans of even lower peak molecular weight (MWp; < 10 kDa) was compared with the effect of untreated OB.
Animals
Female C57BL/6NCrl mice were purchased from Charles River Laboratories (Sulzfeld, Germany). All mice were fed normal chow (R34 rodent chow; Lactamin, Sweden) during an adaptation period of 2 weeks. At 10–12 weeks of age (body weight 17–21 g), mice were randomly divided into experimental groups, and were housed together in cages with ten animals per cage. The mice were kept in a temperature-controlled room with a 12-h light cycle environment, and they had free access to food and water. All experiments were approved by the Malmö/Lund regional ethical committee for laboratory animals. The animals (n 110) tolerated the studies well except for one animal (in Expt 2, fed control diet) that had symptoms of illness and was withdrawn from the study. After 4 weeks on the experimental diet, the mice were killed by cervical dislocation, and the caecal tissue and contents were collected.
Diets
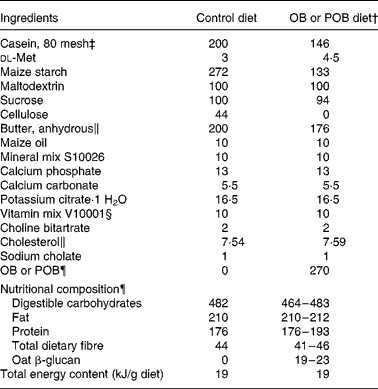
To induce hypercholesterolaemia, an atherogenic diet (0·8 % cholesterol and 0·1 % cholic acid) was designed, which reflected the Western diet with about 41 % energy fat, 16 % energy protein and 43 % energy carbohydrates (Table 1). The different diets were produced in our laboratory from a premix purchased from Research Diets, Inc. (New Brunswick, NJ, USA), in the same way as we have described previously(Reference Andersson, Immerstrand and Swärd14). During preparation of the diets, we assumed that all ingredients were dry, without correcting for traces of water.
Table 1 Formulation of the atherogenic diets (g/kg diet)*
OB, oat bran; POB, processed OB.
* All values are expressed in fresh weight.
† POB (produced from OB) with β-glucan of different peak molecular weights.
‡ Casein is 88 % protein.
§ Containing 97·8 % sucrose.
∥ Anhydrous butter has 230 mg cholesterol/100 g. To compensate for this, extra cholesterol was added so that total amount of cholesterol in all diets was 8 g/kg diet.
¶ Dry-milled OB (Avena sativa, cv. Sang b 1008596) or dry-milled POB, both < 0·8 mm. The nutritional composion of OB and POB is illustrated in Table 2, and it explains the range in nutritional compositon of the OB diet and the POB diets.
In Expt 2, the diet formulae were adjusted to fit the nutrient composition of a new batch of OB (Table 2), in the same way as done when we designed the diets used in the initial control experiment and Expt 1 (Table 1). The experimental diets were fed as powders.
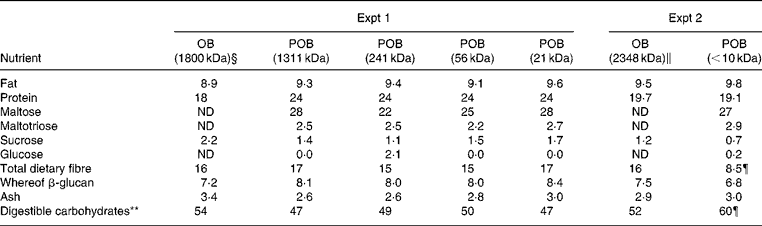
Table 2 Nutrient content of experimental processed oat bran (POB)* products and the oat bran (OB)† used as a starting material (g/100 g)‡
ND, not determined.
* POB (produced from OB) with β-glucan of different peak molecular weights.
† OB used as a starting material for the production of POB (see Materials and methods).
‡ All values are based on DM.
§ Avena sativa (cv. Sang), produced in 2007 by Lantmännen.
∥ A. sativa (cv. 43 % Sang, 10 % Kerstin and 47 % mixed oats containing Belinda in large part); produced in 2008 by Lantmännen.
¶ The deviation of total dietary fibre and consequently total carbohydrates between POB ( < 10 kDa) and OB (2348 kDa) was most probably a consequence of the method used for the total dietary fibre analysis, which is based on an enzymatic digestion of starch and protein followed by precipitation of fibre with 80 % ethanol(Reference Asp, Johansson and Hallmer22). However, fibres of less than ten to twenty monomers are not expected to be quantitatively precipitated.
** Calculated by difference: 100 – protein – fat – ash – dietary fibre (for example, starch or minor sugars).
Processing of oat bran
Two batches of OB were used in the different experiments in mice; they were produced in the same mill (Lantmännen AB, Järna, Sweden) but from different cultivation varieties of oats. The nutrient compositions of the two OB were similar (Table 2), and both were used as starting materials to produce five POB products that would differ only with respect to the molecular weights of β-glucans. In the initial control experiment and in Expt 1 (performed in 2008), we used OB based on a Swedish variety of oats named ‘Sang’ (produced in 2007, batch 1008596). In Expt 2 (performed in 2009), the OB used was based on a mixture of Swedish oat varieties: 43 % Sang, 10 % Kerstin and 47 % mixed oats, mainly Belinda (produced in 2008, batch 1047749). This change was for agricultural reasons. However, we did not see any significant difference in the present results related to the source of the OB (Table 2 and Fig. 2).
Four POB products were produced essentially as described by Triantafyllou Oste(Reference Triantafyllou Oste15), and were treated with different amounts of β-glucanase from Aspergillus sp. (Biocon, Barcelona, Spain) to obtain different molecular weights of β-glucan. One of the batches was produced without the addition of β-glucanase for comparison with untreated OB. An additional, fifth POB product was produced in the same way except that in the β-glucanase step, excess amounts of a β-glucanase from Trichoderma longibrachiatum (Biocon) were used in addition to the β-glucanase from Aspergillus sp. The five products were obtained from OATLY AB (Landskrona, Sweden) as liquid suspensions. Before freeze-drying, a solution of maltodextrin (1:3 in water) was added to the liquid suspension to a final concentration of 19 % in an attempt to prevent the formation of insoluble complex that would remain undissolved during the passage through the intestine in vivo. The mixture of OB and maltodextrin was placed on trays and stored at − 20°C before freeze-drying. DM and minor sugar components were analysed in all suspensions before mixing with maltodextrin. The freeze-drying was kept constant at − 20°C for 162 h, and the temperature was then raised to +5°C (at 4°C per h; Labconco, Ninolab, Upplands Väsby, Sweden). The freeze-dried materials were dry milled to a particle size of less than 0·8 mm (Laboratory Mill 120; Perten Instruments, Huddinge, Sweden).
Analysis of β-glucan in oat products
Samples for molecular weight determination of β-glucan were extracted and analysed as described previously(Reference Immerstrand, Bergenståhl and Trägårdh16). The POB samples were not extracted with ethanol before extraction with 0·1 m-NaOH since the β-glucanases were assumed to be inactivated by processing. This was confirmed by comparing two samples with and without an ethanol extraction; the results showed that the molecular weights were the same in both cases.
The total β-glucan content of solid materials and liquid samples from viscosity measurements were determined by using a kit, following an enzymatic assay method for mixed linkage β-glucans(Reference McCleary and Codd17).
Viscosity measurements
The freeze-dried POB products were solubilised in deionised water for 1 h at room temperature under agitation with a magnetic stirrer (approximately 2·8 g POB per 28 g water). At least two replicates were made for each POB product. The samples were centrifuged at 15 000 g for 10 min, after which the amount of the supernatant was weighed. In order to determine the percentage of solubilised DM and β-glucan, a small aliquot of supernatant was taken and stored at − 20°C until the analysis was done. The viscosity of the supernatant was measured with a stress-controlled rheometer (StressTech, Reologica, Sweden) with a concentric cylinder (25 mm diameter: CC25) at room temperature. Solutions with different sucrose concentrations were used to verify the method. In order to operate within a Newtonian region, the supernatant obtained from POB (1311 kDa) was diluted 1:1 with deionised water before the measurement of viscosity. Different shear stresses were used to provide a range of shear rate from 5 to 50 per s.
Analysis of nutrient composition
Protein content was determined with a Kjeltec System 1003 (Tecator AB, Höganäs, Sweden) or with a carbon/nitrogen analyser (Vario Max CN, Elementar Analysensysteme GmbH, Hanau, Germany). Crude oat protein was calculated as nitrogen content × 6·25. Fat content was determined using the conventional styrene − butadiene rubber solvent extraction method based on the work of Schmid(Reference Schmid18), Bondzynski(Reference Bondzynski19) and Ratzlaff(Reference Ratzlaff20), involving a gravimetric extraction in diethyl ether and petroleum ether (40–60°C, 1:1) after hydrolysis in 7·7 m-HCl and ethanol for 1 h at 75°C. The content of total dietary fibre in the OB samples was determined by Eurofins Foods (Lidköping, Sweden) according to the Association of Official Analytical Chemists (985·29) method of Prosky et al. (Reference Prosky, Asp and Furda21), whereas total dietary fibre in POB products was analysed according to the method of Asp et al. (Reference Asp, Johansson and Hallmer22). Both methods are gravimetric, and are based on the enzymatic digestion of starch and proteins followed by precipitation of the fibre with ethanol. These methods have shown good agreements(Reference Spiller and Spiller23). The sugar analysis of liquid suspensions was performed by means of HPLC using a Zorbax carbohydrate analysis column (4·6 × 150 mm) from Agilent Technologies, Inc. (Santa Clara, CA, USA); elution was done with acrylonitrile–H2O (63:37) at a flow rate of 1 ml/min and at 35°C. Moisture content was determined by drying the samples for 15 h at 105°C, whereupon the DM that remained was weighed after cooling in a desiccator for 1 h.
Plasma cholesterol, TAG and lipoproteins
At baseline and after 4 weeks, blood samples were collected after 4-h fasting(Reference Andersson, Immerstrand and Swärd14). Total plasma cholesterol and TAG were determined with Infinity cholesterol/TAG liquid stable reagent (Thermo Trace, Noble Park, Vic, Australia). Plasma lipoproteins were electrophoretically separated in agarose gels in barbital buffer according to the method of Noble(Reference Noble24). The gels were stained with Sudan black, and densitometric scanning (BioRad GS 800 Calibrated Densitometer and Quantity One quantitation software; BioRad, Hemel Hempstead, Herts, UK) of the intensity of the bands revealed the relative lipid distribution between LDL+VLDL v. HDL. Data are reported as (LDL+VLDL)/(HDL+LDL+VLDL) × 100. The percentage given for the lipoproteins reflects the lipid distribution among lipoproteins (since Sudan black stains cholesterol, TAG and phospholipids) and it does not exactly correspond to HDL- and LDL-cholesterol.
Caecum
The caecum was removed and weighed. The contents were transferred to a sterile tube and stored at − 80°C until analysis of SCFA. The caecal tissue was washed with PBS (pH 7·4), dried between layers of filter paper and weighed. Caecal content was calculated as the weight of the full caecum minus that of caecal tissue.
SCFA
SCFA (i.e. acetic, propionic, butyric, isovaleric, valeric, caproic and heptanoic acids) in the caecal content were analysed using a GLC method(Reference Zhou, Nyman and Jönsson25). A sample of caecal content (0·1 g) was mixed with 1 ml of a solution containing 0·25 m-HCl (to protonise SCFA) and 1 mm-2-ethylbutyric acid (as an internal standard). The sample was homogenised for 1 min with an Ultra Turrax T25 basic (IKA-WERKE, Staufen, Germany), and then was centrifuged (MSE Super Minor, Hugo Tillquist AB, Solna, Sweden). Two hundred microlitres of the supernatant were transferred to a micro-insert bottle, and were injected onto a fused-silica capillary column (DB-FFAP 125-3237; J&W Scientific, Folsom, CA, USA; Agilent Technologies Inc.). Caecal pools (μmol) of the different SCFA were calculated as the concentration of each acid (μmol/g caecal content) multiplied by the caecal content. The total SCFA pool was determined as the sum of SCFA in μmol per caecal content, and the proportions of SCFA were determined as the ratio between the amount of acid (μmol) per caecal content and the total SCFA pool.
Calculations and statistical evaluation
For calculations of the nutrient composition of POB products, the results were corrected for the 19 % of maltodextrin by dividing the values by a factor of 0·81.
The concentration-normalised viscosity was calculated as
where ηsp is the specific viscosity; ηr is the relative viscosity (ηs/η0); ηs is the viscosity of the solution containing the solute (i.e. the β-glucan in the present study); η0 is the viscosity in the absence of the solute and c is the concentration of the solute. The concentration of β-glucan in the solution was converted from weight percentage to g/l using the corresponding density for sucrose solutions.
Data were analysed using the Minitab software package version 14.0 (Minitab, Inc., State College, PA, USA). Unless otherwise stated, results are expressed as means with their standard errors. Outliers were identified as samples deviating from the third quartile with more than 150 % of the interquartile range. The Anderson–Darling test was used to determine the normality of the measurements, where P < 0·05 rejects the null hypothesis that the data are normally distributed. For normally distributed data, one-way ANOVA was used for multiple comparisons (using the general linear model procedure), where Tukey's test for pairwise comparisons of means was used for the significance of difference (P < 0·05). Two sets of data for SCFA were not normally distributed, and therefore median values were calculated and percentiles were presented (Table 5). The non-parametric Kruskal–Wallis test was performed to compare the median values between the groups based on the variance by ranks(Reference Siegel, Castellan and Anker26).
Results
Nutrient content of experimental products
The analysis of nutrient content confirmed that the nutrient composition of the POB products was roughly equal to what was obtained for OB, which was used as a starting material (Table 2). A somewhat greater variation in protein content was seen (18–24 %) and consequently also in the content of digestible carbohydrates (46–53 %). There were no significant differences in the levels of minor sugars (i.e. sucrose, maltotriose, maltose and glucose) between the POB products. The total dietary fibre content measured was clearly lower in POB ( < 10 kDa) than in the starting material (i.e. OB). This was most probably a consequence of the method used for the total dietary fibre analysis, which is based on an enzymatic digestion of starch and protein followed by precipitation of fibre with 80 % ethanol as described by Asp et al. (Reference Asp, Johansson and Hallmer22). Following this method, fibre of less than ten to twenty monomers are not expected to be quantitatively precipitated. Thus, a significant amount, approximately 50 % (8·5/16, see Table 2), of the dietary fibre that is present should be composed of less than ten to twenty monomers.
Physico-chemical properties of processed oat bran
The solubility of the β-glucans in the POB products after a standardised dissolving procedure of the β-glucans in the POB products is presented in Fig. 1(A). There were small differences in the amount of solubilised DM (Fig. 1(B)). Generally, β-glucans from products with low molecular weights dissolve to a greater extent than those from products with high molecular weights.
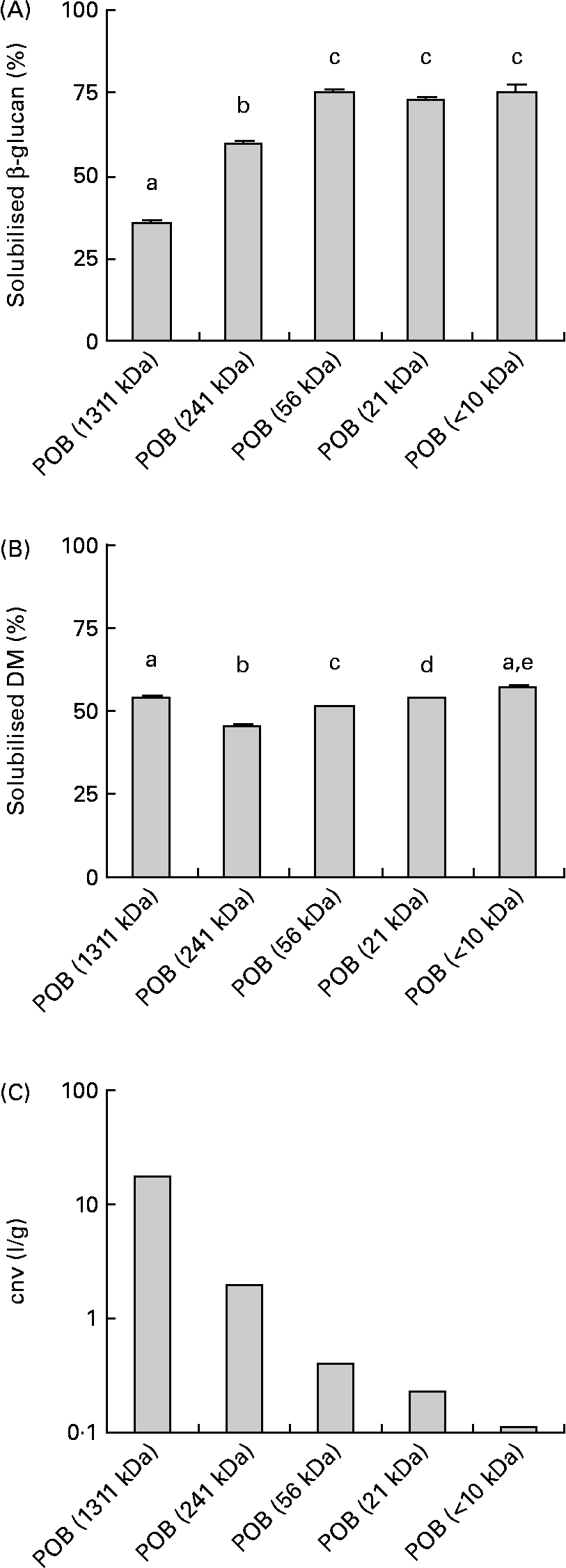
Fig. 1 Physico-chemical properties of processed oat bran (POB) samples with different MWp of β-glucans. The obtained level of water-soluble β-glucan from POB before viscosity measurement ((A), n 4–5). Solubilised DM from POB before viscosity measurement ((B), n 6). Viscous properties of solubilised fractions of POB products, expressed as the concentration-normalised viscosity (cnv) which is equal to ηsp/c β-glucan (see equation 1), where c β-glucan in solution was 1·4 g/l for POB of 1311 kDa, 5·5 g/l for POB of 241 kDa, 5·9 g/l for POB of 56 kDa, 5·8 g/l for POB of 21 kDa and 4·9 g/l for POB < 10 kDa, respectively (C). The results are presented as mean values. Error bars in (A) and (B) show sem. a,b,c,d,e Mean values with unlike letters were significantly different (P < 0·05).
The viscosity of the particle-free supernatant of each product was determined. From the results on viscosity and the β-glucan concentrations, the concentration-normalised viscosity was estimated to compare the thickening efficiency of the different degradation levels of β-glucans. The concentration-normalised viscosity ranged from 1·1 for POB < 10 kDa to 17·7 l/g for POB of 1311 kDa (Fig. 1(C)). Solubilisation of dry-milled OB in deionised water, by using the same conditions as for the POB, resulted in approximately 23 % solubilised β-glucan and 5·7 % solubilised DM.
The MWp value of β-glucan from OB based on ‘Sang’ oats was determined to be approximately 1800 (sem 17) kDa, whereas of β-glucan from for OB based on 43 % Sang was 2348 (sem 25) kDa (means with their standard errors for duplicate samples). MWp values for the different POB products were 1311 (sem 12), 241 (sem 5), 56 (sem 0·0) and 21 (sem 0·2), respectively (expressed in kDa as means with their standard errors for duplicate samples). The MWp value of β-glucan for POB product produced using the most extensive enzyme treatment was not determinable, as we obtained a low fluorescence intensity that was close to the background level. It is known that the fluorescing complex between β-glucan and calcofluor is only formed at molecular weights of β-glucan that are greater than approximately 10 kDa(Reference Gomez, Navarro and Carbonell27). The β-glucans present in the most extensively enzyme-treated POB were therefore most probably equal to or less than about 10 kDa, and thus we refer to this sample as POB < 10 kDa.
Body weight, feed intake and faeces excretion
As in our previous study(Reference Andersson, Immerstrand and Swärd14), the body weight of all mice had increased during the 4 weeks on experimental diets, by an average of 2·8 g per mouse (pooled standard deviation = 0·95).
The mice that were fed oat products generally increased significantly more in body weight than mice that were fed control diet, even though this varied somewhat between the experimental series. Moreover, feed intake and faecal output were similar in the different dietary groups (Table 3).
Table 3 Initial weight, body weight gain, feed intake and dry faeces for mice fed experimental diets for 4 weeks*
(Mean values with their standard errors)

n, Number of observations; OB, oat bran; POB, processed OB with β-glucan of different peak molecular weight.
a,b Mean values with unlike superscript letters were significantly different between groups within each experiment (P < 0·05).
* Statistics were calculated with one-way ANOVA for multiple comparisons (Tukey's test for pairwise comparisons of means).
† The number refers to the number of cages (ten mice housed per cage).
‡ OB, used as a starting material for the production of POB (see Materials and methods).
Plasma cholesterol, lipoproteins and TAG
Distribution plots representing the baseline and the 4-week levels of plasma cholesterol for all dietary groups of mice in each experiment revealed one outlier, which was excluded from further analysis. This mouse belonged to the control group in Expt 2, and had unusually high plasma cholesterol (3·1 mmol/l) at baseline, which became reduced by 0·15 mmol/l after 4 weeks on atherogenic diet.
In the first experiment, we found that all POB products, with β-glucans with different MWp values (1311, 241, 56 and 21 kDa), lowered plasma cholesterol equally (Fig. 2(A)). In an attempt to reveal a loss in efficiency by further reduction of molecular weight, we prepared another batch of POB with an MWp < 10 kDa (see Results: physico-chemical properties). However, the cholesterol-lowering effects of this product were also not significantly different from those of the unprocessed OB (Fig. 2(B)).

Fig. 2 Oat bran (OB) and processed OB (POB) with β-glucans of different peak molecular weight (MWp) values reduce plasma cholesterol equally. Four POB with different β-glucan MWp values had a similar effect on plasma cholesterol ((A), n 10), and a third POB with even smaller β-glucans had the same cholesterol-lowering effects as OB ((B), n 8–10). Baseline values (▨) were not significantly different between the experimental groups. a,b Mean values with unlike letters were significantly different (P < 0·05) after 4 weeks on experimental diets (■). Data are presented as means with their standard errors.
Four weeks on atherogenic diet induced a prominent shift of the lipoprotein profile, where the relative proportion of LDL+VLDL was approximately doubled. No statistically significant reduction in the proportion of LDL+VLDL was found after the addition of OB or any of the POB to the diet (Table 4). Mean TAG levels in Expt 1 were 0·52 mmol/l in control mice after 4 weeks, and they were not significantly affected by the oat products (data not shown), which is in line with the results of our previous study(Reference Andersson, Immerstrand and Swärd14).
Table 4 LDL+VLDL levels in mice at baseline and after 4 weeks on experimental diets*
(Mean values with their standard errors)
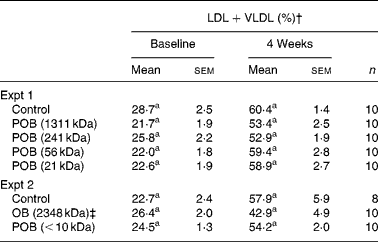
n, Number of observations; POB, processed OB with β-glucan of different peak molecular weights; OB, oat bran.
a Mean values within a column with superscript letter was significantly different between groups within each experiment (P < 0·05).
* Statistical analysis was performed by using one-way ANOVA for multiple comparisons (Tukey's test for pairwise comparisons of means P < 0·05) on normally distributed data.
† Calculated as (LDL+VLDL)/(HDL+LDL+VLDL) × 100.
‡ OB, used as a starting material for the production of POB (see Materials and methods).
Caecal content, caecal tissue weight and formation of SCFA
Weight of caecal tissue and the distribution of SCFA in the contents are given in Table 5. Mice fed POB with the highest MWp value of β-glucan (1311 kDa) had higher caecal content than the control group (P < 0·01). The weight of caecal tissue was higher for mice fed POB with a low MWp value, i.e. 21 kDa (P < 0·01) and 56 kDa (P < 0·05).
Table 5 Caecal content and tissue, and caecal SCFA in mice fed experimental diets (Expt 1)
(Mean values with their standard errors)

n, Number of observations; POB, processed oat bran with β-glucan of different peak molecular weights.
a,b,c Mean values with unlike superscript letters were significantly different (P < 0·05). See Materials and methods for description of statistical procedures.
* The concentration of each acid (μmol/ g caecal content) multiplied with the caecal content.
† Since these data were not normally distributed, a non-parametric test was done (Kruskal–Wallis test). Data are expressed as median and 25th to 75th percentiles.
The total pool of caecal SCFA was higher (P < 0·05) for mice that were fed POB with an MWp value of 1311 or 21 kDa than for mice that were fed the control diet. Acetic acid was the predominant SCFA in all groups, followed by propionic acid and butyric acid. The mean ratio between these three acids was 64:23:13. The caecal pool of propionic acid was higher (P < 0·05) for all POB groups than for the control group (2·6–3·3 v. 1·4 μmol, respectively). Butyric acid, on the other hand, was formed in higher amounts at the high MWp value (P < 0·01) and low MWp value (P < 0·05) compared with the control group.
The ratio of (propionic acid+butyric acid)/acetic acid was significantly higher in all POB groups than in the control group, and increased with the MWp of the β-glucans in the POB products (Table 6).
Table 6 Ratio between the major caecal SCFA formed in mice (Expt 1)*
(Mean values with their standard errors)
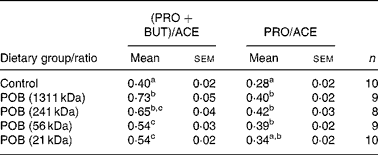
PRO, propionic acid; BUT, butyric acid; ACE, acetic acid; n, number of observations; POB, processed oat bran with β-glucan of different peak molecular weights.
a,b.c Mean values within a column with unlike superscript letters indicate statistical significant difference between groups (P < 0·05).
* Statistical analysis was performed by using one-way ANOVA for multiple comparisons (Tukey's test for pairwise comparisons of means P < 0·05) on normally distributed data.
Discussion
Solubility, viscosity and molecular weight are physico-chemical properties that have been suggested to play crucial roles in the beneficial health effects of β-glucans. It has been demonstrated in human subjects that changes in molecular weight affect the glucose response(Reference Wood, Beer and Butler28), and also have different effects on gastrointestinal hormones(Reference Juvonen, Purhonen and Salmenkallio-Marttila29). The importance of the molecular weight of β-glucans for the cholesterol-lowering effects of oat products is not, however, completely understood. To address this, we evaluated the effects of OB, processed to different MWp values of β-glucan (1311, 241, 56, 21 and < 10 kDa), on plasma cholesterol levels, lipoprotein composition, TAG and intestinal production of SCFA in mice.
It should be noted that the MWp value describes the average of the molecular weight distribution of extracted β-glucans, as the curves appeared to be symmetric(Reference Immerstrand, Bergenståhl and Trägårdh16). The MWp values for β-glucan from the two batches of OB used were 1800 and 2348 kDa, which is in good agreement with previously reported results(Reference Åman, Rimsten and Andersson5, Reference Andersson, Immerstrand and Swärd14, Reference Immerstrand, Bergenståhl and Trägårdh16). The analysis used for molecular weight determination revealed that most of the β-glucans present in the sample with the lowest molecular weight had an MWp < 10 kDa (corresponding to less than sixty-two monomers). Interestingly, results on total dietary fibre indicated that about 50 % of the dietary fibre that is present should be composed of less than ten to twenty monomers. However, we cannot exclude the possibility that cellulose fibre in addition to the β-glucans present was also digested since we used a cellulase during the production of this POB product.
The water solubility of β-glucans increased as the molecular weight decreased, and ranged from 36 to 75 % for POB products (Fig. 1(A)). In comparison, β-glucans from untreated OB (dry milled) dissolved to approximately 23 %.
C57BL/6 mice develop high cholesterol levels when fed an atherogenic diet(Reference Paigen, Mitchell and Reue30). All the mice gained weight on experimental diets, and the molecular weight of β-glucan (from 1311 down to 21 kDa) had no influence on the gain in body weight. The cholesterol-lowering properties of OB preparations were unchanged over the whole range of MWp investigated (Fig. 2). This suggests that β-glucans with MWp as low as 10–20 kDa are functional in lowering cholesterol, so that any limit for a loss in efficiency appears at even lower MWp. We cannot, however, exclude the possibility that the cholesterol-lowering effects were partly caused by oat component(s) other than β-glucans, for example, by arabinoxylans, sterols, lipids and/or antioxidants (e.g. avenanthramides and vitamin E). The present findings regarding the effects on plasma cholesterol agree with previous studies on oat or barley β-glucans in animals(Reference Yokoyama, Knuckles, Wood, Lee and Ho8, Reference Wilson, Nicolsi and Delaney9) and human subjects(Reference Keenan, Goulson and Shamliyan10). Furthermore, a newly published study by Bae et al. (Reference Bae, Lee and Kim31) has shown that there is no difference in cholesterol-lowering properties between oat products with different molecular weights of β-glucan (1450–371 kDa) in male C57BL/6 mice, which is in agreement with the results of the present study. The study by Bae et al. (Reference Bae, Lee and Kim31) used enriched β-glucan at a high concentration (8·6 %), which is hardly attainable in human diets and which may have influenced the nutritional state, as the animals differed significantly in weight gain between the experimental groups.
In the present study, the viscous properties of the five POB were found to vary with MWp of the β-glucans (Fig. 1(C)). One hypothesis might be that these samples create different viscosities of the absorptive layer in the small intestine, and consequently affect the absorption rate as well as the amount of cholesterol absorbed into the plasma differently. We found, however, no correlation between plasma cholesterol levels and the viscous properties of POB. Thus, other mechanisms for the cholesterol-lowering effects must be considered (e.g. intestinal fermentation). Even so, we cannot exclude the possibility that β-glucans increase the viscosity of the intestinal contents in a way that is not strongly dependent on molecular weight or on the viscous properties of the β-glucans themselves. It has, for example, been suggested that increased viscosity of the intestinal contents may be an effect of increased mucus secretion stimulated by the presence of β-glucans. This hypothesis was based on the finding that the small intestinal content was viscous even after a 13-h fasting period, when no β-glucans were detected in the material from the small intestine(Reference Bégin, Vachon and Jones32).
The difference in MWp of β-glucan in the different OB preparations had no statistically significant influence on the measured proportions of LDL+VLDL and HDL (Table 4). The electrophoretic separation of plasma lipoproteins is evaluated by staining of all plasma lipids (cholesterol, TAG and phospholipids) in the bands, and possible changes in HDL v. non-HDL cholesterol may therefore be obscured by the contributions from other lipids. In our previous study, the OB group had significantly lower LDL+VLDL values than the control group(Reference Andersson, Immerstrand and Swärd14).
β-Glucans generally belong to the group of indigestible carbohydrates, which includes NSP, resistant starch and oligosaccharides. These are not digested and absorbed in the small intestine, but are partially or completely fermented to SCFA in the large intestine. However, animal studies have shown that the molecular weight of β-glucans is reduced during passage through the upper gastrointestinal tract, reaching between 35 and 100 kDa in the small intestinal content of pigs(Reference Johansen and Knudsen33), hamsters(Reference Yokoyama, Knuckles, Wood, Lee and Ho8) and rats(Reference Wood, Weisz and Mahn34), which suggests that the molecular weight of β-glucans may have been reduced during passage through the gastrointestinal tract in the present study also.
β-Glucans are generally considered to be water-soluble, fermentable dietary fibre. In contrast, cellulose is a water-insoluble fibre and is much more resistant to fermentation, giving low amounts of SCFA(Reference Berggren, Björk and Nyman35). The effects on colonic fermentation of four POB products, containing β-glucans with different MWp (1311, 241, 56 or 21 kDa), were evaluated in Expt 1 (Table 5). The major fatty acids formed were acetic acid, propionic acid and butyric acid at a mean ratio of 64:23:13. This is in the same range as the ratios found in studies on human intestinal material(Reference Cummings, Pomare and Branch36, Reference Macfarlane and Macfarlane37) (57:22:21) and on rat caecum(Reference Berggren, Björk and Nyman35) (69:21:10).
Butyric acid is usually considered to be important for the health of the colon, and a high degree of butyric acid formation has recently been suggested to have metabolic effects(Reference Marcil, Delvin and Seidman38–Reference Galisteo, Duarte and Zarzuelo40). Drzikova et al. (Reference Drzikova, Dongowski and Gebhardt41) found that the caecal pool of propionate and butyrate was significantly higher in rats fed an OB-based diet than in those fed a cellulose-containing diet, as we also obtained for the POB product with the highest MWp of β-glucans.
Propionic acid has previously been suggested to reduce plasma cholesterol levels in human subjects, but the mechanism behind this is not completely understood(Reference Levrat, Favier and Moundras13, Reference Wolever, Spadafora and Eshuis42–Reference Wolever, Fernandes and Rao44). The acetate produced after fermentation of fibres in the intestine is readily absorbed and transported to the liver where it can act as a substrate for acetyl-CoA formation, the precursor for endogenous cholesterol synthesis. It has been suggested that propionate could possibly impair the acetate utilisation, and thereby also cholesterol biosynthesis(Reference Wolever, Spadafora and Eshuis42, Reference Demigné, Morand and Levrat45, Reference Delzenne and Williams46). In the present study, we found that all POB gave rise to significantly higher pools of propionic acid compared with the control diet. There was no clear effect of β-glucan MWp on the pools of either propionic acid or acetic acid and, except for the lowest MWp, the ratio between propionic acid and acetic acid was significantly higher for all POB groups v. control group. There was, however, a significant positive correlation between the ratio of (propionic acid+butyric acid)/acetic acid and the MWp of β-glucans (Table 6). Since this SCFA ratio was dependent on the MWp of β-glucan but the plasma cholesterol was not, we suggest that caecal formation of specific SCFA may not have been a crucial mechanism for the cholesterol-lowering effects of oats found in the mice. However, it cannot be excluded that the results of SCFA in plasma would have been different.
The caecal content was significantly higher for mice fed β-glucans of the highest molecular weight than for those fed a cellulose-based control diet, which is in agreement with a previous study on rats fed a diet based on OB(Reference Drzikova, Dongowski and Gebhardt41). The weight of caecal tissue was, however, significantly higher in mice fed low-MWp oat products. This might be due to an extensive fermentation of β-glucans and utilisation of SCFA. Another potential explanation is that the formation of SCFA is involved in the proposed stimulation of mucus secretion by β-glucans(Reference Bégin, Vachon and Jones32, Reference Finnie, Dwarakanath and Taylor47).
The results of the present study suggest that the molecular weight of β-glucan and the viscosity of oat products may not be crucial parameters for the cholesterol-lowering effects. However, this does not preclude the possibility that the viscosity of the intestinal contents may be of importance through a more complex mechanism, and that this may be affected by β-glucans without there being any strong relationship concerning the viscosity of β-glucans themselves. Binding of bile acids to β-glucans is another possible mechanism of action that may not be strongly dependent on molecular weight. Regarding the formation of SCFA in the caecum, we found that all POB gave rise to a significantly higher caecal content of propionic acid compared with the control group. There was no clear relationship between MWp and propionic acid content, but the ratio of (propionic acid+butyric acid)/acetic acid increased with increasing MWp of β-glucans.
The cholesterol-lowering effects of oats are most likely a result of different mechanisms, and the present study indicates that development of new oat-based products with beneficial health effects can involve incorporation of β-glucans with a wide range of molecular sizes. However, human trials are needed to confirm the validity of the conclusions drawn from the present study. It would be interesting to define more exactly how individual oat components, such as β-glucans, sterols and various antioxidants, contribute to the cholesterol-lowering effects.
Acknowledgements
The present study was supported by the Functional Food Science Centre at Lund University and by OATLY AB. T. I. was responsible for the preparation and analysis of OB products, production and documentation of diets, analysis of caecal tissue and caecal content, statistical evaluation of all data, and for writing the manuscript. K. E. A. was responsible for the animal studies and plasma lipid analyses. A. R. was responsible for the POB processes. C. W. was responsible for the production of liquid oat suspensions at OATLY AB, and for the analysis of sugar content of POB products, and participated in diet preparation and animal experiments. All authors took part in planning of the experiments and contributed to evaluation of the results and writing of the manuscript. We thank Cathy Wang (Guelph Food Research Centre, Guelph, Canada) for analysing the MWp of β-glucan, Christer Fahlgren (Applied Nutrition and Food Chemistry, Lund University, Lund, Sweden) for analysis of caecal SCFA and Ina Nordström (Department of Experimental Medical Science, Lund University, Lund, Sweden) for analysis of blood lipids. The authors declare no conflict of interest.










