Accurate food intake measurement is a major challenge in nutritional epidemiological studies, as this parameter affects the validity of associations between dietary habits and health outcomes( Reference Prentice 1 – Reference Bingham, Luben and Welch 5 ). Energy intake (EI) under-reporting (UR) is a common source of bias in dietary surveys( Reference Goldberg, Black and Jebb 6 – 10 ); for instance, Black et al. ( Reference Black, Goldberg and Jebb 7 ) found that in surveys employing diet records, diet recall and diet history, UR, respectively, affected 64, 88 and 25 % of the studied samples.
Methods used for the validation of EI are based on the principle that EI is equal to energy expenditure (EE) when body weight is stable. EE can be estimated from the BMR and the energy needed for a given physical activity level (PAL). BMR itself can be predicted from the weight and height of subjects by applying one of the several published equations( Reference Schofield 11 – Reference Henry 16 ), the choice of which can influence the classification of subjects as under-reporters. Goldberg and Black's( Reference Goldberg, Black and Jebb 6 , Reference Black, Goldberg and Jebb 7 , Reference Black 9 ) method for identifying under-reporters is based on the comparison of the ratio of the reported EI to the BMR with the PAL. To determine whether a given mean or individual value of reported EI:BMR is acceptable, the ratio has to fall within the 95 % confidence limit of a value defined by the Goldberg and Black equation; values below or above this cut-off are considered to correspond to under-reporters or over-reporters, respectively.
A better understanding of the factors that affect EI reporting is important for improving dietary assessment and perhaps even nutritional survey design. Previous investigations of the characteristics of under-reporters( Reference Lafay, Basdevant and Charles 17 – Reference Mendez, Popkin and Buckland 31 ) have shown that women( Reference Macdiarmid and Blundell 18 , Reference Yannakoulia, Panagiotakos and Pitsavos 25 – Reference Lutomski, Van Den Broeck and Harrington 28 ), the elderly( Reference Macdiarmid and Blundell 18 , Reference Johansson, Wikam and Ahrén 21 , Reference Rasmussen, Matthiessen and Biltoft-Jensen 26 – Reference Lutomski, Van Den Broeck and Harrington 28 ) and overweight individuals( Reference Macdiarmid and Blundell 18 , Reference Johansson, Wikam and Ahrén 21 , Reference Yannakoulia, Panagiotakos and Pitsavos 25 , Reference Poslusna, Ruprich and De Vries 27 , Reference Mendez, Wynter and Wilks 30 , Reference Mendez, Popkin and Buckland 31 ) are more likely to under-report their EI. In contrast, data on the effects of several other factors (such as food habits, moderate-to-vigorous physical activity and sedentary behaviour( Reference Lioret, Touvier and Balin 29 , Reference Meng, Kerr and Zhu 32 ), socio-economic factors( Reference Macdiarmid and Blundell 18 , Reference Johansson, Wikam and Ahrén 21 , Reference Yannakoulia, Panagiotakos and Pitsavos 25 , Reference Poslusna, Ruprich and De Vries 27 , Reference Schoch and Raynor 33 ) and body-image perception( Reference Yannakoulia, Panagiotakos and Pitsavos 25 , Reference Poslusna, Ruprich and De Vries 27 , Reference Lutomski, Van Den Broeck and Harrington 28 )) are scarce or inconsistent.
The reasons for UR may differ from one country to another, as nutritional habits are partly determined by cultural and geographical factors( Reference Harrison, Galal and Ibrahim 34 , Reference Wyndels, Dallongeville and Simon 35 ). In France, previous studies have estimated UR among the nationally representative Individuelle Nationale des Consommations Alimentaires 2 (INCA2) dietary survey participants and have characterised UR in specific populations of children( Reference Lioret, Touvier and Balin 29 ) and adults( Reference Lafay, Basdevant and Charles 17 , Reference Lafay, Mennen and Basdevant 20 , Reference Sallé, Ryan and Ritz 24 ). On the basis of the INCA2 survey, the primary objective of the present study was to identify factors associated with energy UR in adults, including several that have not been well characterised to date (geographical area of residence, sedentary behaviour and the place where lunch is eaten). The secondary objective was to assess the effect of estimating BMR with two different equations – namely the Oxford( Reference Henry 16 ) and the Schofield equations( Reference Schofield 11 ) – on the prevalence of UR and the characteristics of under-reporters (see Supplementary Tables 1 and 2, available online).
Study design and subjects
The French INCA2 cross-sectional survey was carried out between December 2005 and May 2007 by the French Food Safety Agency (Agence française de sécurité sanitaire des aliments)( 36 ). The survey was primarily designed to assess food intake patterns. A complex sampling design was used to obtain a nationally representative sample of French people( 36 – Reference Dufour, Lafay and Volatier 38 ). Briefly, two independent, random samples (adults aged between 18 and 79 years and children aged between 3 and 17 years) were obtained using a multistage cluster sampling technique. The sampling frame was based on the national census published by the French National Institute of Statistics and Economic Studies (Institut National de la Statistique et des Études Économiques). First, 181 primary geographical units (stratified by the region of residence and the number of inhabitants in the urban area) were randomly selected with probability proportional to size. Second, households were randomly drawn from each primary unit, and two independent sampling frames were set up: the first was restricted to households including at least one child and the second included households regardless of the presence of children. Lastly, either a child or an adult was randomly selected from each household. The participation rate for subjects aged 18 years or above was 63 %, which yielded a sample of 2624 adults (women: 58·6 %).
The INCA2 protocol was approved by the French Data Protection Authority (Commission Nationale Informatique et Liberté) and the French National Council for Statistical Information (Conseil National de l'Information Statistique), especially the Committee of Label for Statistical Surveys (Comité du Label des Enquêtes Statistiques). Verbal informed consent was obtained from all the subjects. Verbal consent was witnessed and formally recorded.
Measurements
Food intake was assessed using a 7 d, open-ended, estimated food diary. Data on behavioural, demographic and socio-economic variables were collected through self-questionnaires and face-to-face questionnaires. The region of residence was recorded during a face-to-face interview. A trained, certified investigator handed over the food diary and the self-questionnaire at each respondent's home and spent 45–60 min explaining how to fill them out. The investigator returned to the respondents' home after the weeklong survey and checked the accuracy of the information recorded in the documents.
Age and weight status
Age (in years) was coded as a qualitative variable according to the categories in the Schofield and Oxford equations; this resulted in three groups: 18–30; 30–60; >60. Anthropometric data were collected during home visits by trained interviewers. Weight was measured in light clothes and without shoes to the nearest 0·1 kg using electronic scales (Teraillon). Height was measured to the nearest centimetre (in the standing position and without shoes) with a portable gauge. Based on the guidelines of World Health Organization( 39 ), BMI was categorised into thin (BMI < 18·5 kg/m2), normal (BMI 18·6–24·9 kg/m2), overweight (BMI 25·0–29·9 kg/m2) and obese (BMI ≥ 30 kg/m2).
Geographical area of residence
The participants were classified according to their region of residence into a three-class variable: ‘northern France’ (Champagne, Picardie, Haute-Normandie, Centre, Basse-Normandie, Bourgogne, Nord, Lorraine, Alsace, Franche-comté, Pays de Loire and Bretagne); ‘southern France’ (Poitou Charente, Aquitaine, Midi-Pyrénées, Limousin, Rhône-Alpes, Auvergne, Languedoc and Provence côte d'azur); ‘Paris region’. The latter class covered the ‘Ile de France’ administrative region of France.
Dietary intake data
Dietary intake was recorded in the 7 d food diaries. In the diaries, each day was divided into three main meal (i.e. breakfast, lunch and dinner) periods and three between-meal periods. The participants estimated portion sizes using the validated Supplémentation en Vitamines et Minéraux Anti-oxydants (SU-VI-MAX)( Reference Hercberg, Deheeger and Preziosi 40 ) photographic booklet. If they preferred, the participants could also choose to express the amounts eaten in grams using their own household measures and scales. Dietary intake was evaluated using the food composition tables from the French Data Centre on Food Quality (Centre d'information sur la qualité des aliments)( Reference Favier, Ireland and Toque 41 , Reference Ireland, du Chaffaut and Oseredczuk 42 ). The average daily EI (in MJ/d) and the percentage of contribution of carbohydrates, fat and proteins to total EI were assessed. The contribution of nutrients to EI was addressed as categorical variables based on the tertiles of their distribution, leading to three levels (‘low’, ‘intermediate’ and ‘high’). The frequencies of consumption of (1) cereal products, (2) meat, poultry, fish and eggs and (3) dairy products (milk, milk products and cheese) were also assessed from the 7 d food diaries and coded into the following three categories: ‘every day’; ‘every week’; ‘don't know’.
Food habits and concerns
The possible places at which lunch was eaten were as follows: ‘at home’; ‘staff canteen’; ‘at work but not in the canteen’; ‘at a friend's or family member's home’; ‘at a fast-food outlet or restaurant’. Whenever food or drink was consumed, the participants had to mention whether this was taken as a meal or as a snack. ‘Snacking frequency’ was defined as episodes of eating between the main meals and was coded as ‘regularly’ ( ≥ once a day), ‘occasionally or never’ ( ≥ once a week or never) or ‘don't know’. The usual frequency of fast-food consumption was coded as ‘regularly’ ( ≥ once a week), ‘occasionally or never’ ( ≥ once a year or never) or ‘don't know’. The usual frequency of purchasing food from a vending machine was coded as ‘regularly’ ( ≥ once a day), ‘occasionally or never’ ( ≥ once a week or never) or ‘don't know’.
Socio-economic status
Occupational status was divided into four categories: ‘farmers or junior managers’; ‘workers’; ‘senior managers’; ‘homemakers or students’. Educational level was divided into three categories: ‘primary school’; ‘secondary school’; ‘university’. Household living standards were addressed as follows: ‘having gone away on holiday for more than 4 d within the last 3 months’ (yes/no); ‘perception of financial situation’ (‘good’, ‘moderate’ or ‘poor’); ‘financial access to desired food products’ (‘good’, ‘moderate’ or ‘poor’).
Food and weight concerns
The respondents were also asked about their food concerns: the variable ‘perception of diet quality’ was divided into three categories (‘good’, ‘not good’ and ‘don't know’).
The participants were asked to state whether they were on a restrictive diet at the time of the survey (‘yes’/‘no’) and whether they had been on a weight-loss diet during some time in the past and how they perceived their current weight (‘normal’, ‘overweight’, ‘too thin’ or ‘don't know’).
Sedentary behaviour and physical activity
Sedentary behaviour was measured in min/d, as described elsewhere( Reference Lioret, Touvier and Balin 29 ). Briefly, a global index labelled ‘total screen time’ (based on the time spent watching television and the time spent in front of a computer or playing video games) was divided into tertiles of sedentary behaviour (‘high sedentariness’, ‘moderate sedentariness’ and ‘low sedentariness’). The moderate-to-vigorous intensity physical activity of each participant was assessed using the validated International Physical Activity Questionnaire( Reference Lee, Macfarlane and Lam 43 ) and treated as a qualitative variable (‘high’, ‘moderate’ and ‘low’).
Classification of under-reporters, normal reporters and over-reporters
UR and over-reporting were investigated using Goldberg's CUT-OFF 2 criterion( Reference Goldberg, Black and Jebb 6 , Reference Black 9 ). A hypothesis of a moderately inactive lifestyle was set for the entire sample, leading to a PAL value of 1·55( 44 ). The BMR was estimated using the Schofield( Reference Schofield 11 ) and Oxford equations( Reference Henry 16 ) for age and sex categories. Since the BMR values obtained with each of these equations were highly correlated (R 2 0·97), the results obtained using the Oxford equation were considered in the primary analysis. Goldberg's CUT-OFF 2 was established as follows:
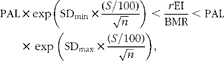 $$\begin{eqnarray} PAL\times exp\left (SD_{min}\times \frac {( S /100)}{\sqrt { n }}\right )< \frac { r EI}{BMR}< PAL\times \,exp\,\left (SD_{max}\times \frac {( S /100)}{\sqrt { n }}\right ), \end{eqnarray}$$
$$\begin{eqnarray} PAL\times exp\left (SD_{min}\times \frac {( S /100)}{\sqrt { n }}\right )< \frac { r EI}{BMR}< PAL\times \,exp\,\left (SD_{max}\times \frac {( S /100)}{\sqrt { n }}\right ), \end{eqnarray}$$
where n is equal to 1 (for data on the individual level), the standard deviation (sd min) is ± 1·96 (corresponding to the lower and upper boundaries of the 95 % CI) and S is calculated as follows:
where d is the number of recording days (seven in most cases), CVwEI is the within-subject variation in EI (23 %), CVwBMR is the precision of the estimated BMR relative to the measured BMR (8·5 %) and CVtP is the between-subject variation in PAL (15 %). The participants were classified by creating dichotomous outcomes (1 = under-reporters; 0 = not under-reporters) or (1 = over-reporters; 0 = not over-reporters).
Statistical analyses
All the analyses were carried out using the SAS software (version 9.3; SAS Institute Inc.). Data were weighted for unequal sampling probabilities: a complex unproportional three-degree design with stratification was applied to the sampled subjects included in the survey. The primary units were constituted of geographical areas, and stratification was applied to the degree of urbanisation. The secondary units were constituted of housing, with no stratification on rural area and stratification on urban area. The third degree was constituted of subjects inside housing. According to the INCA2 adult population (n 2624), 234 men (21 %) and 577 women (37 %) were on a weight-loss diet and 28 (1·8 %) women were pregnant at the time of data collection. Anthropometric data (weight and/or height) were missing for 9 % of the participants (n 254). Therefore, all these subjects were excluded from the analysis, to comply with Goldberg's steady-state criterion and to improve the accuracy of the data( Reference Goldberg, Black and Jebb 6 , Reference Black 9 ). Some of the excluded participants were characterised by more than one of these criteria. In this step, over-reporters accounted for 3·4 % (n 60) of the sample and were also excluded (leading to the final sample of 1567 individuals). At this step, the sample was stratified by sex and adjusted for age to analyse associations with UR. Continuous variables are presented as means and standard deviations, while categorical variables are presented as relative frequencies and 95 % CI. Associations between categorical variables were assessed with a χ2 test. Sex-stratified, unconditional logistic regressions (‘proc surveylogistic’ in SAS) were then carried out to identity factors associated with UR in men and in women. The following confounders were considered in univariate and multivariate analyses: sex; age; BMI; region of residence; educational level; lunch place; snacking frequency; cereal product intake; dairy product intake; high-protein product intake; perception of diet quality; past dieting; sedentary behaviour. All the variables associated with UR with a P value ≤ 0·20 in the univariate analysis were introduced into the logistic regression model. Collinearity between variables was tested. When two variables were strongly correlated, only one was chosen (depending on its significance level). A backward procedure was used, with a P value of 0·10 required for retention in the model. Analyses were adjusted for age. Odds ratios and 95 % CI were calculated. A P value < 0·05 was considered to be statistically significant.
Results
Characteristics of the study population
Sex distribution in the study sample (n 1567) was similar to that calculated for the French adult population by the Institut National de la Statistique et des Études Économiques( 45 ) (men = 48·2 % and women = 51·7 % v. men = 48·6 % and women = 51·4 %, respectively), which suggests that valid inferences can be drawn on the basis of the present results.
The characteristics of the participants (stratified by sex) are summarised in Table 1. The mean weight, height, BMI, EI and BMR were higher in men than in women (all P< 0·0001). In contrast, there were no significant inter-sex differences in age (P= 0·16) or EI/BMR (P= 0·96). The prevalence of UR was 24 % (95 % CI 20·4, 26·8) in men and 21 % (95 % CI 17·6, 23·8) in women with no statistical difference (P= 0·17).
Table 1 Characteristics of the participants* (Mean values with their standard errors)
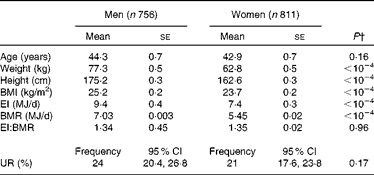
EI, energy intake; EI:BMR, ratio of energy intake to BMR; UR, under-reporting.
* Participants of the Individual and National Study on Food Consumption 2 except pregnant women, people on a diet, people who only reported weight and height without measurements, and over-reporters according to the Oxford equation.
† P according to surveyreg.
Relationships between under-reporting and covariates
The results of the univariate analysis are presented in Table 2 (for anthropometric, physical activity and socio-economic characteristics) and Table 3 (for dietary habits and perceptions). In men, the anthropometric and socio-economic factors associated with UR were age, BMI and occupation (all P< 0·05). The dietary factors associated with UR were snacking frequency, contributions of proteins and lipids to EI, perception of weight, perception of diet quality and previous weight-loss dieting (all P< 0·05). In women, the anthropometric and socio-economic characteristics associated with UR were BMI, geographical area of residence, educational level, occupation, holidays within the last 3 months, perception of financial situation and financial access to desired foods (all P< 0·05). Dietary factors associated with UR were snacking frequency, cereal product intake, contributions of proteins and carbohydrates to EI, perception of weight and perception of diet quality (all P< 0·05).
Table 2 Socio-economic and physical activity characteristics of the participants according to declaring status (Oxford's equation for the prediction of BMR), with sex stratification* (Percentages and 95 % confidence intervals)

NR, normal reporters; UR, under-reporters; MVPA, moderate-to-vigorous intensity physical activity.
* Participants of the Individual and National Study on Food Consumption 2 except pregnant women, people on a diet, people who only reported weight and height without measurements, and over-reporters according to the Oxford equation.
† χ2 test between the UR and NR groups, within each sex.
Table 3 Food habits and perception characteristics of the participants according to declaring status (Oxford's equation for the prediction of BMR), with sex stratification* (Percentages and 95 % confidence intervals)

NR, normo-reporters; UR, under-reporters; EI, energy intake.
* Participants of the Individual and National Study on Food Consumption 2 except pregnant women, people on a diet, people who only reported weight and height without measurements, and over-reporters according to the Oxford equation.
† χ2 test.
‡ At work but not in the canteen.
§ Milk, ultra-spawns dairy and cheese.
∥ Meat, poultry, fish and eggs.
The results of the multivariate analysis are given in Table 4. UR was independently and negatively associated with age (P= 0·01 and P= 0·006, respectively, in men and women) and positively associated with overweight, obesity (P< 0·0001 and P= 0·0002, respectively, in men and women) and protein intake (P< 0·0001 in both men and women). Men who reported eating lunch in a staff canteen were less likely to be under-reporters (P= 0·004), whereas men who stated frequently consuming dairy products (P= 0·04) or having a history of restrictive diet consumption were more likely to be under-reporters (P= 0·002). Women were less likely to under-report their EI if they lived in the Paris region (P< 0·0001, v. the other regions), were more educated (P= 0·03), preferred to eat at a family's or friend's home (P= 0·04) (rather than at home) or reported regular snacking (rather than occasional snacking) (P= 0·0001). Women were more likely to under-report if they usually ate lunch at work but not in a staff canteen (P= 0·04), when they reported weekly consumption of cereal products (P= 0·003), when they perceived their diet quality to be poor (P= 0·01) or when they had a moderate level of sedentary behaviour (P= 0·009).
Table 4 Under-reporting according to sociodemographic, anthropometric, behavioural and nutritional variables (Oxford equation for the prediction of BMR)* (Odds ratios and 95 % confidence intervals)

* Participants of the Individual and National Study on Food Consumption 2 except pregnant women, people on a diet, people who only reported weight and height without measurements, and over-reporters according to the Oxford equation.
† Stepwise multivariate logistic regression analysis (P of the model). Only the OR of the variables selected by the stepwise logistic regression are given.
‡ At work but not in the canteen.
§ Milk, ultra-spawns dairy and cheese.
∥ Meat, poultry, fish and eggs.
Comparison between the Oxford and Schofield equations for the prediction of BMR
We repeated the same analyses on the basis of the Schofield equation. The BMR, respectively, estimated by the Schofield and Oxford equations displayed a high degree of agreement in terms of the classification of participants according to their reporting status (Supplementary Table 1, available online). Overall, the characteristics associated with UR (age, weight status, contribution of proteins to EI) were the same (Supplementary Table 2, available online). However, there were some minor differences for both men and women. This was especially true for the two variables assessing the PAL, as sedentary behaviour was no longer significantly associated with UR in women and the International Physical Activity Questionnaire score was negatively associated with UR in men.
Discussion
In the present study of a representative sample of the French adult population based on the collection of validated food intake data, the overall prevalence of UR was 22·5 %. This value was similar in men and women; however, several characteristics of under-reporters differed according to sex. Earlier studies( Reference Black, Goldberg and Jebb 7 , Reference Livingstone and Black 23 – Reference Poslusna, Ruprich and De Vries 27 , Reference Mendez, Popkin and Buckland 31 , Reference Subar, Kipnis and Troiano 46 – Reference Tomoyasu, Toth and Poehlman 55 ) have reported overall UR rates ranging from about 10 % to up to 60 %, depending on methodological constraints (e.g. dietary intake data collection and EE measurement or estimation methods) and the characteristics of the study population (sex, age, BMI and PAL)( Reference Poslusna, Ruprich and De Vries 27 ). The prevalence of 22·5 % found in the present study falls within the range of values recorded in other European populations – especially when a similar methodology is used( Reference Yannakoulia, Panagiotakos and Pitsavos 25 , Reference Mendez, Popkin and Buckland 31 , Reference Ferrari, Slimani and Ciampi 47 , Reference Becker, Foley and Shelley 49 , Reference Murakami, Sasaki and Okubo 50 ). For instance, the prevalence of UR was 26 % in a survey of 2000 Swedish men and women aged from 1 to 74 years, using a 7 d record and a PAL cut-off of 1·27( Reference Becker, Foley and Shelley 49 ). In a study that used the ‘gold standard’ doubly labelled water method for the evaluation of EE, the prevalence of UR in a similar Anglo-Saxon population of adults was 20 %( Reference Subar, Kipnis and Troiano 46 ), which is close to the prevalence found for the French adult population in the present study.
The method used for the estimation of UR must be sensitive, specific and accurate. Of the various BMR prediction equations published to date, those of Mifflin et al. ( Reference Mifflin, St Jeor and Hill 14 ), Schofield( Reference Schofield 11 ), Müller et al. ( Reference Müller, Bosy-Westphal and Klaus 15 ) and Oxford( Reference Henry 16 ) are used particularly frequently. An equation is considered as validated if, when applied to a population other than the one originally used for derivation, 70–75 % of the participants are correctly assigned an energy requirement within 10 % of the measured value( 56 ). Thus, it is particularly important to choose a prediction equation derived from a population that is as similar as possible to the study population. In the present study, we chose the Oxford equation because the derivative population was broader than that used by Schofield( Reference Schofield 11 , Reference Henry 16 , 56 ). However, the prevalence of UR estimated using the Oxford equation – used in the present study for the first time for a representative sample of the adult population across France – provided much the same results as those obtained using the Schofield equation (in terms of both prevalence and classification). This suggests that the use of the Oxford equation would not distort comparisons between the successive INCA surveys.
We found a similar prevalence of UR in men and women. The literature on the effect of sex on UR remains unclear; women are more inclined to under-report than men in most( 10 , Reference Macdiarmid and Blundell 18 , Reference Yannakoulia, Panagiotakos and Pitsavos 25 – Reference Lutomski, Van Den Broeck and Harrington 28 ) but not in all studies( Reference Poslusna, Ruprich and De Vries 27 , Reference Dlugosz and Wadolowska 52 – Reference Livingstone, Prentice and Strain 57 ). The reasons for this disparity may be related to differences in study design (age range and exclusion criteria for the final sample), cultural factors related to each participant's perception of his/her dietary intake or inter-country differences in social pressure for body-shape aesthetic references. Indeed, earlier research on sex differences in energy reporting has suggested that women are more sensitive to the social pressure to conform to an expected image, which may result in UR of EI( Reference Macdiarmid and Blundell 18 , 44 , Reference Herbert, Clemow and Pbert 58 , Reference Herbert, Yunsheng and Clemow 59 ). Recent reports have suggested that this pressure is now much the same for men and women in France, which may explain a similar prevalence of UR in men and women in the present study( 60 ).
In addition to estimating the prevalence of UR, we sought to characterise under-reporters. We found that BMI, age, the usual lunch place, a lower frequency of consumption of some food groups and the contribution of proteins to EI were associated with UR. In agreement with the findings of most of the other studies, overweight was strongly and positively associated with UR( Reference Macdiarmid and Blundell 18 , Reference Johansson, Wikam and Ahrén 21 , Reference Poslusna, Ruprich and De Vries 27 , Reference Mendez, Popkin and Buckland 31 , Reference Becker, Foley and Shelley 49 , Reference Freisling, van Bakel and Biessy 51 , Reference Sichert-Hellert, Kersting and Schoch 61 ); for instance, Freisling et al. ( Reference Freisling, van Bakel and Biessy 51 ) estimated that the probability of being identified as an under-reporter increased by 66 % for each five unit in adjusted BMI. Similarly, older people were less likely to under-report their EI, an observation that remains subject to debate( Reference Macdiarmid and Blundell 18 , Reference Yannakoulia, Panagiotakos and Pitsavos 25 , Reference Sichert-Hellert, Kersting and Schoch 61 ), as elderly subjects are more likely to display recall bias during data collection( Reference Livingstone and Black 23 , Reference Yannakoulia, Panagiotakos and Pitsavos 25 ). However, others have shown that healthy older adults report as accurately as young adults do( Reference De Vries, de Groot and van Staveren 62 ). To the best of our knowledge, the influence of a person's usual lunch place has never been investigated previously in adults. In the present study, men/women were less likely to under-report ‘when they usually ate lunch in a staff canteen (rather than at home)’/‘when then usually ate lunch in a friend's or family member's home’. Similarly, in the INCA2 study of children( Reference Lioret, Touvier and Balin 29 ), eating regularly in the school canteen was found to be inversely associated with UR. These associations may reflect more structured eating behaviours, which may decrease the rate of UR. Lastly, UR was associated with the intake of certain foods and/or nutrients. Indeed, self-reporting of food and nutrient intake tends to depend on how socially desirable they are perceived to be( Reference Lafay, Basdevant and Charles 17 , Reference Macdiarmid and Blundell 18 , Reference Lafay, Mennen and Basdevant 20 , Reference Schoch and Raynor 33 , Reference Herbert, Clemow and Pbert 58 , Reference Herbert, Yunsheng and Clemow 59 ). Similar differences have been observed in both men and women, with a lower frequency of consumption (rather than the reported portion size) being related to UR( Reference Millen, Tooze and Subar 63 ). Notably, the contribution of protein-containing foods to EI was positively and strongly associated with UR in both men and women, which may suggest that these participants under-report their carbohydrate and/or lipid intake. The use of ‘gold standard’ biomarkers is still the only way to accurately measure macronutrient intake. For instance, the true prevalence of UR for protein intake in the Observing Energy and Protein Nutrition (OPEN) study (in which urinary N was used as a biomarker of protein intake v. 24 h dietary recall) ranged from 11 to 15 %( Reference Subar, Kipnis and Troiano 46 ).
Educational level and geographical area of residence were independently associated with UR in women. Most studies carried out in adults have shown that UR is more prevalent in groups with a lower educational level – probably due to the skills needed to complete diet questionnaires accurately and less time investment in nutritional matters( Reference Macdiarmid and Blundell 18 , Reference Lutomski, Van Den Broeck and Harrington 28 , Reference Lioret, Touvier and Balin 29 ). The association between UR and the geographical area of residence is poorly documented. In the present study, women living in the Paris region were less likely to under-report than those living in northern or southern France. Similar regional associations have been reported in Japan( Reference Murakami, Sasaki and Okubo 50 ) and Poland( Reference Dlugosz and Wadolowska 52 ), which may be related to cultural factors or urban lifestyle. A higher snacking frequency was associated with less UR, which may be related to a higher EI Indeed, this observation first demonstrates that the EI of snacking women is high enough to balance their EE( Reference Black 8 , Reference Black 9 , Reference Macdiarmid and Blundell 18 , Reference Summerbell 64 ). Then, further analyses should be carried out to discriminate the respective contribution of snacks and that of meals to the total EI and to identify eventual specific food patterns or specific behaviour in this population. On the other hand, poor perception of one's diet quality was also related to UR, which may suggest that foods with a poor image tend to be omitted from food records more frequently. Lastly, the present study is the first to report that female under-reporters are also characterised by a moderate level of sedentary behaviour, which may be related to health awareness attitudes. Similarly, a history of slimming was specifically associated with UR( Reference Tooze, Subar and Thompson 65 ).
Besides the inherent limitations of a cross-sectional design and the possible existence of confounding factors that were not taken into account, few other limitations have to be mentioned. First, BMR was estimated using prediction equations and not measured with ‘gold standard’ methods (such as doubly labelled water). Biomarker-based methods have been used sometimes in large national populations( Reference Schleicher, McCoy and Powers 66 – Reference Tinker, Sarto and Howard 69 ) or in calibration studies among multicentre epidemiological surveys( Reference Slimani, Bingham and Runswick 70 ). However, their use remains limited because of their cost burden. Second, the value of PAL entered into Goldberg's criterion was chosen from the theoretical estimations provided by the World Health Organization some years ago( 44 ) rather than from specific studies on the energy expended in physical activity by the study population. Nevertheless, the best method for handling this fundamental issue is still subject to debate, and different methods and values of PAL have been suggested by various task forces, researchers( Reference Lee, Macfarlane and Lam 43 , Reference Briefel, Sempos and McDowell 71 , Reference Whybrow and Kirk 72 ) and European expert panels( 56 , 73 ). The difficulty in choosing a value of PAL judged to be appropriate to a studied population partly lies in the main methods used to measure or to estimate PAL: biomarker-based methods( 44 , 56 , 73 – Reference Ekelund, Aman and Yngve 75 ); adequate bibliography when available( Reference Herbert, Clemow and Pbert 58 ); theoretical values proposed by panel reports( 44 , 73 – Reference Shetty, Henry and Black 76 ). It has also to be pointed out that the measures of moderate-to-vigorous intensity physical activity levels, determined using a lifestyle questionnaire such as the International Physical Activity Questionnaire, that were estimated were not adequate for estimating the value of the PAL component of total EE with accuracy( 73 ). However, in the present study, as the theoretical value of PAL proposed by the World Health Organization( 44 ) for a sedentary lifestyle population was chosen, we assume that a misclassification of more active participants could exist. Lastly, an inherent limitation of the design of the present study is that it was not able to distinguish between restraint-eaters( Reference Macdiarmid and Blundell 18 ) and under-reporters.
A major strength of the present study is the use of a nationally representative sample (the INCA2 survey participants) for which many parameters related to food intake, socio-economic status, weight status, weight perception, physical activity and sedentary behaviour were recorded. The representativeness of the sample was ensured by (1) the sampling design, with randomisation and weighting, (2) the good participation rate (63 %) and (3) the low missing data rate. The present study constitutes a wide-ranging investigation of UR covariates and provides novel insights into this issue in adults. Moreover, the large number of participants enabled us to stratify by sex and analyse UR for the three age classes in the BMR prediction equations. The usual day-to-day variability in food intake was taken into account during 7 d recordings in most cases, which also constitutes a strength. Lastly, the fact that we objectively measured weight and height improved the robustness of two important criteria (the definition of UR and the BMI values of the participants)( Reference Hill and Roberts 77 – Reference Craig and Adams 79 ).
In conclusion, the present study highlights the diversity of the characteristics associated with UR in a French adult population and provides new insights, which should be investigated deeply. Some of these characteristics are related to dietary habits (e.g. snacking), suggesting that the methods used to collect dietary intake data should focus specifically on these factors. Other identified factors were related to the physiological characteristics of the participants (e.g. weight status), which influence the prediction of BMR.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114513003759
Acknowledgements
The authors thank Francis Guillemin for discussions and Colin Haslock for proof reading the manuscript for English language.
The study was supported by ANSES for the design and conduct of the study, analysis of the data, interpretation of the findings and preparation of the manuscript.
The authors' contributions are as follows: I. B. V. designed the study, analysed and interpreted the data, and wrote the manuscript; J. D. oversaw the conceptualisation of the study, interpretation of the results and writing of the manuscript; A. B. contributed to the statistical analyses; A. D. contributed to the conceptualisation of the study. J.-L. V. contributed to the literature search and the statistical analyses. All authors contributed to and approved the final draft of the paper.
None of the authors has any conflicts of interest to declare.






