INTRODUCTION
Hepatitis delta virus (HDV), first discovered by Rizzetto et al. [Reference Rizzetto1] in a patient with chronic hepatitis B virus (HBV) infection, is a unique single-stranded RNA virus that requires the helper function of HBV for infection [Reference Smedile2]. It is established now that co-infection or superinfection of HBV and HDV may cause severe liver disease [Reference Taylor3, Reference Wu4] and HDV may increase hepatocellular carcinoma development three-fold compared to HBV infection without delta virus [Reference Fattovich5]. The severity of liver disease, however, varies by area and among clinical groups [Reference Hadler6], and may be related to the differences in the frequencies of HDV viraemia among anti-HDV-positive cases or to the different genotypes of HDV [Reference Wu, Chiang and Sheen7]. Three genotypes of HDV (I–III) have been identified with different geographic distributions: Genotype I is found worldwide with predominance in North America, Europe, Africa, the Middle East and East Asia [Reference Shakil8]; genotype II has been isolated only in East Asia (Taiwan and Japan) [Reference Sakugawa9] while genotype III has been restricted to Northern South America (Peru and Colombia) [Reference Casey10].
Lebanon is considered moderately endemic for hepatitis B with an overall carrier rate of 2·2% [Reference Nabulsi, ElSaleeby and Araj11]. However, there are no data on the significance of HDV infection in the Lebanese population except for one hospital-based study published two decades ago on the prevalence of HDV [Reference Farci12]. In this study, HDV infection and HDV genotypes were investigated by testing HBsAg-positive sera from Lebanese patients and blood donors collected from various parts of the country using reverse transcriptase–polymerase chain reaction (RT–PCR) and restriction fragment length polymorphism (RFLP).
PATIENTS AND METHODS
Patients
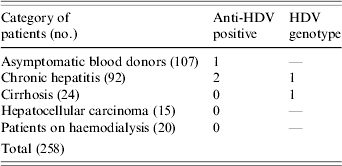
Recently 167 Lebanese individuals (103 males, 64 female) with HBV infection seen at nine medical centres representing the whole country between June 2002 and August 2004 were investigated for the prevalence of HBV genotypes in Lebanon [Reference Sharara13]; The mean age of the patients was 42±12 years. A questionnaire designed to gather demographic, clinical and laboratory data was completed for each individual. The demographic data included sex, age, place of birth and travel history. The clinical information included mode of presentation (asymptomatic, symptoms of chronic liver disease), presumed source of infection (sexual, parenteral, other), liver histology and treatment. The manifestations of HBV infection in the 167 individuals were as follows: 46 were asymptomatic blood donors, 82 had symptomatic chronic hepatitis, 24 had cirrhosis and 15 had hepatocellular carcinoma (Table). In addition to the 167 patients mentioned, samples from 91 HBsAg-positive patients collected from one medical centre were also included in the study (61 blood donors, 10 with chronic hepatitis and 20 on haemodialysis; 64 males, 27 females, mean age 36±10 years) (Table). All 258 samples were tested for anti-HDV and for HDV-RNA. Genotyping was determined by RFLP and confirmed by direct sequencing.
Table. Categories of HBsAg-positive patients tested for antibody to HDV (anti-HDV) and HDV genotypes
Serological assay of HBV and HDV
All samples were confirmed HBsAg in their respective centres and were tested for HBeAg and antibody to HBeAg at the Molecular Virology Laboratory, Faculty of Health Sciences, American University of Beirut using a commercially available enzyme-linked immunosorbent assay (ELISA) (VIDAS HBe/Anti-HBe; bioMérieux, Boxtel, The Netherlands). Testing for anti-HDV was performed by Abbott ELISA kits (Abbott Diagnostic Division, Wiesbaden, Germany).
Determination of Genotypes of HDV
HDV-RNA detection and genotyping
RNA was extracted by the High Pure Viral RNA kit (Roche Diagnostics GmbH, Mannheim, Germany) according to manufacturer's instructions. HDV RNA detection was done using the Ready-To-Go RT–PCR kit (Amersham, Pharmacia Biotech Inc., Uppsala, Sweden) following the manufacturer's specifications with primers 120 (homologous to the sequence of nucleotides 889–912) and 214 (complementary to the sequence of nucleotides 1334–1313) according to the corresponding delta-antigen-coding region [Reference Chao14]. HDV genotyping was investigated using RFLP analysis of the amplified region of nucleotides 911–1260, which is generally accepted to be ideal for the genotyping [Reference Casey10]. In brief, 10 μl from the extracted RNA was used for RT–PCR. The reaction mixture was subjected to 40 cycles of amplification; each cycle consisted of 95°C, 1 min; 42°C, 1 min; 72°C, 1·5 min [Reference Chao14]. The amplified DNA was digested by XhoI (Roche Diagnostics) and SacII (Roche Diagnostics) at 37°C for 3 h. The digested fragments were separated by electrophoresis in 3% agarose gel and stained by ethidium bromide. The restriction pattern was read according to the description of Wu et al. [Reference Wu15]. Genotypes I and II were included in all runs as a control.
Sequencing
Nucleotide sequencing was performed on the only positive sample we had; the result was comparable with the published sequence of HDV genotype I [Reference Wu15].
RESULTS AND DISCUSSION
To the best of our knowledge, our study is the first national study on the frequency of HDV infection and on distribution of HDV genotypes in Lebanon. The results of our study show that the prevalence of delta antibody among HBsAg-positive Lebanese patients and blood donors is ∼1% (three patients: one blood donor and two with chronic hepatitis) and contrasts sharply with the earlier report from Lebanon where HDV infection was studied [Reference Farci12]. In that older study, testing for HDV was done on 43 hospitalized patients with acute or chronic active HBV infection revealing absence of HDV infection in patients with acute HBV infection and in 12 asymptomatic HBsAg-positive carriers. Conversely, HDV infection was found in 40% of patients with chronic active hepatitis. The cohort included in this study consisted of hospitalized patients and might, therefore, not represent a true prevalence of HDV infection in Lebanon.
Of interest, our results are in contrast to the situation in neighbouring Arab [Reference Toukan16–Reference Saudy18] and non-Arab countries in the Mediterranean region [Reference Rezvan19–Reference Dan21]. For example, anti-HDV positivity was reported in 8·6% and 3·3% of Saudi patients and blood donors respectively [Reference Al-Traif17] compared to 32·7% and 5·2% in Turkish patients and blood donors respectively [Reference Balik20]. HDV infection is common in Asian populations [Reference Mumtz22] and is also more common in intravenous drug users [Reference Dan21, Reference Kao23]. None of our patients was an intravenous drug user. The prevalence of HDV has been reported to have decreased significantly over the past decade in various studies [Reference Huo24]. The cause for decreasing HDV infection can be attributed to many factors including anti-HBV vaccination [Reference Mast25], introduction and wide use of disposable needles for blood sampling and other medical purposes in high-risk populations [Reference Huo26], in addition to a natural decline in the HDV endemicity level in some communities [Reference Sagnelli27]. A combination of these factors may have contributed to the decrease in the prevalence of HDV infection in our population.
Although a small number of anti-HDV-positive samples were analysed in this study (three samples), only one was HDV-RNA positive and was characterized as HDV genotype I. This genotype is reported to be the predominant genotype in Egypt [Reference Saudy18], Turkey [Reference Rozdayi28], Iran [Reference Behzadian29] and is perhaps the predominant genotype in the Mediterranean region. Genotype I of HDV has been reported to occur worldwide and to cause chronic hepatitis with a wide spectrum of severity [Reference Cotrina30]. This is in contrast to HDV genotype II reported in East Asia, Japan and Taiwan which often results in mild chronic hepatitis [Reference Ivaniushina31] and to HDV genotype III – confined mainly to North and South America – and which usually results in severe infection [Reference Nakano32]. Recently we have shown that genotype D was the only genotype found in Lebanese patients with chronic hepatitis B [Reference Sharara13]. Parallel to genotype D of HBV, genotype I of HDV seems to be the unique genotype found in the Lebanese population. No genotypic diversity was found in Lebanese patients with chronic hepatitis B and D. Our study, along with others from neighbouring countries [Reference Al-Traif17, Reference Kao23–Reference Mast25] point to the predominance of both HBV genotype D and HDV genotype I in countries of the Middle East.
With the introduction of mandatory HBV vaccination to all newborns in Lebanon in 1992–1993 along with other preventive measures, it is anticipated that the decrease in the prevalence of HBV infection may deplete the carrier reservoir of HBV and may lead to complete control and possibly eradication of HDV infection in Lebanon.
ACKNOWLEDGEMENTS
This work was supported partially by a grant from Schering-Plough and partially by the University Research Board (URB) of the American University of Beirut. The authors thank Drs B. Farhat, M. Bahlawan, U. Farhat, M. Alameddine, E. Nour, R. Sayegh, C. Yaghi, H. Assi, A. Ferzli and R. Shatila. Special thanks go to Mrs Maha Abul Naja for typing the manuscript.
DECLARATION OF INTEREST
None.



